Proteomics Provide Insight into the Interaction between Selenite and the Microalgae Dunaliella salina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Culture Conditions
2.2. Algal Growth and Toxicity Test
2.3. Measurement of the Chlorophyll Fluorescence Parameters
2.4. Antioxidant Enzymes Assay
2.5. Determination of Total and Organic Se Contents
2.6. Protein Extraction and Proteomic Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Toxicity and Effects of Selenite on the Growth Dunaliella salina
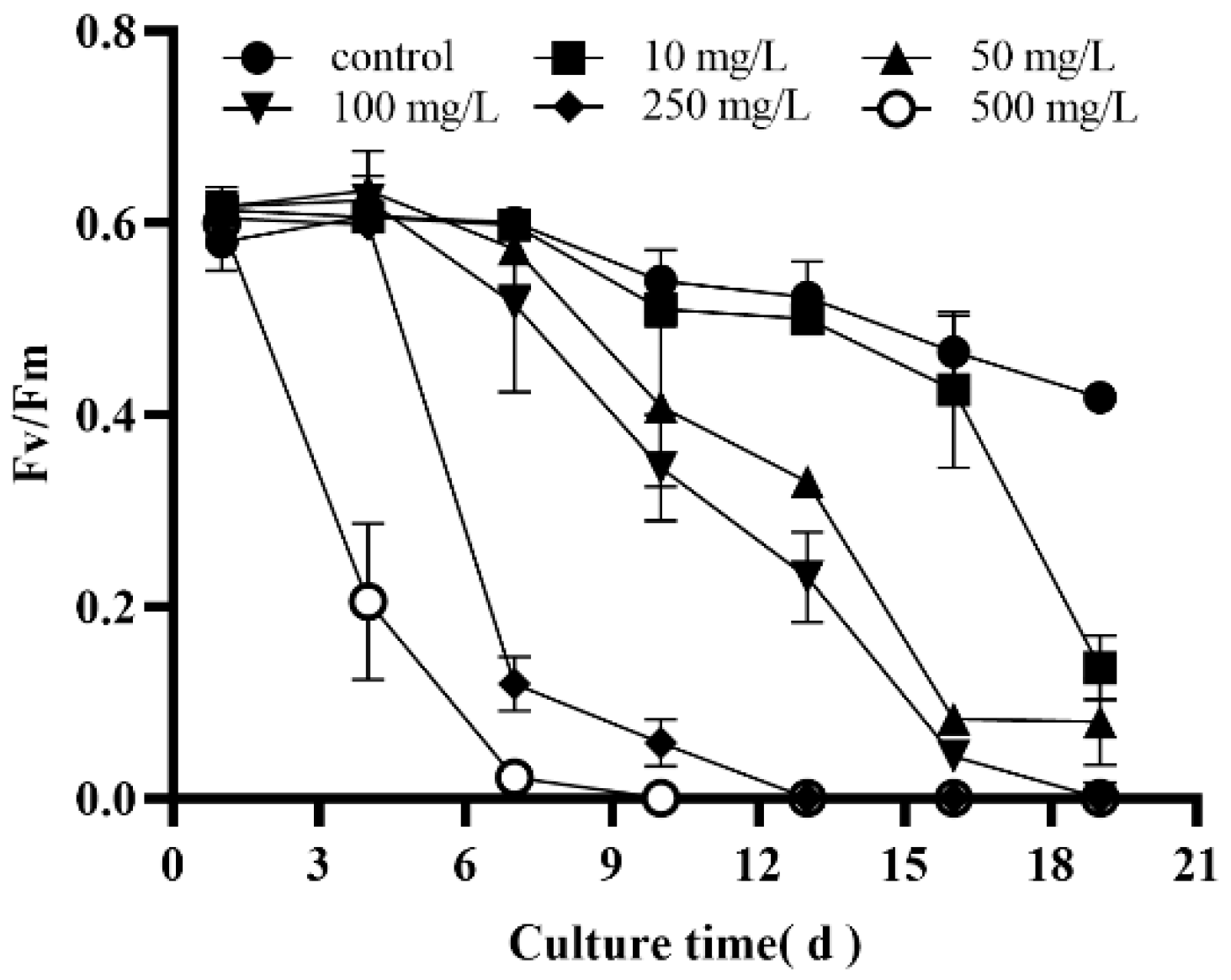
3.2. Effects of Selenite on the Photosynthesis of Dunaliella salina
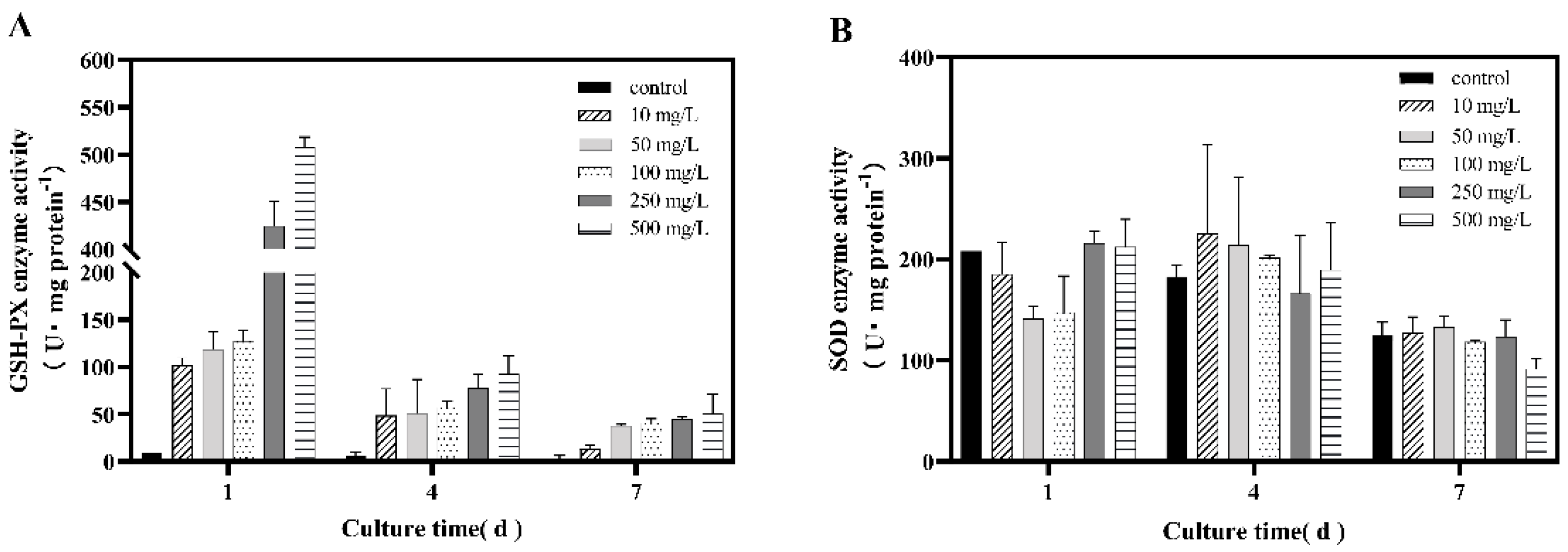
3.3. Effect of Selenite on the Activities of Antioxidant Enzymes in Dunaliella salina
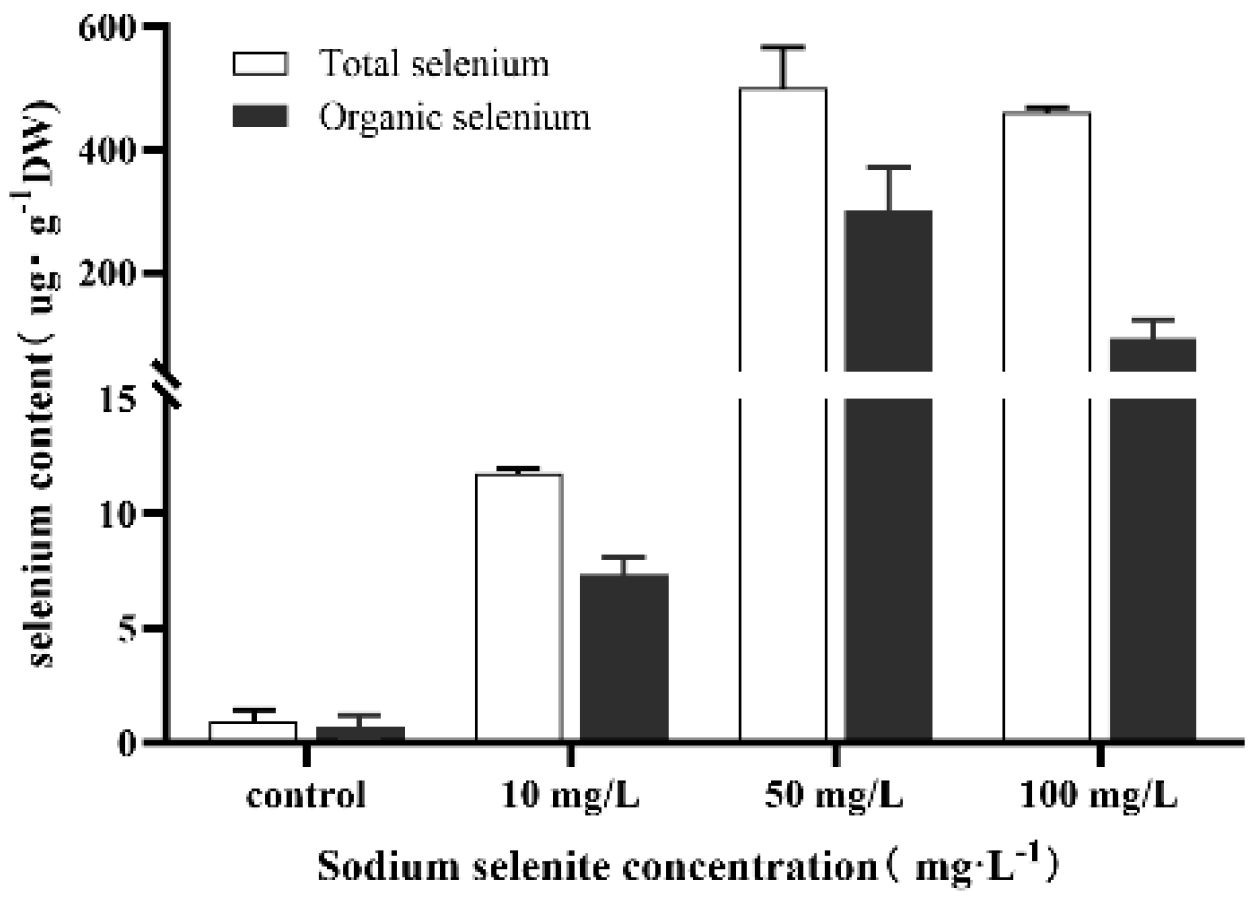
3.4. Intracellular Se Bioaccumulation
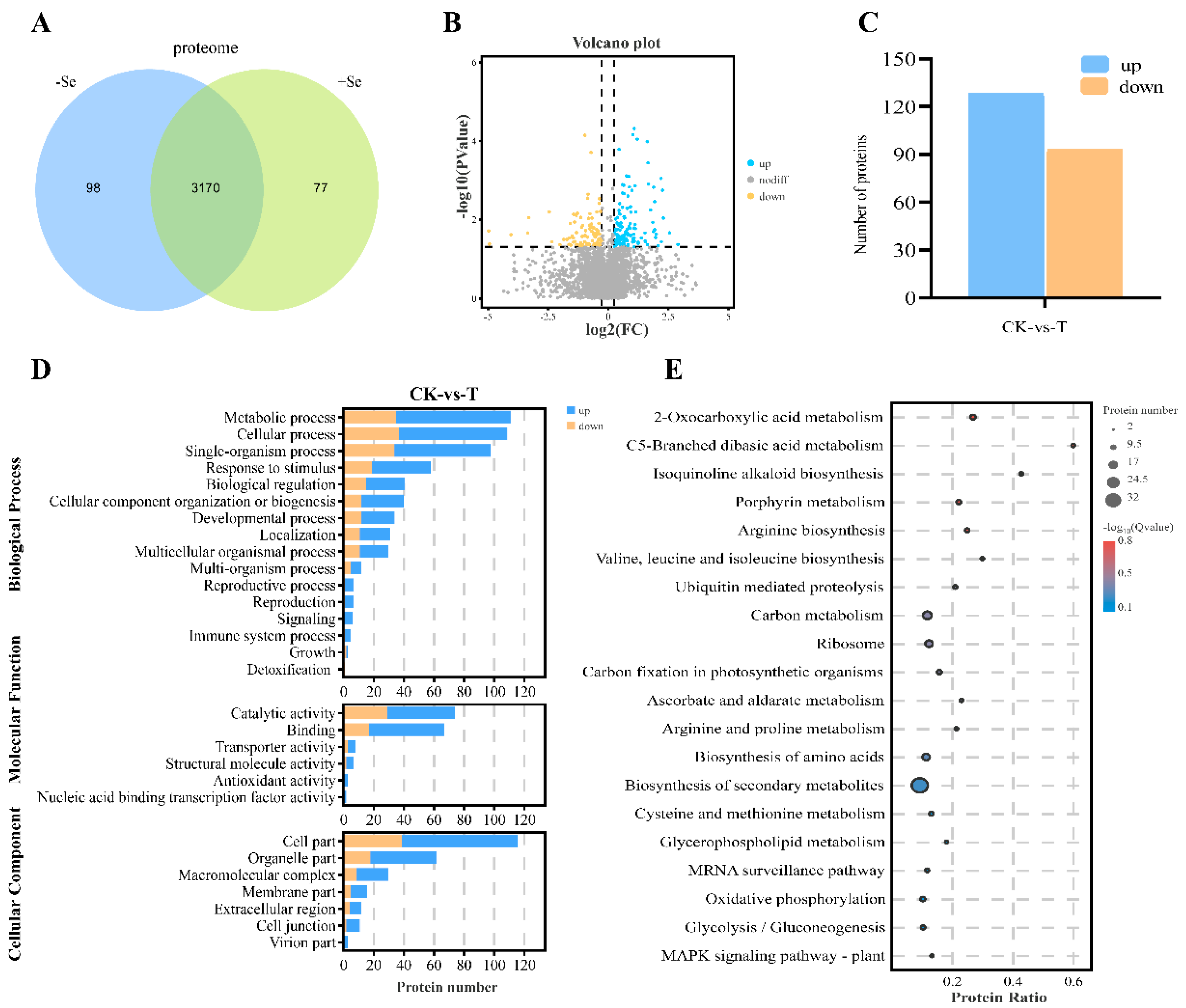
3.5. Quantitative Proteomic Analysis
3.6. Redox and Antistress-Related Proteins in Response to Selenite
3.7. Responses of Amino Acid Synthesis-Related Proteins to Selenite
3.8. Energy and Carbohydrate Metabolism-Related Proteins in Response to Selenite
3.9. Ubiquitin–Proteasome—Related Proteins in Response to Selenite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Ren, H.; Li, X.; Guo, L.; Wang, L.; Hao, X.; Zeng, J. Integrative Transcriptome and Proteome Analysis Reveals the Absorption and Metabolism of Selenium in Tea Plants [Camellia sinensis (L.) O. Kuntze]. Front. Plant Sci. 2022, 13, 848349. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Vindry, C.; Ohlmann, T.; Chavatte, L. Translation regulation of mammalian selenoproteins. Biochim. Biophys. Acta BBA Gen. Subj. 2018, 1862, 2480–2492. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Norsworthy, B.K.; Hill, K.E.; Motley, A.K.; Byrne, D.W. Effects of Chemical Form of Selenium on Plasma Biomarkers in a High-Dose Human Supplementation Trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 804–810. [Google Scholar] [CrossRef]
- Morlon, H.; Fortin, C.; Floriani, M.; Adam, C.; Garnier-Laplace, J.; Boudou, A. Toxicity of selenite in the unicellular green alga Chlamydomonas reinhardtii: Comparison between effects at the population and sub-cellular level. Aquat. Toxicol. 2005, 73, 65–78. [Google Scholar] [CrossRef]
- Novoselov, S.; Rao, M.; Onoshko, N.V.; Zhi, H.; Kryukov, G.; Xiang, Y.; Weeks, D.P.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. EMBO J. 2002, 21, 3681–3693. [Google Scholar] [CrossRef] [PubMed]
- Vriens, B.; Behra, R.; Voegelin, A.; Zupanic, A.; Winkel, L.H.E. Selenium Uptake and Methylation by the Microalga Chlamydomonas reinhardtii. Environ. Sci. Technol. 2016, 50, 711–720. [Google Scholar] [CrossRef]
- Wu, H.-L.; Chen, T.-F.; Yin, X.; Zheng, W.-J. Spectrometric characteristics and underlying mechanisms of protective effects of selenium on Spirulina platensis against oxidative stress. Guang Pu Xue Yu Guang Pu Fen Xi 2012, 32, 749–754. [Google Scholar]
- Zhao, Y.; Song, X.; Cao, X.; Wang, Y.; Si, Z.; Chen, Y. Toxic effect and bioaccumulation of selenium in green alga Chlorella pyrenoidosa. J. Appl. Phycol. 2019, 31, 1733–1742. [Google Scholar] [CrossRef]
- Umysová, D.; Vítová, M.; Doušková, I.; Bišová, K.; Hlavová, M.; Čížková, M.; Machát, J.; Doucha, J.; Zachleder, V. Bioaccumulation and toxicity of selenium compounds in the green alga Scenedesmus quadricauda. BMC Plant Biol. 2009, 9, 58. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Tao, M.; Li, J.; Hu, Z. Effects of selenite on green microalga Haematococcus pluvialis: Bioaccumulation of selenium and enhancement of astaxanthin production. Aquat. Toxicol. 2017, 183, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu-Aruxandei, D.; Vlaicu, A.; Marinaș, I.C.; Vintilă, A.C.N.; Dimitriu, L.; Oancea, F. Effect of betaine and selenium on the growth and photosynthetic pigment production in Dunaliella salina as biostimulants. FEMS Microbiol. Lett. 2019, 366, 257. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; Cheng, Z.D.; Li, S.Q.; Gao, Y.H. Toxicity and Accumulation of Selenite in Four Microalgae. Chin. J. Oceanol. Limnol. 2003, 21, 280–285. [Google Scholar]
- Guimarães, B.O.; de Boer, K.; Gremmen, P.; Drinkwaard, A.; Wieggers, R.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. Selenium enrichment in the marine microalga Nannochloropsis oceanica. Algal Res. 2021, 59, 102427. [Google Scholar] [CrossRef]
- Zou, H.; Huang, J.-C.; Zhou, C.; He, S.; Zhou, W. Mutual effects of selenium and chromium on their removal by Chlorella vulgaris and associated toxicity. Sci. Total Environ. 2020, 724, 138219. [Google Scholar] [CrossRef]
- Morlon, H.; Fortin, C.; Adam, C.; Garnier-Laplace, J. Selenite Transport and its Inhibition in the Unicellular Green Alga Chlamydomonas Reinhardtii. Environ. Toxicol. Chem. 2006, 25, 1408–1417. [Google Scholar] [CrossRef]
- Ponton, D.E.; Fortin, C.; Hare, L. Organic selenium, selenate, and selenite accumulation by lake plankton and the alga Chlamydomonas reinhardtii at different pH and sulfate concentrations. Environ. Toxicol. Chem. 2018, 37, 2112–2122. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Mou, C.; Hao, X.; Liu, X.; Chen, X.; Chen, D. Cell Factory of CarotenoidsProgress in Dunaliella Cultivation and Its Research. Adv. Mar. Sci. 2010, 28, 554–562. [Google Scholar]
- Soto, J.O. Dunaliella Identification Using DNA Fingerprinting Intron-Sizing Method and Species-Specific Oligonucleotides. Handbook of Marine Microalgae; Academic Press: Cambridge, MA, USA, 2015; pp. 559–568. [Google Scholar] [CrossRef]
- Ebenezer, V.; Ki, J.-S. Quantification of the Sub-lethal Toxicity of Metals and Endocrine-disrupting Chemicals to the Marine Green Microalga Tetraselmis suecica. Fish. Aquat. Sci. 2013, 16, 187–194. [Google Scholar] [CrossRef]
- Karasinski, J.; Wrobel, K.; Escobosa, A.R.C.; Konopka, A.; Bulska, E.; Wrobel, K. Allium cepa L. Response to Sodium Selenite (Se(IV)) Studied in Plant Roots by a LC-MS-Based Proteomic Approach. J. Agric. Food Chem. 2017, 65, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Araie, H.; Shiraiwa, Y. Selenium Utilization Strategy by Microalgae. Molecules 2009, 14, 4880–4891. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Vecchia, F.D. Selenium accumulation and metabolism in algae. Aquat. Toxicol. 2017, 189, 11. [Google Scholar] [CrossRef]
- Geoffroy, L.; Gilbin, R.; Simon, O.; Floriani, M.; Adam, C.; Pradines, C.; Cournac, L.; Garnier-Laplace, J. Effect of selenate on growth and photosynthesis of Chlamydomonas reinhardtii. Aquat. Toxicol. 2007, 83, 149–158. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Tang, C.; Jia, H.; Wu, L. Speeding up selenite bioremediation using the highly selenite-tolerant strain Providencia rettgeri HF16-A novel mechanism of selenite reduction based on proteomic analysis. J. Hazard. Mater. 2020, 406, 124690. [Google Scholar] [CrossRef]
- Vítová, M.; Bišová, K.; Hlavová, M.; Zachleder, V.; Rucki, M.; Čížková, M. Glutathione peroxidase activity in the selenium-treated alga Scenedesmus quadricauda. Aquat. Toxicol. 2011, 102, 87–94. [Google Scholar] [CrossRef]
- Pilonsmits, E.; Pilon, M. Sulfur metabolism in plastids. Advances in Photosynthesis and Respiration. In Advances in Photosynthesis and Respiration; Wise, R.R., Hoober, J.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 387–402. [Google Scholar]
- Yokota, A.; Shigeoka, S.; Onishi, T.; Kitaoka, S. Selenium as Inducer of Glutathione Peroxidase in low-CO2-Grown Chlamydomonas reinhardtii. Plant Physiol. 1988, 86, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, Y.; Chen, T.; Zheng, W.; Yang, Y. Effects of selenium on antioxidant enzymes and photosynthesis in the edible seaweed Gracilaria lemaneiformis. J. Appl. Phycol. 2018, 31, 1303–1310. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, Y.; Huang, Z.; Yang, Y. Selenium Accumulation in Unicellular Green Alga Chlorella vulgaris and Its Effects on Antioxidant Enzymes and Content of Photosynthetic Pigments. PLoS ONE 2014, 9, e112270. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Dall’acqua, S.; Mietto, A.; Pilon-Smits, E.A.; Sambo, P.M.A. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanumlycopersicon L.). Aquat. Toxicol. 2012, 61, 10542–10545. [Google Scholar] [CrossRef]
- Gómez-Jacinto, V.; García-Barrera, T.; Garbayo, I.; Vílchez, C.; Gómez-Ariza, J.L. Metallomic study of selenium biomolecules metabolized by the microalgae Chlorella sorkiniana in the biotechnological production of functional foods enriched in selenium. Pure Appl. Chem. 2012, 84, 269–280. [Google Scholar] [CrossRef]
- Neumann, P.M.; DE Souza, M.P.; Pickering, I.J.; Terry, N. Rapid microalgal metabolism of selenate to volatile dimethylselenide. Plant Cell Environ. 2003, 26, 897–905. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Wang, X.; Ngai, S.-M.; Wong, Y.-S. Comparative Proteomics Analysis of Selenium Responses in Selenium-Enriched Rice Grains. J. Proteome Res. 2013, 12, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Song, L.; Nechushtai, R.; Mendoza-Cozatl, D.G.; Mittler, R. The Cluster Transfer Function of AtNEET Supports the Ferredoxin–Thioredoxin Network of Plant Cells. Antioxidants 2022, 11, 1533. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Song, L.; Sengupta, S.; McInturf, S.A.; Grant, D.G.; Marjault, H.; Castro-Guerrero, N.A.; Burks, D.; Azad, R.K.; Cozatl, D.G.M.; et al. Expression of a dominant-negative AtNEET-H89C protein disrupts iron–sulfur metabolism and iron homeostasis in Arabidopsis. Plant J. 2019, 101, 1152–1169. [Google Scholar] [CrossRef]
- Moon, H.; Lee, B.; Choi, G.; Shin, D.; Prasad, D.T.; Lee, O.; Kwak, S.-S.; Kim, D.H.; Nam, J.; Bahk, J.; et al. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc. Natl. Acad. Sci. USA 2002, 100, 358–363. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Kadam, U.S.; Hong, J.C. Selenoprotein: Potential Player in Redox Regulation in Chlamydomonas reinhardtii. Antioxidants 2022, 11, 1630. [Google Scholar] [CrossRef]
- Jimenez, A.; Hernandez, J.A.; del Rio, L.A.; Sevilla, F. Evidence for the Presence of the Ascorbate-Glutathione Cycle in Mitochondria and Peroxisomes of Pea Leaves. Plant Physiol. 1997, 114, 275–284. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Jang, H.H.; Lee, J.R.; Sung, N.R.; Bin Lee, H.; Lee, D.H.; Park, D.-J.; Kang, C.H.; Chung, W.S.; Lim, C.O.; et al. Oligomerization and chaperone activity of a plant 2-Cys peroxiredoxin in response to oxidative stress. Plant Sci. 2009, 177, 227–232. [Google Scholar] [CrossRef]
- Rey, P.; Cuiné, S.; Eymery, F.; Garin, J.; Court, M.; Jacquot, J.-P.; Rouhier, N.; Broin, M. Analysis of the proteins targeted by CDSP32, a plastidic thioredoxin participating in oxidative stress responses. Plant J. 2004, 41, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Paeng, S.K.; Kang, C.H.; Chi, Y.H.; Chae, H.B.; Lee, E.S.; Park, J.H.; Wi, S.D.; Bin Bae, S.; Phan, K.A.T.; Lee, S.Y. AtTPR10 Containing Multiple ANK and TPR Domains Exhibits Chaperone Activity and Heat-Shock Dependent Structural Switching. Appl. Sci. 2020, 10, 1265. [Google Scholar] [CrossRef]
- Ho, C.-L.; Saito, K. Molecular biology of the plastidic phosphorylated serine biosynthetic pathway in Arabidopsis thaliana. Amino Acids 2001, 20, 243–259. [Google Scholar] [CrossRef] [PubMed]
- DE LA Torre, F.; Cañas, R.A.; Pascual, M.B.; Avila, C.; Cánovas, F.M. Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J. Exp. Bot. 2014, 65, 5527–5534. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-Aspartate: An Essential Metabolite for Plant Growth and Stress Acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef]
- Okunev, R.V. Free Amino Acid Accumulation in Soil and Tomato Plants (Solanum lycopersicum L.) Associated with Arsenic Stress. Water Air Soil Pollut. 2019, 230, 253. [Google Scholar] [CrossRef]
- Garcia, M.D.; Nouwens, A.; Lonhienne, T.G.; Guddat, L.W. Comprehensive understanding of acetohydroxyacid synthase inhibition by different herbicide families. Proc. Natl. Acad. Sci. USA 2017, 114, E1091–E1100. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, Y.-H.; Jiang, S.-C.; Lu, K.; Lu, Y.-F.; Feng, X.-J.; Wu, Z.; Liang, S.; Yu, Y.-T.; Wang, X.-F.; et al. Light-harvesting chlorophyll a/b-binding proteins, positively involved in abscisic acid signalling, require a transcription repressor, WRKY40, to balance their function. J. Exp. Bot. 2013, 64, 5443–5456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niyogi, K.K.; Li, X.-P.; Rosenberg, V.; Jung, H.-S. Is PsbS the site of non-photochemical quenching in photosynthesis? J. Exp. Bot. 2004, 56, 375–382. [Google Scholar] [CrossRef]
- Xian, J.; Zhang, M.; Sun, C.; Wang, Y.; Wang, K.; Chen, J.; Zhao, M.; Wang, Y. Research Progress of Soluble Pyrophosphatase. Genom. Appl. Biol. 2019, 38, 4030–4035. [Google Scholar]
- Castineira, J.R.P.; Serrano, A. The H+-Translocating Inorganic Pyrophosphatase from Arabidopsis thaliana Is More Sensitive to Sodium Than Its Na+-Translocating Counterpart from Methanosarcina mazei. Front. Plant Sci. 2020, 11, 1240. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-Q.; Xue, H.-W. The ubiquitin-proteasome system in plant responses to environments. Plant Cell Environ. 2019, 42, 2931–2944. [Google Scholar] [CrossRef]
- Sharma, S.; Prasad, A.; Sharma, N.; Prasad, M. Role of ubiquitination enzymes in abiotic environmental interactions with plants. Int. J. Biol. Macromol. 2021, 181, 494–507. [Google Scholar] [CrossRef]
- Vallentine, P.; Hung, C.-Y.; Xie, J.; Van Hoewyk, D. The ubiquitin-proteasome pathway protects Chlamydomonas reinhardtii against selenite toxicity, but is impaired as reactive oxygen species accumulate. AoB Plants 2014, 6, plu062. [Google Scholar] [CrossRef]
- Wiborg, J.; O’Shea, C.; Skriver, K. Biochemical function of typical and variant Arabidopsis thaliana U-box E3 ubiquitin-protein ligases. Biochem. J. 2008, 413, 447–457. [Google Scholar] [CrossRef]
- Li, Q.-S.; Lin, X.-M.; Qiao, R.-Y.; Zheng, X.-Q.; Lu, J.-L.; Ye, J.-H.; Liang, Y.-R. Effect of fluoride treatment on gene expression in tea plant (Camellia sinensis). Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Yang, L.; Wang, Y.; Jiang, F.; Lai, J.; Pan, K. Proteomics Provide Insight into the Interaction between Selenite and the Microalgae Dunaliella salina. Processes 2023, 11, 563. https://doi.org/10.3390/pr11020563
Jiang X, Yang L, Wang Y, Jiang F, Lai J, Pan K. Proteomics Provide Insight into the Interaction between Selenite and the Microalgae Dunaliella salina. Processes. 2023; 11(2):563. https://doi.org/10.3390/pr11020563
Chicago/Turabian StyleJiang, Xiaoyu, Liu Yang, Yinghui Wang, Fajun Jiang, Junxiang Lai, and Kailin Pan. 2023. "Proteomics Provide Insight into the Interaction between Selenite and the Microalgae Dunaliella salina" Processes 11, no. 2: 563. https://doi.org/10.3390/pr11020563




