Alkali Etching Hydrochar-Based Adsorbent Preparation Using Chinese Medicine Industry Waste and Its Application in Efficient Removal of Multiple Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Hydrothermal Carbonization and Alkali Etching
2.3. Analytical Method
2.4. Adsorption Experiment
2.5. Adsorption Kinetics Experiment
3. Results and Discussion
3.1. Pore Structure Analyses
3.2. Surface Microstructures Analyses
3.3. Functional Groups Analyses
3.4. Adsorption Properties Analyses of AE-CMIW Hydrochar for Multiple Pollutants
3.5. Adsorption Difference Analyses of AE-CMIW Hydrochar for Multiple Pollutants
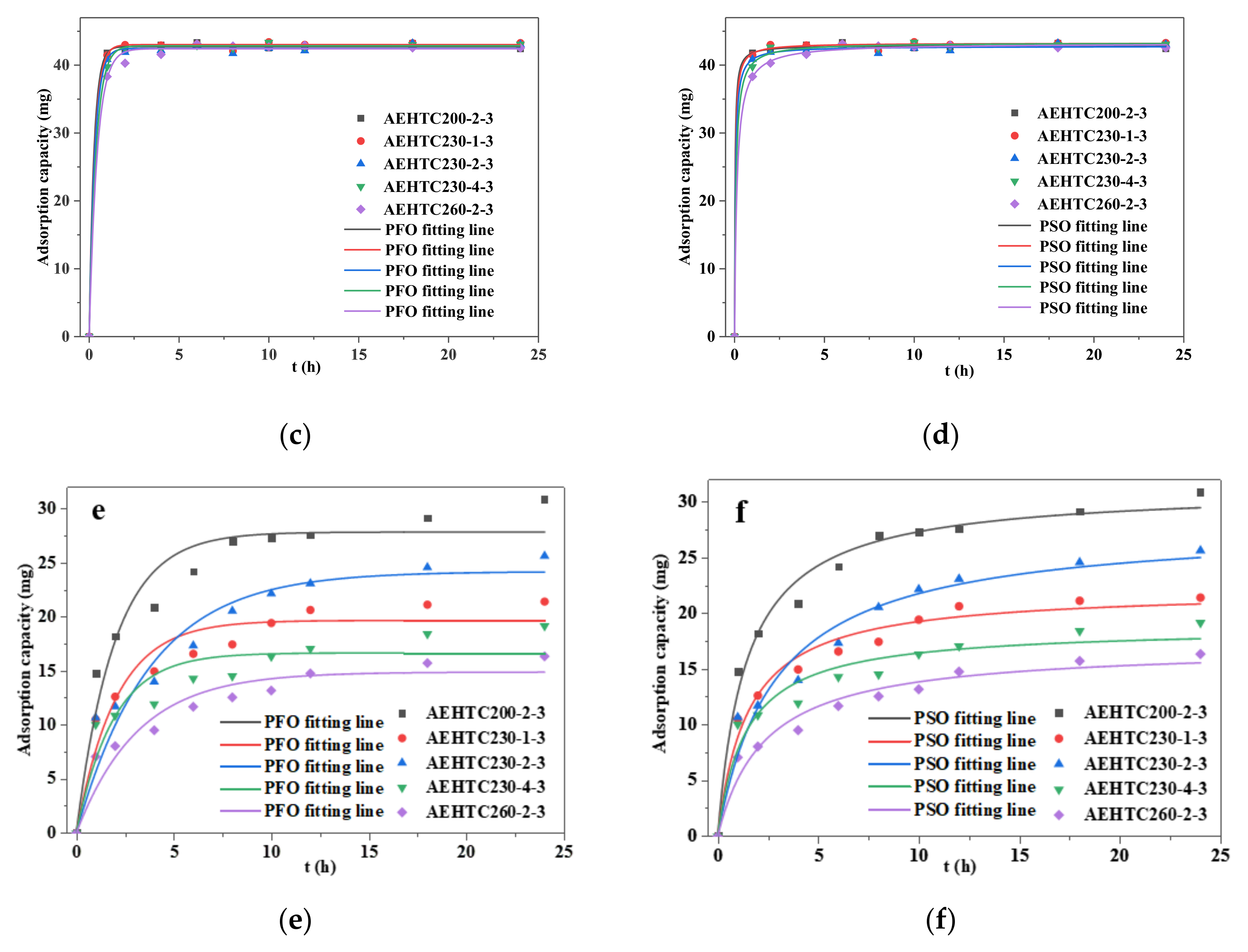
3.6. Adsorption Kinetics Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.K.; Jin, Q.Z.; Ye, X.M.; Lei, H.Y.; Jia, J.D.; Zhao, Z.H. Effect of process wastewater recycling on the chemical evolution and formation mechanism of hydrochar from herbaceous biomass during hydrothermal carbonization. J. Clean. Prod. 2020, 277, 123281. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Wang, L.J.; Liang, p.; He, Z.L.; Yan, G.H.; Liang, P.; He, Z.L.; Yan, G.H. Analysis of the status quo and future prospects of Chinese medicinal resources sustainable development. World Chin. Med. 2018, 13, 1752–1755. [Google Scholar]
- Chen, Z.Y.; Chen, H.S.; Wu, X.Y.; Zhang, J.H.; Evrendilek, D.E.; Liu, J.Y. Temperatureand heating rate-dependent pyrolysis mechanisms and emissions of Chinese medicine residues and numerical reconstruction and optimization of their nonlinear dynamics. Renew. Energ. 2021, 164, 1408–1423. [Google Scholar] [CrossRef]
- Shen, Q.B.; Wang, Z.Y.; Yu, Q.; Cheng, Y.; Liu, Z.D.; Zhang, T.P. Removal of tetracycline from an aqueous solution using manganese dioxide modified biochar derived from Chinese herbal medicine residues. Environ. Res. 2020, 183, 109195. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, D.; Niu, X.; Guo, H.; Yu, Y.; Tang, Z.; Lin, Z.; Fu, M. Insights into CO2 adsorption on KOH-activated biochars derived from the mixed sewage sludge and pine sawdust. Sci. Total Environ. 2022, 826, 154133. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. The Law of the People’s Republic of China on the Prevention and Control of Environmental Pollution by Solid Waste. 2020. Available online: https://www.mee.gov.cn/ywgz/fgbz/fl/202004/t20200430_777580.shtml (accessed on 30 April 2020).
- Zhamiyeva, R.; Sultanbekova, G.; Balgimbekova, G.; Mussin, K.; Abzalbekova, M.; Kozhanov, M. Problems of the effectiveness of the implementation of international agreements in the field of waste management: The study of the experience of Kazakhstan in the context of the applicability of European legal practices. Int. Environ. Agreem. 2022, 22, 177–199. [Google Scholar] [CrossRef]
- Liu, L.M.; Zhai, Y.B.; Liu, X.M.; Liu, X.p.; Wang, Z.X.; Zhu, Y.P.; Wang, Z.X.; Zhu, Y.; Xu, M. Features and mechanisms of sewage sludge hydrothermal carbonization aqueous phase after ferrous/persulfate-based process: The selective effect of oxidation and coagulation. J. Clean. Prod. 2022, 366, 132831. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G.; Zhu, Y. Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.H.; Duc Nguyen, D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qin, Q.Y.; Liu, X.; Wang, W.L. Effects of stepwise microwave synergistic process water recirculation during hydrothermal carbonization on properties of wheat straw. Front. Energy Res. 2022, 10, 846752. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qin, Q.Y.; Sun, X.; Wang, W.L. Hydrothermal Treatment: An Efficient Food Waste Disposal Technology. Front. Nutr. 2022, 9, 986705. [Google Scholar] [CrossRef] [PubMed]
- Panequea, M.; De la Rosaa, J.M.; Kernb, J.; Rezac, M.T.; Knicker, H. Hydrothermal carbonization and pyrolysis of sewage sludges: What happen to carbon and nitrogen? J. Anal. Appl. Pyrolysis 2017, 128, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Zhai, Y.B.; Zhu, Y.; Xu, B.B.; Wang, T.F.; Li, C.T.; Zeng, G.M. Production of char from sewage sludge employing hydrothermal carbonization: Char properties, combustion behavior and thermal characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Rangabhashiyam, S.; Dulta, K.; Umeh, C.T.; Iwuozor, K.O.; Aniagor, C.O.; Eshiemogie, S.O.; Iwuchukwu, F.U.; Igwegbe, C.A. Recent advances in hydrochar application for the adsorptive removal of wastewater pollutants. Chem. Eng. Res. Des. 2022, 184, 419–456. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, L.; Mo, H.; Chen, Y.; Hu, X. Microwaveassisted catalytic hydrothermal carbonization of Laminaria japonica for hydrochars catalyzed and activated by potassium compounds. Bioresour. Technol. 2021, 341, 125835. [Google Scholar] [CrossRef] [PubMed]
- Khoshbouy, R.; Takahashi, F.; Yoshikawa, K. Preparation of high surface area sludge-based activated hydrochar via hydrothermal carbonization and application in the removal of basic dye. Environ. Res. 2019, 175, 457–467. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, Z.H.; Chen, H.G.; Cai, T.; Liu, Z.D. Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: A critical review. Environ. Pollut. 2021, 268, 115910. [Google Scholar] [CrossRef]
- Ge, S.; Foong, S.Y.; Ma, N.L.; Liew, R.K.; Mahari, W.A.W.; Xia, C.; Yek, p.N.Y.; Peng, W.; Nam, W.L.; Lim, X.Y.; et al. Vacuum pyrolysis incorporating microwave heating and base mixture modification: An integrated approach to transform biowaste into eco-friendly bioenergy products. Renew. Sustain. Energy Rev. 2020, 127, 109871. [Google Scholar] [CrossRef]
- Verma, M.; Lee, I.Y.; Hong, Y.M.; Kumar, V.; Kim, H. Multifunctional β-Cyclodextrin-EDTA-Chitosan polymer adsorbent synthesis for simultaneous removal of heavy metals and organic dyes from wastewater. Environ. Pollut. 2022, 292, 118447. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Z.; Cui, L.; Li, Y.; Ngo, H.H.; Wang, Y.; Wei, Q.; Ma, H.; Yan, L.; Du, B. Fabrication of hyperbranched polyamine functionalized graphene for high-efficiency removal of Pb(II) and methylene blue. Chem. Eng. J. 2016, 287, 545–556. [Google Scholar] [CrossRef]
- Deng, J.H.; Zhang, G.M.; Gong, J.L.; Niu, Q.Y.; Liang, J. Simultaneous removal of Cd (II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem. Eng. J. 2013, 226, 189–200. [Google Scholar] [CrossRef]
- Qin, X.M.; Bai, L.; Tan, Y.Z.; Li, L.; Song, F.; Wang, Y.Z. β-Cyclodextrin-crosslinked polymeric adsorbent for simultaneous removal and stepwise recovery of organic dyes and heavy metal ions: Fabrication, performance and mechanisms. Chem. Eng. J. 2019, 372, 1007–1018. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Wu, L.; Hu, G.; Cui, Y. SERS detection and removal of mercury(II)/silver(I) using oligonucleotide-Functionalized core/shell magnetic silica sphere@Au nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 7371–7379. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kumar, V.; Vlaskin, M.S. Environmental Technology & Innovation Hydropyrolysis of freshwater macroalgal bloom for bio-oil and biochar production: Kinetics and isotherm for removal of multiple heavy metals. Environ. Technol. Innov. 2021, 22, 101440. [Google Scholar]
- Lakard, S.; Magnenet, C.; Mokhter, M.A.; Euvrard, M.; Buron, C.C.; Lakard, B. Retention of Cu(II) and Ni(II) ions by filtration through polymer-modified membranes. Separ. Purif. Technol. 2015, 149, 1–8. [Google Scholar] [CrossRef]
- Luo, S.; Qin, F.; Zhao, H.; Liu, Y.; Chen, R. Fabrication uniform hollow Bi2S3 nanospheres via Kirkendall effect for photocatalytic reduction of Cr (VI) in electroplating industry wastewater. J. Hazard. Mater. 2017, 340, 253–262. [Google Scholar] [CrossRef]
- Verma, M.; Mitan, M.; Kim, H.; Vaya, D. Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. J. Phys. Chem. Solid. 2021, 155, 110125. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, J.; Pan, B. Fabrication of novel magnetic nanoparticles of multifunctionality for water decontamination. Environ. Sci. Technol. 2016, 50, 881–889. [Google Scholar] [CrossRef]
- Guo, D.M.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Yang, D.J. Efficient removal of Pb(II), Cr(VI) and organic dyes by polydopamine modified chitosan aerogels. Carbohydr. Polym. 2018, 202, 306–314. [Google Scholar] [CrossRef]
- Hadi, p.; Guo, J.; Barford, J.; Mckay, G. Multilayer dye adsorption in activated carbons-facile approach to exploit vacant sites and interlayer charge interaction. Environ. Sci. Technol. 2016, 50, 5041–5049. [Google Scholar] [CrossRef]
- Li, C.; Yan, Y.; Zhang, Q.; Zhang, Z.; Huang, L.; Zhang, J.; Xiong, Y.; Tan, S. Adsorption of Cd2+ and Ni2+ from aqueous single-metal solutions on graphene oxide-chitosan-poly (vinyl alcohol) hydrogels. Langmuir 2019, 35, 4481–4490. [Google Scholar] [CrossRef] [PubMed]
- Pap, S.; Kirk, C.; Bremner, B.; Turk Sekulic, M.; Shearer, L.; Gibb, S.W.; Taggart, M.A. Low-cost chitosan-calcite adsorbent development for potential phosphate removal and recovery from wastewater effluent. Water Res. 2020, 173, 115573. [Google Scholar] [CrossRef]
- Verma, M.; Singh, K.P.; Kumar, A. Reactive magnetron sputtering based synthesis of WO3 nanoparticles and their use for the photocatalytic degradation of dyes. Solid State Sci. 2020, 99, 105847. [Google Scholar] [CrossRef]
- Hou, Z.; Wen, Z.; Wang, D.; Wang, J.; François-Xavier, C.p.; Wintgens, T.P.; Wintgens, T. Bipolar jet electrospinning bi-functional nanofibrous membrane for simultaneous and sequential filtration of Cd2+ and BPA from water: Competition and synergistic effect. Chem. Eng. J. 2018, 332, 118–130. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gao, B.; Zhao, S.N.; Wu, p.F.; Han, L.J.; Liu, X.; Wu, P.F.; Han, L.J.; Liu, X. Optimization of a “coal-like” pelletization technique based on the sustainable biomass fuel of hydrothermal carbonization of wheat straw. J. Clean. Prod. 2020, 242, 118426. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, Y.F.; Wang, M.Y.; Han, L.J.; Liu, X. Effects of hydrothermal carbonization conditions on the combustion and kinetics of wheat straw hydrochar pellets and efficiency improvement analyses. Energ. Fuel. 2020, 34, 587–598. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Peng, W.Q.; Han, L.J.; Xiao, W.H.; Liu, X. Effects of different pretreatments on compression molding of wheat straw and mechanism analysis. Bioresour. Technol. 2018, 251, 210–217. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liu, S.S.; Wang, M.M.; Ma, X.L.; Sun, X.; Liu, X.; Wang, L.S.; Wang, W.L. Hydrochar magnetic adsorbent derived from Chinese medicine industry waste via one-step hydrothermal route: Mechanism analyses of magnetism and adsorption. Fuel 2022, 326, 125110. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhang, X.Y.; Zhang, p.Z.; Liu, X.; Han, L.J.; Zhang, P.Z.; Liu, X.; Han, L.J. Facile fabrication of magnetic bio-derived chars by co-mixing with Fe3O4 nanoparticles for effective Pb2+ adsorption: Properties and mechanism. J. Clean. Prod. 2020, 262, 121350. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A review of treatment Technologies for the Mitigation of the toxic environmental effects of acid mine drainage (AMD). Process Saf. Environ. Prot. 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Mohamed, A.K.; Salam, M.A. Self-decoration of N-doped graphene oxide 3-D hydrogel onto magnetic shrimp shell biochar for enhanced removal of hexavalent chromium. J. Hazard. Mater. 2021, 408, 124951. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Liu, Y.C.; Qian, F.; Zhou, C.; Zhang, S.C.; Chen, J.M. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol. 2014, 154, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Mihajlović, M.; Petrović, J.; Maletić, S.; Isakovski, M.K.; Stojanović, M.; Lopičić, Z.; Trifunović, S. Hydrothermal carbonization of Miscanthus × giganteus: Structural and fuel properties of hydrochars and organic profile with the ecotox-icological assessment of the liquid phase. Energy Convers. Manag. 2018, 159, 254–263. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, Y.; Wang, T.; Peng, C.; Li, S.; Wang, B.; Liu, X.; Li, C. Effect of temperature on the sulfur fate during hydrothermal carbonization of sewage sludge. Environ. Pollut. 2020, 260, 114067. [Google Scholar] [CrossRef]
- Liu, X.; Zhai, Y.; Li, S.; Wang, B.; Wang, T.; Liu, Y.; Qiu, Z.; Li, C. Hydrothermal carbonization of sewage sludge: Effect of feed-water pH on hydrochar’s physicochemical properties, organic component and thermal behavior. J. Hazard. Mater. 2020, 388, 122084. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Huang, G.; Xu, C.; Lin, B. Hydrothermal carbonization synthesis of cassava slag biochar with excellent adsorption performance for Rhodamine B. J. Clean. Prod. 2020, 251, 119717. [Google Scholar] [CrossRef]
- Tu, R.; Jiang, E.C.; Yan, S.; Xu, X.W.; Rao, S. The pelletization and combustion properties of terrified Camellia shell via dry and hydrothermal torrefaction: A comparative evaluation. Bioresour. Technol. 2018, 264, 78–89. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, L.; Gao, Y.; Xu, J.; Chen, H.; Zhu, Y.; Zhu, C. Characterization and pelletization of cotton stalk hydrochar from HTC and combustion kinetics of hydrochar pellets by TGA. Fuel 2019, 244, 479–491. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Wan, J.; Xue, W.; Wen, X.; Liu, X.; Chen, S.; Lei, L.; Zhang, Q. Recent advances of biochar materials for typical potentially toxic elements management in aquatic environments: A review. J. Clean. Prod. 2020, 255, 119523. [Google Scholar] [CrossRef]
- Peng, H.; Gao, p.; Chu, G.; Pan, B.; Peng, J.; Xing, B.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; et al. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Karami, K.; Beram, S.M.; Bayat, p.; Siadatnasab, F.; Ramezanpour, A.; Bayat, P.; Siadatnasab, F.; Ramezanpour, A. A novel nanohybrid based on metal–organic framework MIL101−Cr/PANI/Ag for the adsorption of cationic methylene blue dye from aqueous solution. J. Mol. Struct. 2022, 1247, 131352. [Google Scholar] [CrossRef]
- Siruru, H.; Syafii, W.; Wistara, I.N.J.; Pari, G.; Budiman, I. Properties of sago waste charcoal using hydrothermal and pyrolysis carbonization. Biomass. Convers. Bior. 2020, 12, 5543–5554. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.M.; Yang, R.N.; Yang, B.C.; Quan, S.; Jiang, X. Effect of free carboxylic acid groups in UiO-66 analogues on the adsorption of dyes from water: Plausible mechanisms for adsorption and gate-opening behavior. J. Mol. Liq. 2019, 283, 160–166. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Choudhary, p.; Das, S.K.; Choudhary, P.; Das, S.K. Biomaterial functionalized graphene-magnetite nanocomposite: A novel approach for simultaneous removal of anionic dyes and heavy-metal ions. ACS Sustain. Chem. Eng. 2018, 6, 6328–6341. [Google Scholar] [CrossRef]
- Usman, M.; Ahmed, A.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Simultaneous adsorption of heavy metals and organic dyes by β-Cyclodextrin-Chitosan based cross-linked adsorbent. Carbohydr. Polym. 2021, 255, 117486. [Google Scholar] [CrossRef]
- Sumalinog, D.A.G.; Capareda, S.C.; de Luna, M.D.G. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 210, 255–262. [Google Scholar] [CrossRef]
- Essandoh, M.; Kunwar, B.; Pittman, C.U.; Mohan, D.; Mlsna, T. Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem. Eng. J. 2015, 265, 219–227. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.Z.; Liu, J.L.; Fang, L.; Lv, J.Q.; Lv, K. Removal of aqueous-phase lead ions by dithiocarbamate-modified hydrochar. Sci. Total Environ. 2020, 714, 136897. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, H.; Guo, Y.; Liu, Z.; Jiao, W. Immobilization of heavy metals in contaminated soils by modified hydrochar: Efficiency, risk assessment and potential mechanisms. Sci. Total Environ. 2019, 685, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Chen, H.; Feng, Q.; Yao, D.; Chen, H.; Wang, H.; Zhou, Z.; Li, H.; Tian, Y.; Lu, X. Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod. 2017, 165, 221–230. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Rocher, V.; Kyriakou, G.; Greenway, G.M. Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of prosopis africana shell. J. Ind. Eng. Chem. 2014, 20, 3467–3473. [Google Scholar] [CrossRef]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef]
- Manjunath, S.V.; Baghel, R.S.; Kumar, M. Antagonistic and synergistic analysis of antibiotic adsorption on Prosopis juliflora activated carbon in multicomponent systems. Chem. Eng. J. 2020, 381, 122713. [Google Scholar] [CrossRef]
- Suteu, D.; Zaharia, C.; Badeanu, M. Kinetic modeling of dye sorption from aqueous solutions onto apple seed powder. Cell. Chem. Technol. 2016, 50, 1085–1091. [Google Scholar]
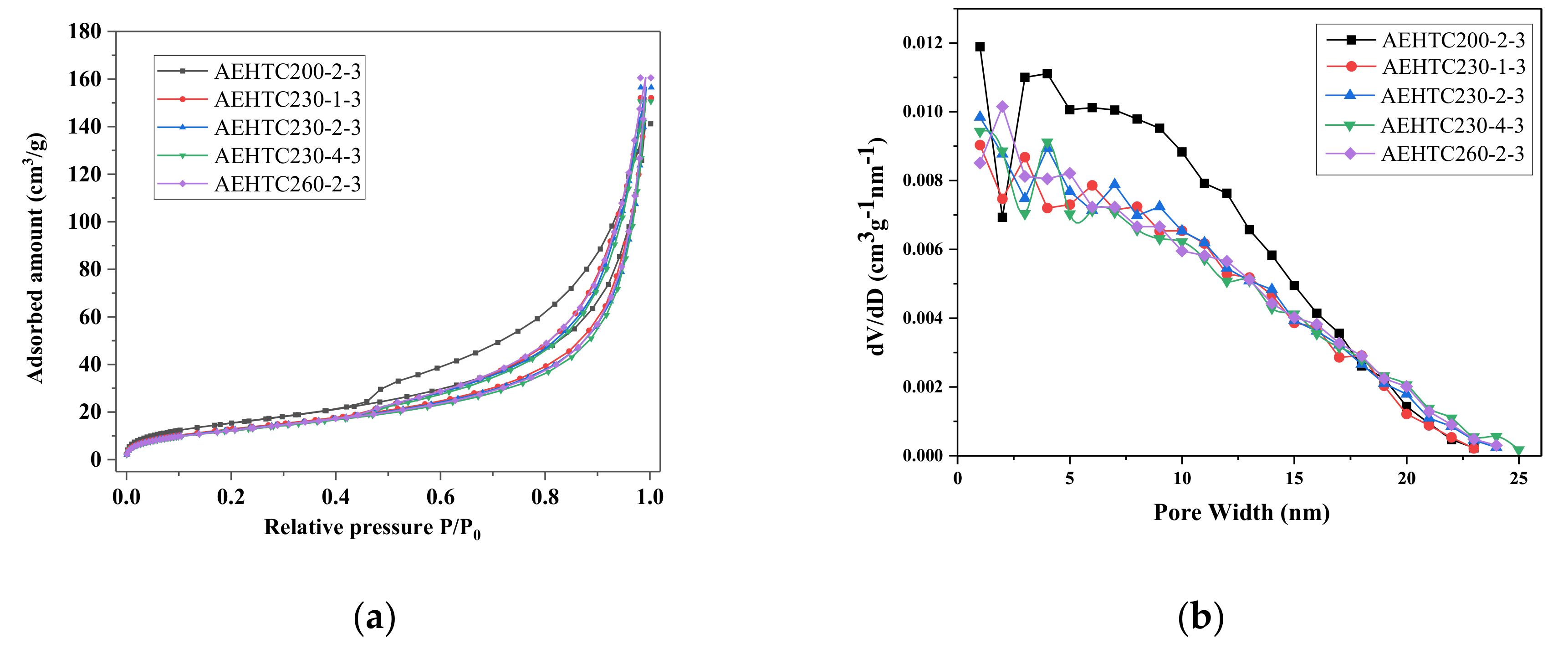
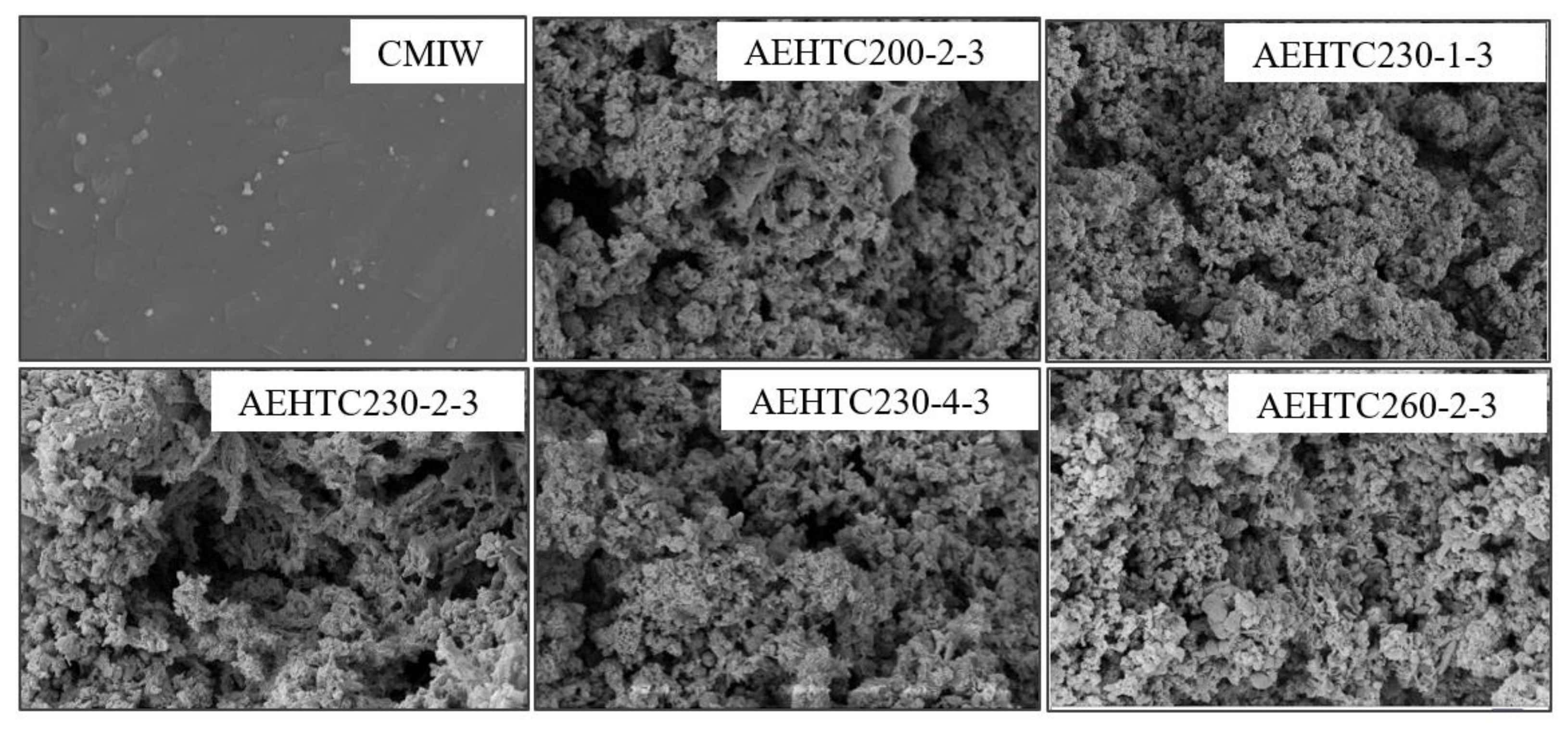
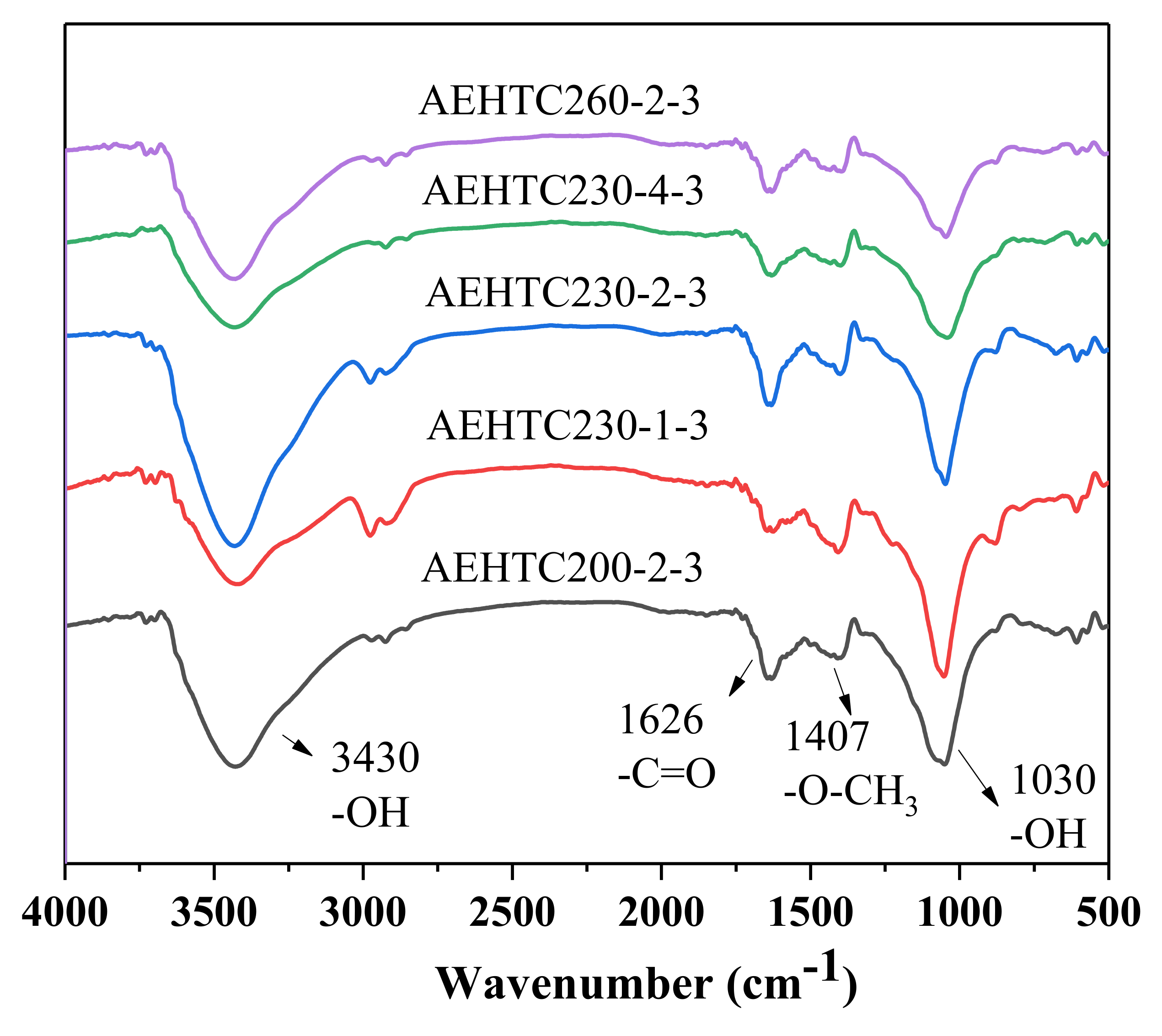
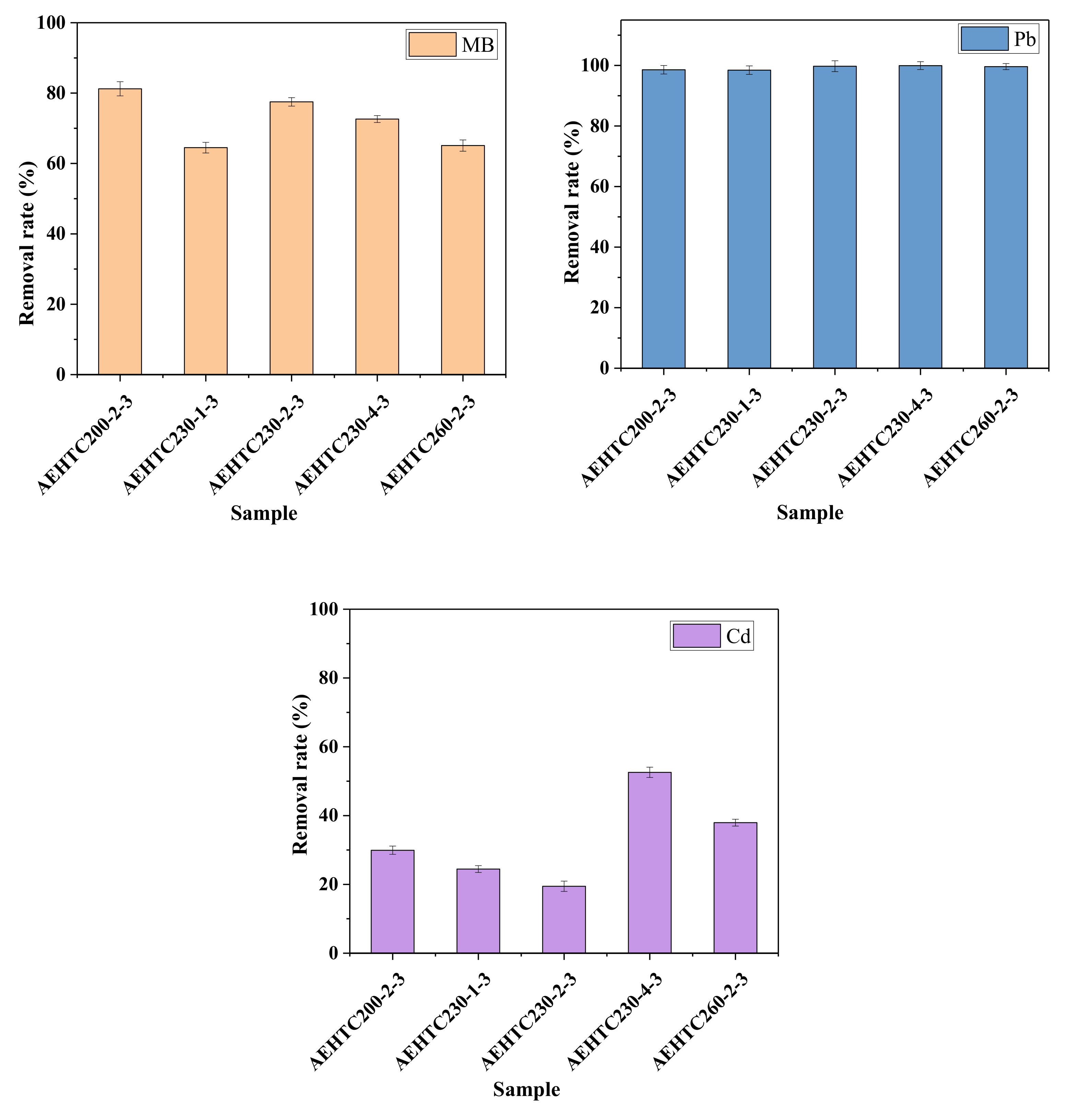
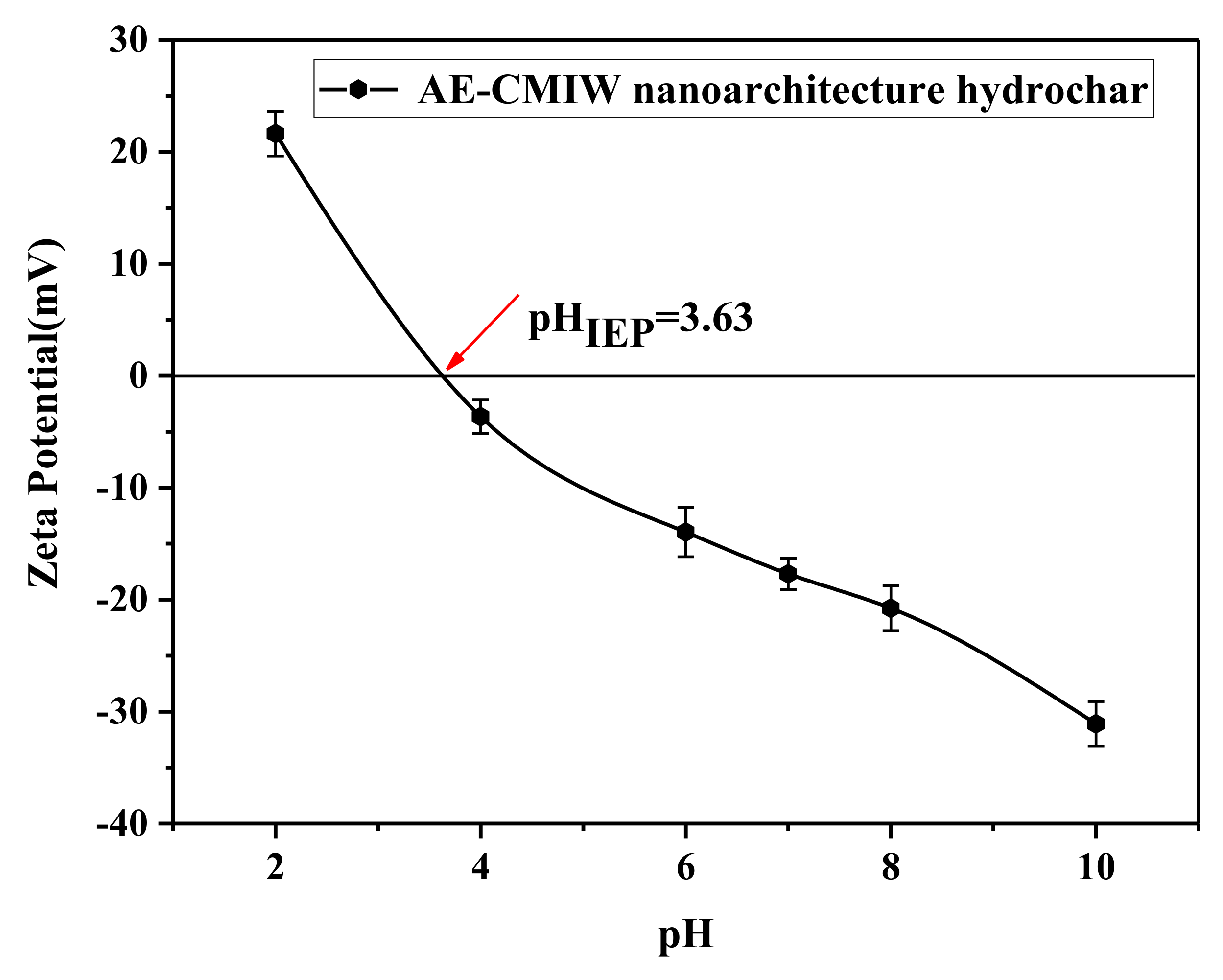

| Sample | SBET (m2/g) | Vtotal (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| CMIW | 19.121 ± 0.05 f | 0.055 ± 0.01 c | 11.445 ± 0.11 bc |
| AEHTC 200-2-3 | 53.466 ± 0.04 c | 0.213 ± 0.03 b | 14.729 ± 0.15 d |
| AEHTC 230-1-3 | 44.633 ± 0.06 e | 0.225 ± 0.02 b | 18.437 ± 0.13 bc |
| AEHTC 230-2-3 | 43.381 ± 0.07 b | 0.226 ± 0.02 b | 18.977 ± 0.10 bc |
| AEHTC 230-4-3 | 42.023 ± 0.05 a | 0.225 ± 0.04 a | 19.527 ± 0.09 a |
| AEHTC 260-2-3 | 42.611 ± 0.06 d | 0.236 ± 0.05 a | 20.056 ± 0.12 bc |
| Element Contents (w.t.%) | C | O | Fe | Cd | Pb |
|---|---|---|---|---|---|
| AEHTC 200-2-3 | 11.87 ± 0.01 a | 31.31 ± 0.02 a | 35.92 ± 0.01 a | 3.20 ± 0.07 a | 17.69 ± 0.02 a |
| AEHTC 230-1-3 | 5.03 ± 0.05 b | 22.69 ± 0.03 b | 42.38 ± 0.04 b | 2.54 ± 0.04 b | 27.37 ± 0.05 b |
| AEHTC 230-2-3 | 5.95 ± 0.02 a | 24.56 ± 0.05 a | 41.44 ± 0.06 a | 2.17 ± 0.02 a | 25.89 ± 0.04 a |
| AEHTC 230-4-3 | 6.44 ± 0.01 b | 24.73 ± 0.03 b | 44.78 ± 0.01 b | 5.21 ± 0.01 b | 18.84 ± 0.05 b |
| AEHTC 260-2-3 | 7.90 ± 0.01 a | 27.82 ± 0.02 a | 39.13 ± 0.02 a | 4.59 ± 0.01 a | 20.55 ± 0.02 a |
| Sample | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (h−1) | R2 | qe (mg/g) | k2 (g/mg · h) | R2 | ||
| AEHTC 200-2-3 | MB | 29.471 | 1.109 | 0.986 | 31.076 | 0.067 | 0.995 |
| Pb2+ | 42.738 | 3.772 | 0.999 | 42.922 | 0.858 | 0.999 | |
| Cd2+ | 27.823 | 0.510 | 0.939 | 31.207 | 0.022 | 0.983 | |
| AEHTC 230-1-3 | MB | 27.232 | 1.328 | 0.996 | 28.380 | 0.100 | 0.997 |
| Pb2+ | 43.028 | 3.321 | 0.999 | 43.255 | 0.622 | 0.999 | |
| Cd2+ | 19.661 | 0.493 | 0.924 | 22.122 | 0.030 | 0.975 | |
| AEHTC 230-2-3 | MB | 28.949 | 1.056 | 0.987 | 30.622 | 0.063 | 0.996 |
| Pb2+ | 42.465 | 3.238 | 0.998 | 42.786 | 0.490 | 0.999 | |
| Cd2+ | 24.169 | 0.267 | 0.924 | 27.949 | 0.013 | 0.959 | |
| AEHTC 230-4-3 | MB | 28.638 | 1.149 | 0.966 | 30.470 | 0.104 | 0.988 |
| Pb2+ | 42.860 | 2.626 | 0.999 | 43.315 | 0.299 | 0.999 | |
| Cd2+ | 16.662 | 0.524 | 0.866 | 18.760 | 0.038 | 0.941 | |
| AEHTC 260-2-3 | MB | 26.482 | 1.097 | 0.953 | 28.204 | 0.066 | 0.985 |
| Pb2+ | 42.421 | 2.253 | 0.997 | 43.223 | 0.178 | 0.999 | |
| Cd2+ | 14.889 | 0.888 | 0.907 | 17.059 | 0.025 | 0.959 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, S.; Qin, Q.; Chen, G.; Wang, W. Alkali Etching Hydrochar-Based Adsorbent Preparation Using Chinese Medicine Industry Waste and Its Application in Efficient Removal of Multiple Pollutants. Processes 2023, 11, 412. https://doi.org/10.3390/pr11020412
Zhang X, Liu S, Qin Q, Chen G, Wang W. Alkali Etching Hydrochar-Based Adsorbent Preparation Using Chinese Medicine Industry Waste and Its Application in Efficient Removal of Multiple Pollutants. Processes. 2023; 11(2):412. https://doi.org/10.3390/pr11020412
Chicago/Turabian StyleZhang, Xinyan, Shanshan Liu, Qingyu Qin, Guifang Chen, and Wenlong Wang. 2023. "Alkali Etching Hydrochar-Based Adsorbent Preparation Using Chinese Medicine Industry Waste and Its Application in Efficient Removal of Multiple Pollutants" Processes 11, no. 2: 412. https://doi.org/10.3390/pr11020412









