Special Issue on “Enzymatic Synthesis and Characterization of Polymers”
Funding
Data Availability Statement
Conflicts of Interest
References
- Pellis, A.; Acero, E.H.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The Closure of the Cycle: Enzymatic Synthesis and Functionalization of Bio-Based Polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Loos, K. Enzymatic Synthesis of Biobased Polyesters and Polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef] [PubMed]
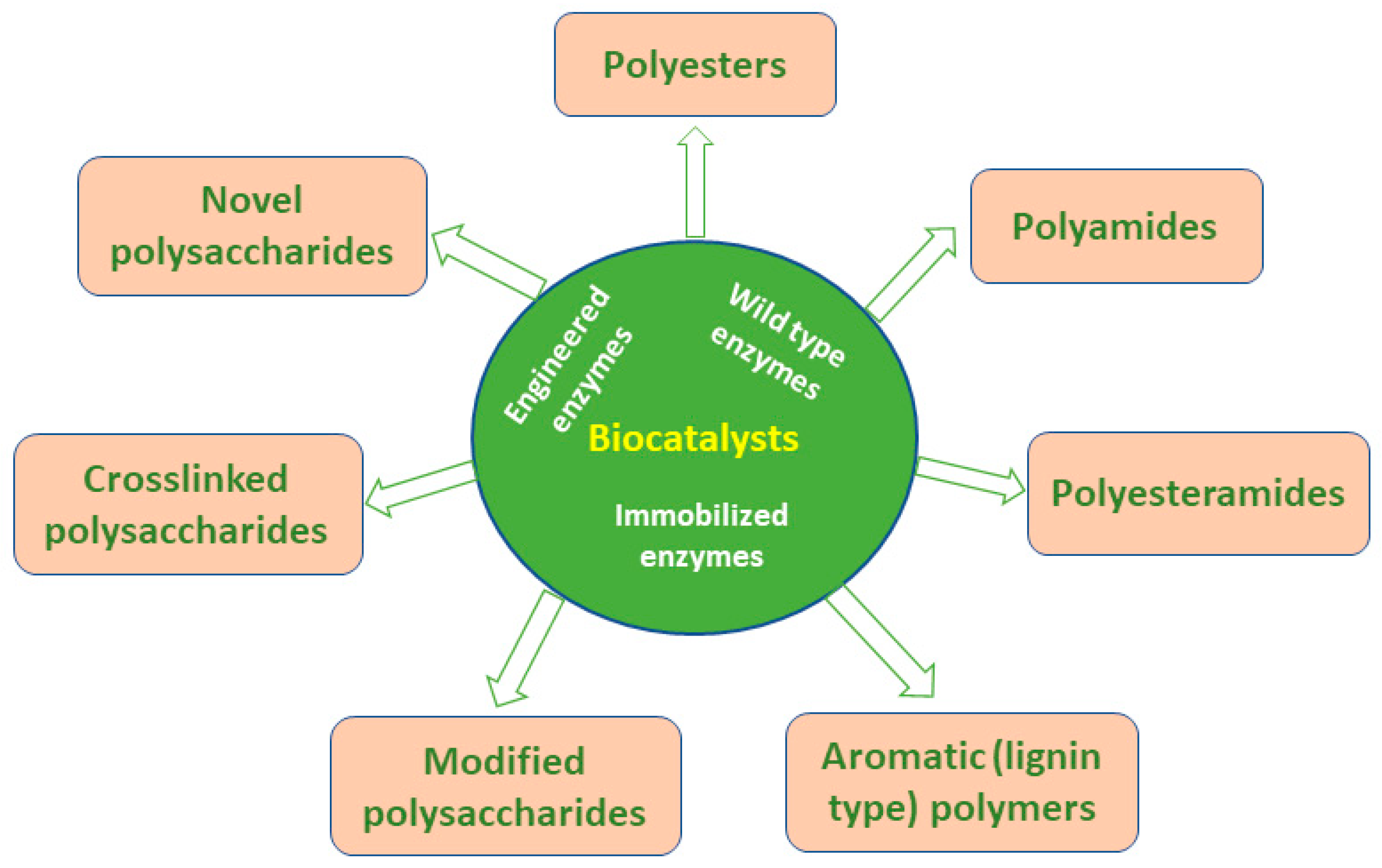
- Nikulin, M.; Švedas, V. Prospects of Using Biocatalysis for the Synthesis and Modification of Polymers. Molecules 2021, 26, 2750. [Google Scholar] [CrossRef] [PubMed]
- Todea, A.; Dreavă, D.M.; Benea, I.C.; Bîtcan, I.; Peter, F.; Boeriu, C.G. Achievements and Trends in Biocatalytic Synthesis of Specialty Polymers from Biomass-Derived Monomers Using Lipases. Processes 2021, 9, 646. [Google Scholar] [CrossRef]
- Pospiech, D.; Choińska, R.; Flugrat, D.; Sahre, K.; Jehnichen, D.; Korwitz, A.; Friedel, P.; Werner, A.; Voit, B. Enzymatic Synthesis of Poly(alkylene succinate)s: Influence of Reaction Conditions. Processes 2021, 9, 411. [Google Scholar] [CrossRef]
- Dreavă, D.M.; Benea, I.C.; Bîtcan, I.; Todea, A.; Șișu, E.; Puiu, M.; Peter, F. Biocatalytic Approach for Novel Functional Oligoesters of ε-Caprolactone and Malic Acid. Processes 2021, 9, 232. [Google Scholar] [CrossRef]
- Buzatu, A.R.; Frissen, A.E.; van den Broek, L.A.M.; Todea, A.; Motoc, M.; Boeriu, C.G. Chemoenzymatic Synthesis of New Aromatic Esters of Mono- and Oligosaccharides. Processes 2020, 8, 1638. [Google Scholar] [CrossRef]
- Kadokawa, J.-i.; Nakamura, S.; Yamamoto, K. Thermostable α-Glucan Phosphorylase-Catalyzed Enzymatic Copolymerization to Produce Partially 2-Deoxygenated Amyloses. Processes 2020, 8, 1070. [Google Scholar] [CrossRef]
- Khalighi, S.; Berger, R.G.; Ersoy, F. Cross-Linking of Wheat Bran Arabinoxylan by Fungal Laccases Yields Firm Gels. Processes 2020, 8, 36. [Google Scholar] [CrossRef]
- Khalighi, S.; Berger, R.G.; Ersoy, F. Cross-Linking of Fibrex Gel by Fungal Laccase: Gel Rheological and Structural Characteristics. Processes 2020, 8, 16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, F.; Boeriu, C.G. Special Issue on “Enzymatic Synthesis and Characterization of Polymers”. Processes 2023, 11, 504. https://doi.org/10.3390/pr11020504
Peter F, Boeriu CG. Special Issue on “Enzymatic Synthesis and Characterization of Polymers”. Processes. 2023; 11(2):504. https://doi.org/10.3390/pr11020504
Chicago/Turabian StylePeter, Francisc, and Carmen Gabriela Boeriu. 2023. "Special Issue on “Enzymatic Synthesis and Characterization of Polymers”" Processes 11, no. 2: 504. https://doi.org/10.3390/pr11020504
APA StylePeter, F., & Boeriu, C. G. (2023). Special Issue on “Enzymatic Synthesis and Characterization of Polymers”. Processes, 11(2), 504. https://doi.org/10.3390/pr11020504





