Production of Biogas from Food Waste Using the Anaerobic Digestion Process with Biofilm-Based Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Media Culture of Biofilm Producing Strain
2.2. Collection and Preparation of Food Waste
2.3. Anaerobic Inoculum Collection and Preparation
2.4. Characteristic of Anaerobic Inoculum and Food Waste
2.5. Immobilization of Biofilm on Granular Activated Carbon and Hydrolysis of Food Waste
2.6. Development of Anaerobic Digestion of Domestic Food Waste
2.6.1. Semi-Continuous Experimental Design and Setup
2.6.2. Inoculum and Start-Up Time of Food Waste Anaerobic Digester
2.7. Evaluation of Biogas Production
2.7.1. Optimization of AD Parameters by OFAT: Effect of Inoculum-to-Feed Ratio
2.7.2. Effect of HRT on Biogas Production
3. Results and Discussion
3.1. Study of the Anaerobic Digestion Process of Food Waste
3.1.1. Biofilm Immobilization
3.1.2. Hydrolysis of Food Waste with Biofilm Immobilized on Granular Activated Carbon
The Changes in Total Solids and Volatile Solids
The Changes in Total Dissolved Solid (TDS) of One-Factor-at-Time
The Changes in Soluble COD of One Factor-at-Time
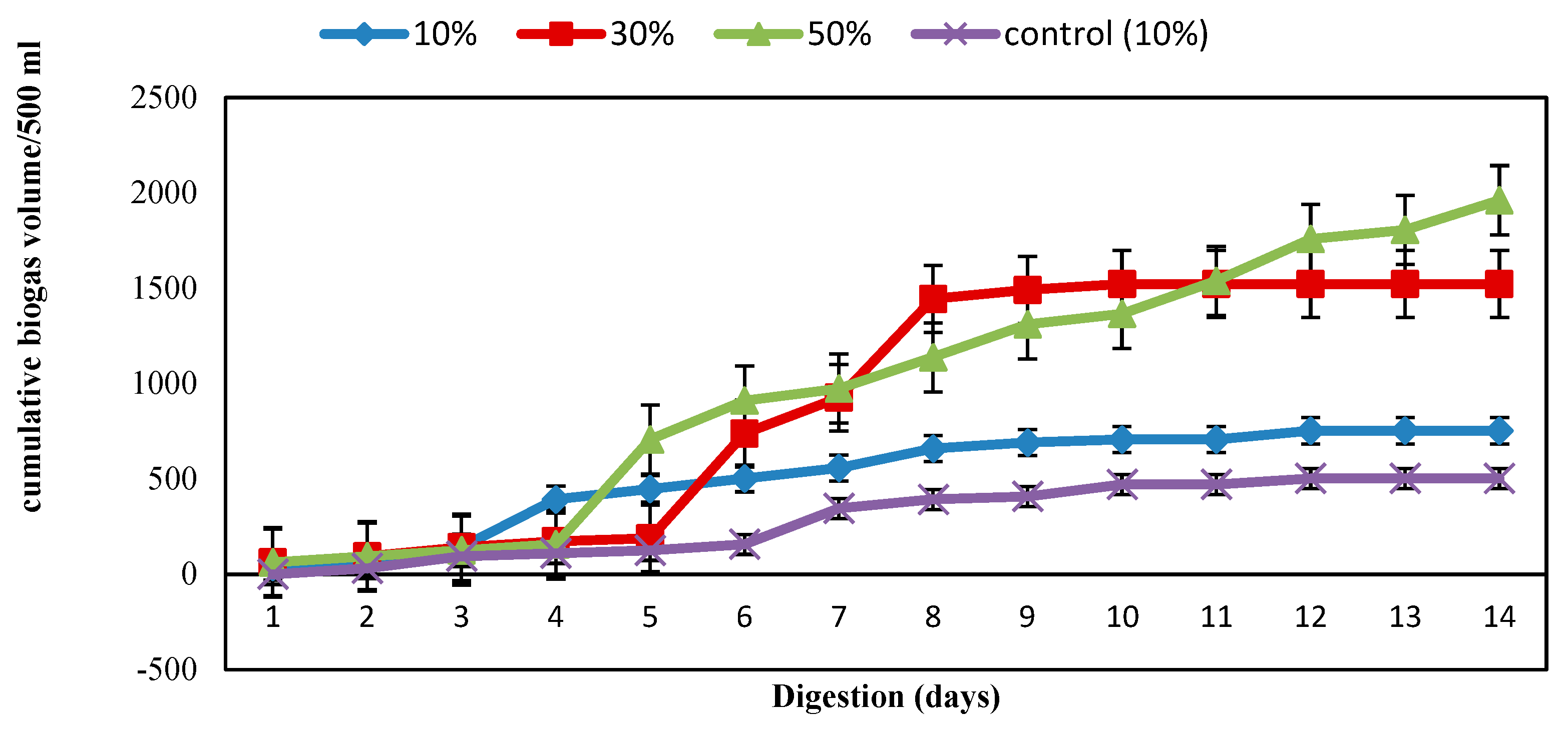
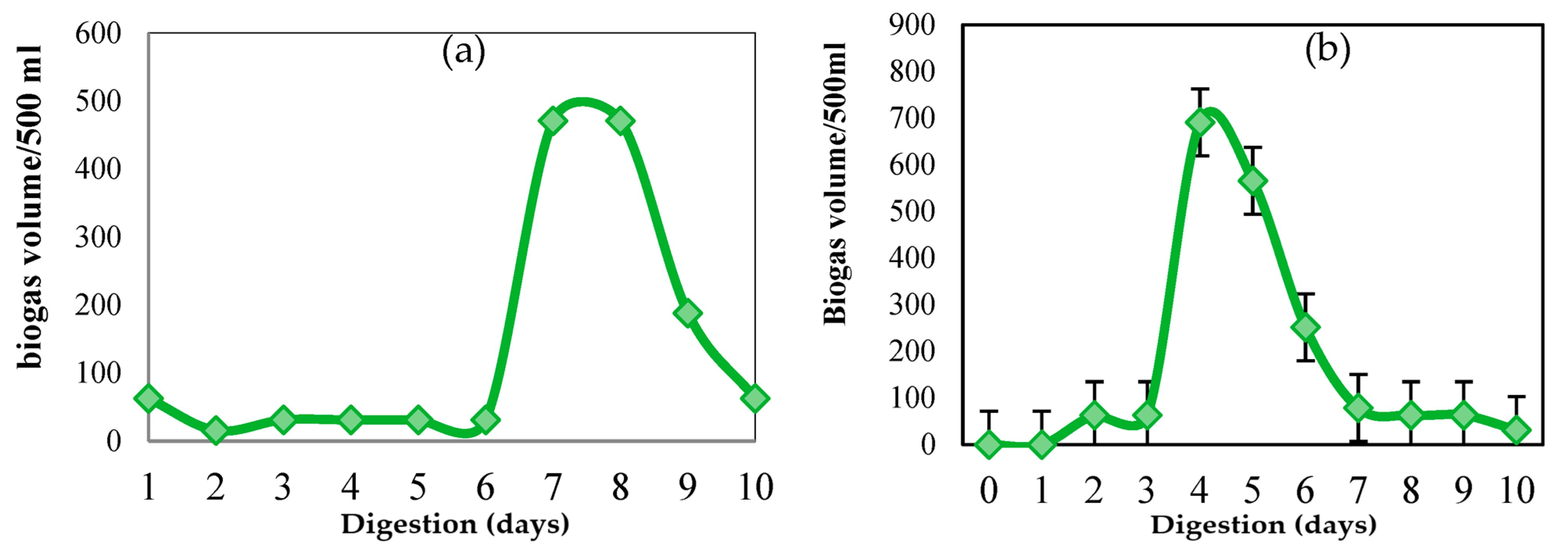
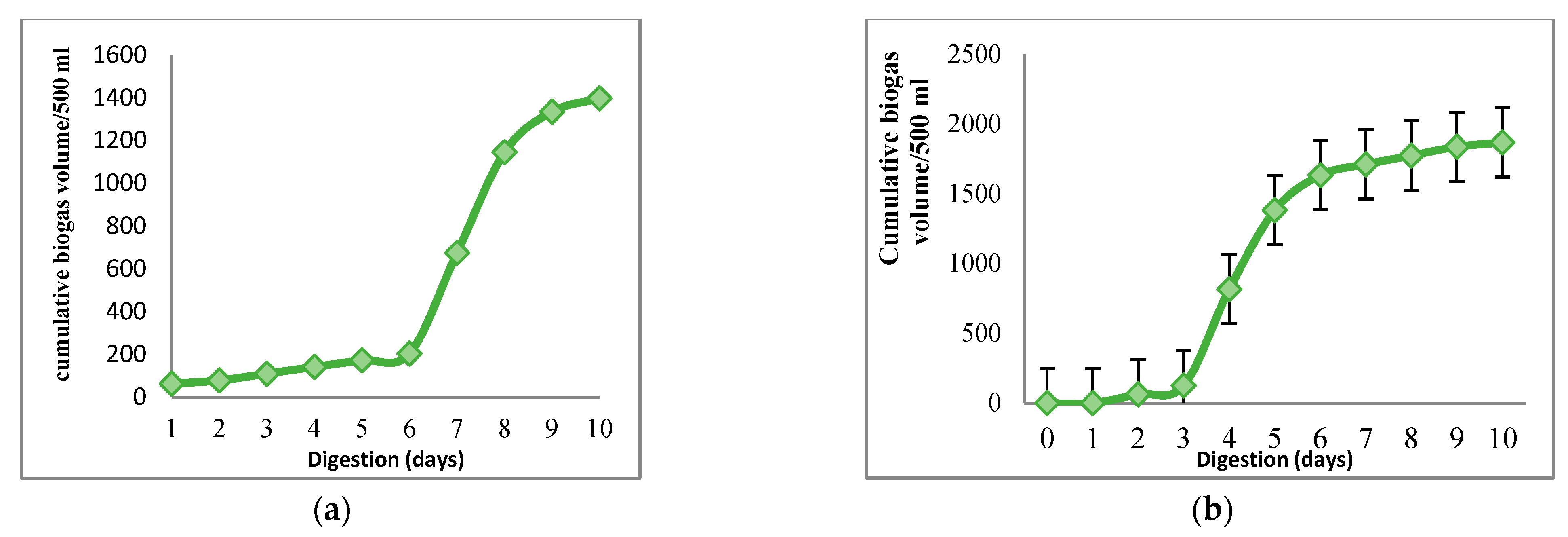
3.2. Biogas Production
3.3. Performance Parameters of the Digestion Process
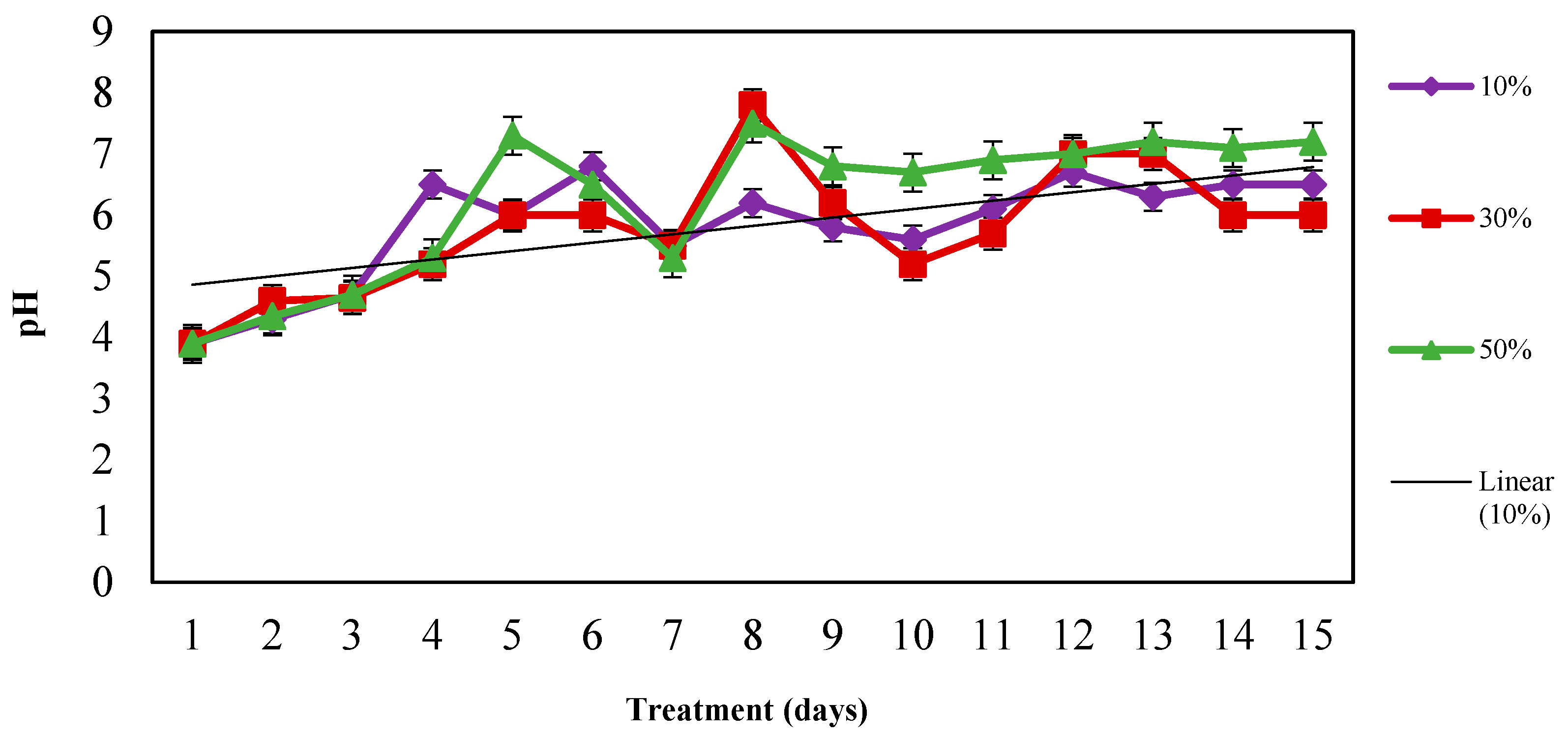
3.3.1. pH
3.3.2. Total Volatile Solid Content (TVS) and TVS Reduction
3.3.3. TCOD and TCOD Reduction
3.4. Optimum HRT for Anaerobic Digestion by OFAT
4. Conclusions
- i.
- Although this biofilm study on food waste anaerobic digestion for biogas production has been successfully developed, a pilot and full-scale study could be conducted based on this study.
- ii.
- Biofilm studies on the stability and monitoring parameters of the EPS matrix of biofilm should be studied.
- iii.
- Determining biogas composition and monitoring VFA are important to consider further for evaluating product efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunditsakulchai, P.; Liu, C. Integrated strategies for household food waste reduction in Bangkok. Sustainability 2021, 13, 7651. [Google Scholar] [CrossRef]
- Shaba Mohammed, I.; Na, R.; Shimizu, N. Modeling Anaerobic Co-Digestion of Corn Stover Hydrochar and Food Waste for Sustainable Biogas Production. Fermentation 2022, 8, 110. [Google Scholar] [CrossRef]
- Khan, I. Waste to biogas through anaerobic digestion: Hydrogen production potential in the developing world-a case of Bangladesh. Int. J. Hydrog. Energy 2020, 45, 15951–15962. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production–A review. Biores. Tech. 2018, 248, 29–36. [Google Scholar] [CrossRef]
- Mehdaoui, I.; Majbar, Z.; Atemni, I.; Elhaji, M.; Abbou, M.B.; Jennan, S.; Rais, Z. Agronomic valorization of the composts with olive waste. Moroc. J. Chem. 2022, 10, 606–621. [Google Scholar]
- Ghosh, P.; Shah, G.; Sahota, S.; Singh, L.; Vijay, V.K. Biogas production from waste: Technical overview, progress, and challenges. Bioreactors 2020, 89–104. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of substrate pretreatment on biogas production through anaerobic digestion of food waste. Int. J. Hydrog. Energy 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Enhancing biogas production from caribbean pelagic Sargassum utilising hydrothermal pretreatment and anaerobic co-digestion with food waste. Chemosphere 2021, 275, 130035. [Google Scholar] [CrossRef]
- Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Genovese, F. Recent updates on the use of agro-food waste for biogas production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Park, J.G.; Shin, W.B.; Kim, B.S.; Byun, B.S.; Jun, H.B. Maximizing biogas production by pretreatment and by optimizing the mixture ratio of co-digestion with organic wastes. Environ. Eng. Res. 2019, 24, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Bedoić, R.; Špehar, A.; Puljko, J.; Čuček, L.; Ćosić, B.; Pukšec, T.; Duić, N. Opportunities and challenges: Experimental and kinetic analysis of anaerobic co-digestion of food waste and rendering industry streams for biogas production. Renew. Sust. Energy Rev. 2020, 130, 109951. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sust. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Zhang, Y.; Liu, C. Improve biogas production from low- organic-content sludge through high-solids anaerobic co-digestion with food waste. Biores. Tech. 2016, 219, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the heating method on the efficiency of biomethane production from expired food products. Fermentation 2021, 7, 12. [Google Scholar] [CrossRef]
- Parajuli, A.; Khadka, A.; Sapkota, L.; Ghimire, A. Effect of Hydraulic Retention Time and Organic-Loading Rate on Two-Staged, Semi-Continuous Mesophilic Anaerobic Digestion of Food Waste during Start-Up. Fermentation 2022, 8, 620. [Google Scholar] [CrossRef]
- Cayetano, R.D.A.; Kim, G.B.; Park, J.; Yang, Y.H.; Jeon, B.H.; Jang, M.; Kim, S.H. Biofilm formation as a method of improved treatment during anaerobic digestion of organic matter for biogas recovery. Biores. Tech. 2022, 344, 126309. [Google Scholar] [CrossRef]
- Fazil, N.A.; Alam, M.Z.; Azmi, A.S.; Mansor, M.F. Isolation and screening of bacteria with biofilm formation ability and characterization with hydrolytic enzyme production for enhanced biogas production. Malays. J. Microbiol. 2018, 14, 96–101. [Google Scholar]
- Alam, M.Z.; Hanid, N.A. Development of Indigenous biofilm for enhanced biogas production from palm oil mill effluent. J. Adv. Res. Fluid Mech. Therm. Sci. 2017, 39, 1–8. [Google Scholar]
- Bouh, I.G.; Alam, M.Z.; Kabbashi, N.A.; Mohamed, A. Enhancement of Biogas Production from Sewage Sludge by Biofilm Pretreatment Method. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 57, 141–147. [Google Scholar]
- Abbas, Y.; Jamil, F.; Rafiq, S.; Ghauri, M.; Khurram, M.S.; Aslam, M.; Mubeen, M. Valorization of solid waste biomass by inoculation for the enhanced yield of biogas. Clean Technol. Environ. Policy 2020, 22, 513–522. [Google Scholar] [CrossRef]
- Malinowsky, C.; Nadaleti, W.; Debiasi, L.R.; Moreira, A.J.G.; Bayard, R.; de Castilhos Junior, A.B. Start-up phase optimization of two-phase anaerobic digestion of food waste: Effects of organic loading rate and hydraulic retention time. J. Environ. Manag. 2021, 296, 113064. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Wang, J. An overview on biogas generation from anaerobic digestion of food waste. Int. J. Green Energy 2016, 13, 119–131. [Google Scholar] [CrossRef]
- Ambrose, H.W.; Philip, L.; Suraishkumar, G.K.; Karthikaichamy, A.; Sen, T.K. Anaerobic co-digestion of activated sludge and fruit and vegetable waste: Evaluation of mixing ratio and impact of hybrid (microwave and hydrogen peroxide) sludge pre-treatment on two-stage digester stability and biogas yield. J. Water Process Eng. 2020, 37, 101498. [Google Scholar] [CrossRef]
- Schermuly, J.; Walk, S.; Oyedele, V.; Arroyo Cuara, A.E.; Körner, I.; Deegener, S. Report on Results for Household Food Waste Collection and Decentralised Shredding in the “Lübeck-Case”: Decisive Deliverable D3. 6: A Decentralised Management Scheme for Innovative Valorisation of Urban Biowaste; Hamburg University of Technology: Hamburg, Germany, 2018. [Google Scholar]
- Morales-Polo, C.; Cledera-Castro, M.D.M.; Moratilla Soria, B.Y. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.; Teymouri, P.; Ghalebi, M.; Jaafarzadeh, N.; Alavi, N.; Askari, A.; Foladivanda, M. Sludge characterization of an industrial water treatment plant, Iran. Des. Water Treat. 2014, 52, 5306–5316. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater. Std. Methods, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; p. 541. [Google Scholar]
- Bi, S.; Hong, X.; Yang, H.; Yu, X.; Fang, S.; Bai, Y.; Wang, Y. Effect of hydraulic retention time on anaerobic co-digestion of cattle manure and food waste. Renew. Energy. 2020, 150, 213–220. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.C.; Li, W.; Lim, J.W.; Dai, Y.; Tong, Y.W. Three-stage anaerobic digester for food waste. Appl. Energy 2017, 194, 287–295. [Google Scholar] [CrossRef]
- Haryanto, A.; Triyono, S.; Wicaksono, N.H. Effect of Hydraulic Retention Time on Biogas Production from Cow Dung in A Semi Continuous Anaerobic Digester. Inter. J. Ren. Energy Dev. 2018, 7, 93–100. [Google Scholar] [CrossRef]
- Manser, N.D.; Mihelcic, J.R.; Ergas, S.J. Semi-continuous mesophilic anaerobic digester performance under variations in solids retention time and feeding frequency. Biores. Tech. 2015, 190, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; He, Q.; Ma, Y.; Wang, X.; Peng, X. A mesophilic anaerobic digester for treating food waste: Process stability and microbial community analysis using pyrosequencing. Microb. Cell Factories 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.S.; Onik, M.H.; Kumar, D.; Rahman, M.A.; Yousuf, A.; Uddin, M.R. Impact of temperature, inoculum flow pattern, inoculum type, and their ratio on dry anaerobic digestion for biogas production. Sci. Rep. 2022, 12, 6162. [Google Scholar] [CrossRef]
- Khadka, A.; Parajuli, A.; Dangol, S.; Thapa, B.; Sapkota, L.; Carmona-Martínez, A.A.; Ghimire, A. Effect of the substrate to inoculum ratios on the kinetics of biogas production during the mesophilic anaerobic digestion of food waste. Energies 2022, 15, 834. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association Water Works Association (APHA); American Water Works Association (AWWA); Environment Federation (WEF): Washington, DC, USA, 2017. [Google Scholar]
- Tasnim, A.; Al Mamun, M.R.; Hossen, M.A.; Rahman, M.T.; Soeb, M.J.A. Comparison Effect on Biogas Production from Vegetable and Fruit Waste with Rumen Digesta Through Co-Digestion Process. Eur. J. Energy Res. 2022, 2, 1–7. [Google Scholar] [CrossRef]
- Elsayed, M.; Diab, A.; Soliman, M. Methane production from anaerobic co-digestion of sludge with fruit and vegetable wastes: Effect of mixing ratio and inoculum type. Biomass Convers. Biorefinery 2021, 11, 989–998. [Google Scholar] [CrossRef]
- Alcántara-Hernández, R.J.; Taş, N.; Carlos-Pinedo, S.; Durán-Moreno, A.; Falcón, L.I. Microbial dynamics in anaerobic digestion reactors for treating organic urban residues during the start-up process. Lett. Appl. Microbiol. 2017, 64, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.W.; Wong, S.W.K.; Dai, Y.; Tong, Y.W. Effect of seed sludge source and start-up strategy on the performance and microbial communities of thermophilic anaerobic digestion of food waste. Energy 2020, 203, 117922. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H.; Lee, D.J.; Tung, K.L.; Jin, P.; Wu, Y. Challenges in biogas production from anaerobic membrane bioreactors. Renew. Energy 2016, 98, 120–134. [Google Scholar] [CrossRef]
- Poh, P.E.; Gouwanda, D.; Mohan, Y.; Gopalai, A.A.; Tan, H.M. Optimization of wastewater anaerobic digestion using mechanistic and meta-heuristic methods: Current limitations and future opportunities. Water Conserv. Sci. Eng. 2016, 1, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Onthong, U.; Juntarachat, N. Evaluation of biogas production potential from raw and processed agricultural wastes. Energy Procedia 2017, 138, 205–210. [Google Scholar] [CrossRef]
- Admasu, A.; Bogale, W.; Mekonnen, Y.S. Experimental and simulation analysis of biogas production from beverage wastewater sludge for electricity generation. Sci. Rep. 2022, 12, 9107. [Google Scholar] [CrossRef] [PubMed]
- Nwaigwe, K.N.; Enweremadu, C.C. Analysis of Chemical Oxygen Demand (COD) removal rate using Upflow Bioreactor with Central Substrate Dispenser (UBCSD). In Proceedings of the 4th Int’l Conference on Advances in Engineering Sciences & Applied Mathematics (ICAESAM’2015), Kuala Lumpur, Malaysia, 8–9 December 2015; pp. 61–64. [Google Scholar]
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Gaby, J.C.; Zamanzadeh, M.; Horn, S.J. The effect of temperature and retention time on methane production and microbial community composition in staged anaerobic digesters fed with food waste. Biotechnol. Biofuels 2017, 10, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavini, T.D.; Chia, A.I.; John, A. Evaluation of the effect of total solids concentration on biogas yields of agricultural wastes. Int. Res. J. Environ. Sci. 2014, 3, 70–75. [Google Scholar]
- Orhorhoro, E.K.; Ebunilo, P.O.; Sadjere, G.E. Experimental determination of effect of total solid (TS) and volatile solid (VS) on biogas yield. Am. J. Mod. Energy 2017, 3, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Mehdaoui, I.; Majbar, Z.; Atemni, I.; Jennan, S.; Ainane, T.; Gaga, Y.; Chetouani, A. What effects does an organic amendment to olive waste have on the soil and crop yield? Moroc. J. Chem. 2021, 9, 4–9. [Google Scholar]
- Lopes, W.S.; Leite, V.D.; Prasad, S. Influence of inoculum on performance of anaerobic reactors for treating municipal solid waste. Biores. Tech. 2004, 94, 261–266. [Google Scholar] [CrossRef]
- Neves, L.; Oliveira, R.; Alves, M.M. Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios. Proc. Biochem. 2004, 39, 2019–2024. [Google Scholar] [CrossRef] [Green Version]
- Pavi, S.; Kramer, L.E.; Gomes, L.P.; Miranda, L.A.S. Biogas production from co-digestion of organic fraction of municipal solid waste and fruit and vegetable waste. Bioresour. Technol. 2017, 228, 362–367. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Zhao, M.; Huang, Z.; Miao, H.; Xu, Z.; Ruan, W. Enhancing biogas generation performance from food wastes by high-solids thermophilic anaerobic digestion: Effect of pH adjustment. Int. Biodeterior. Biodegrad. 2015, 105, 153–159. [Google Scholar] [CrossRef]
- Atemni, I.; Mehdaoui, I.; Majbar, Z.; Ainane, T.; Haji, M.E.; Abbou, M.B.; Rais, Z. Monitoring composting process of olive by-products and assessment of compost maturity. Environ. Eng. Man. J. (EEMJ) 2022, 21, 44–51. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Biores. Tech. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.; Rahman, S. A review of process parameters influence in solid-state anaerobic digestion: Focus on performance stability thresholds. Renew. Sustain. Energy Rev. 2022, 167, 112756. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, H.; Li, J. Effects of organic composition on mesophilic anaerobic digestion of food waste. Biores. Tech. 2017, 244, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.K.; Joshi, R.; Dhar, H.; Verma, S.; Awasthi, M.K.; Varjani, S.; Kumar, S. Improving methane yield and quality via co-digestion of cow dung mixed with food waste. Biores. Tech. 2018, 251, 259–263. [Google Scholar] [CrossRef]
- Khammour, F.; Abdoul-Latif, F.M.; Ainane, A.; Mohamed, J.; Ainane, T. Eco-friendly adsorbent from waste of mint: Application for the removal of hexavalent chromium. J. Chem. 2021, 2021, 8848964. [Google Scholar] [CrossRef]
- Uma, S.; Thalla, A.K.; Jayanthi, S. Performance Evaluation on anaerobic digestion of banana waste along with domestic wastewater. In Proceedings of the National Conference on Technological Innovations for Sustainable Infrastructure, Calicut, India, 13–14 March 2015; Volume 13, p. 14. [Google Scholar]
- Kumar, G.; Sivagurunathan, P.; Park, J.H.; Kim, S.H. Anaerobic digestion of food waste to methane at various organic loading rates (OLRs) and hydraulic retention times (HRTs): Thermophilic vs. mesophilic regimes. Environ. Eng. Res. 2015, 21, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Ounsaneha, W.; Rattanapan, C.; Suksaroj, T.T.; Kantachote, D.; Klawech, W.; Rakkamon, T. Biogas production by co-digestion of municipal wastewater and food waste: Performance in semi-continuous and continuous operation. Water Environ. Res. 2021, 93, 306–315. [Google Scholar] [CrossRef]
- Nayono, S.E.; Gallertb, C.; Winter, J. Co-digestion of press water and food waste in a biowaste digester for improvement of biogas production. Biores. Tech. 2010, 101, 6987–6993. [Google Scholar] [CrossRef]
- Shi, X.S.; Dong, J.J.; Yu, J.H.; Yin, H.; Hu, S.M.; Huang, S.X.; Yuan, X.Z. Effect of hydraulic retention time on anaerobic digestion of wheat straw in the semi-continuous continuous stirred-tank reactors. Biomed. Res. Int. 2017, 2017, 2457805. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Sun, S.; Yang, D.; Sheng, W.; Ma, Y.; He, W.; Li, G. Anaerobic digestion: An alternative resource treatment option for food waste in China. Sci. Total Environ. 2021, 779, 146397. [Google Scholar] [CrossRef]
- Liu, X.; Khalid, H.; Amin, F.R.; Ma, X.; Li, X.; Chen, C.; Liu, G. Effects of hydraulic retention time on anaerobic digestion performance of food waste to produce methane as a biofuel. Environ. Technol. Innov. 2018, 11, 348–357. [Google Scholar] [CrossRef]
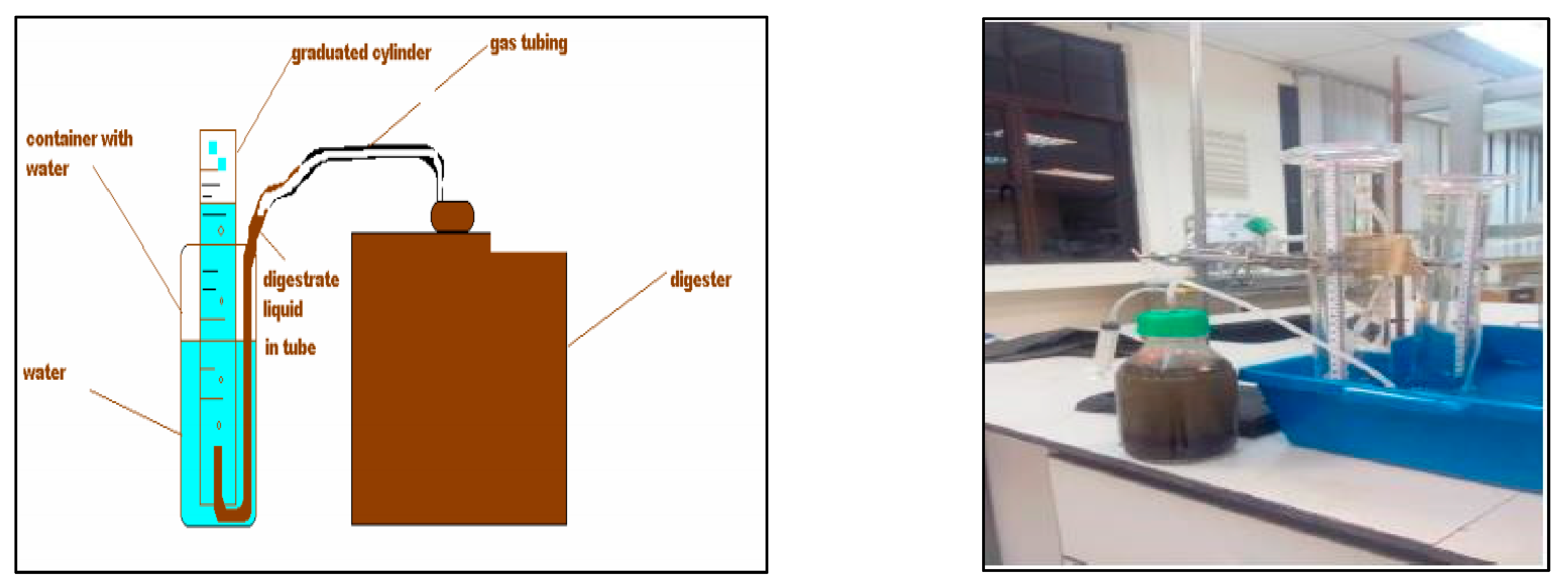



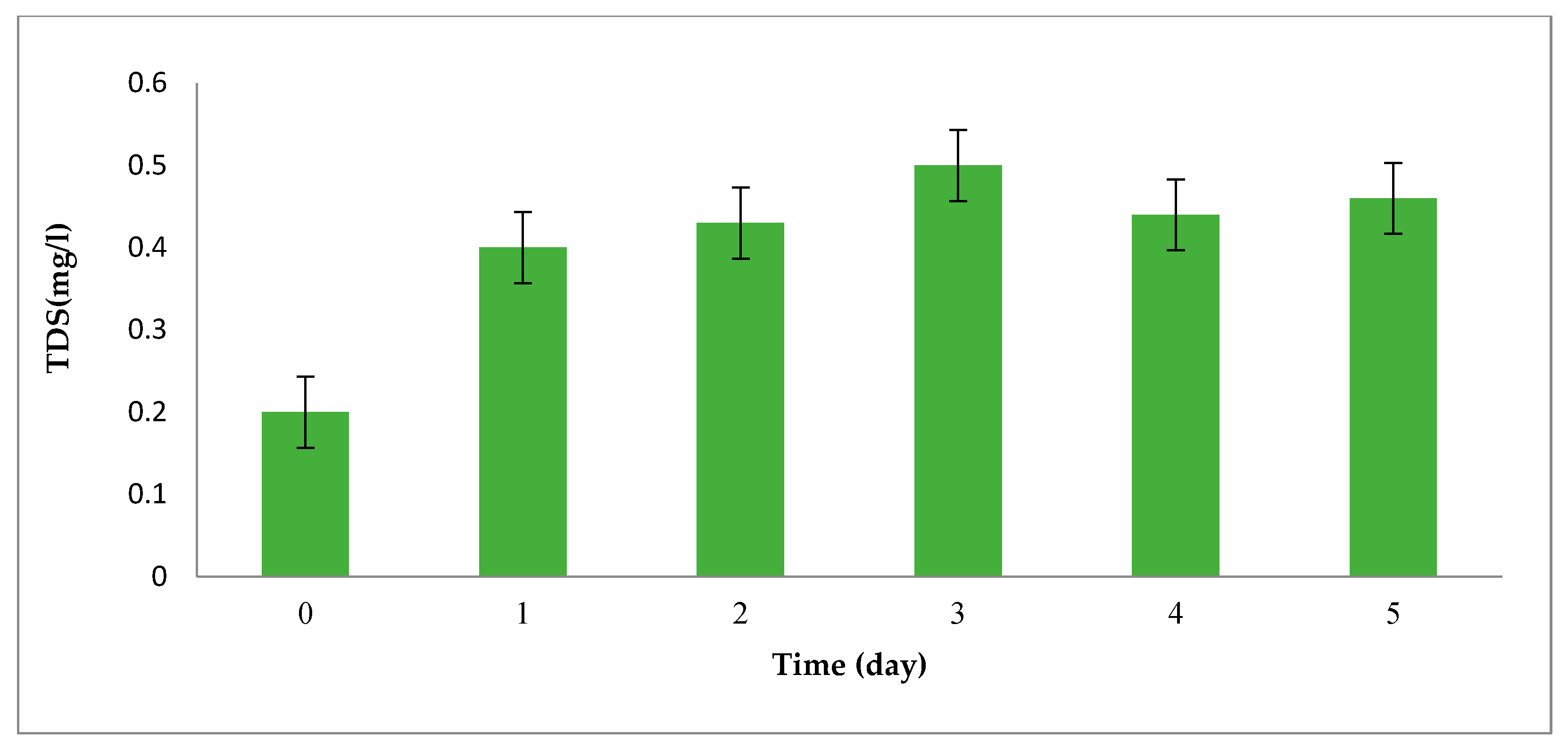
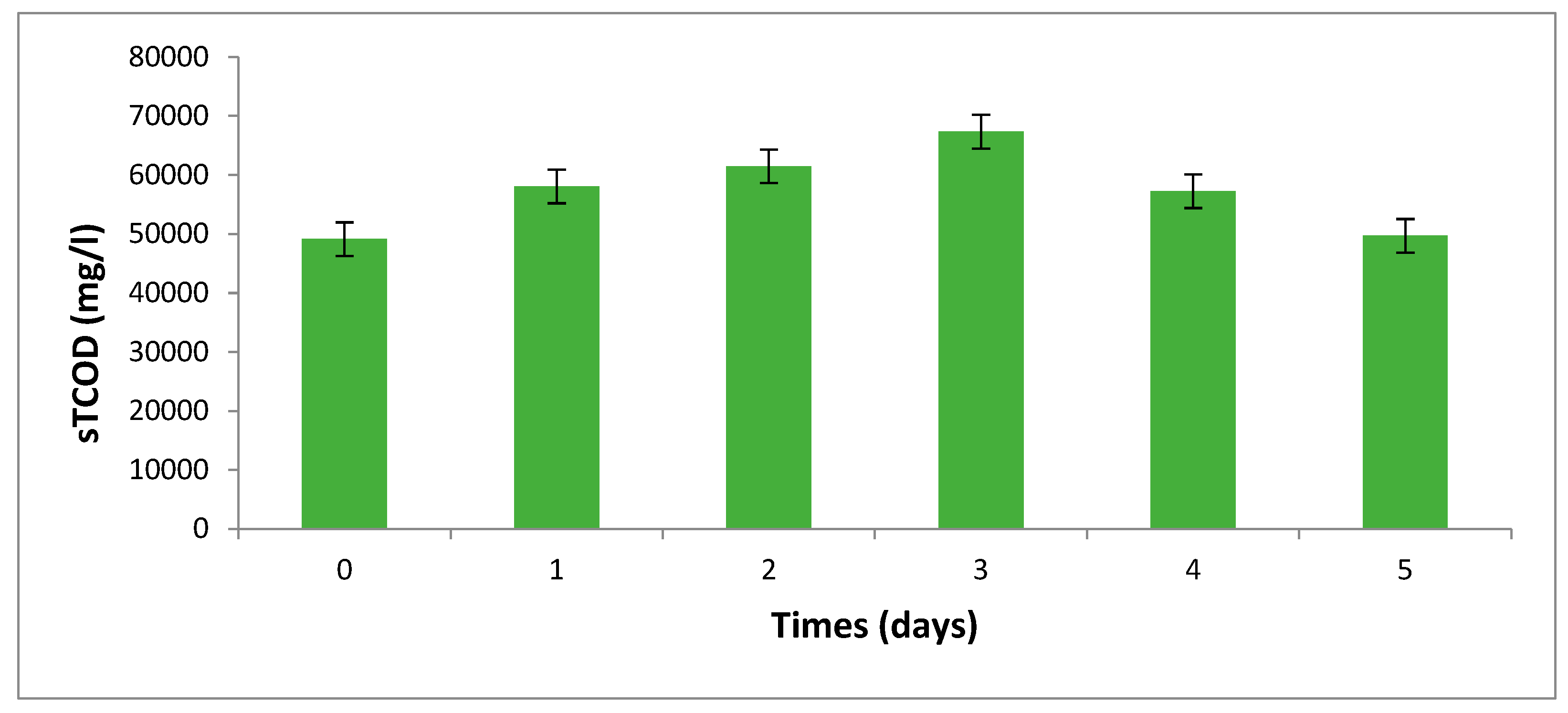
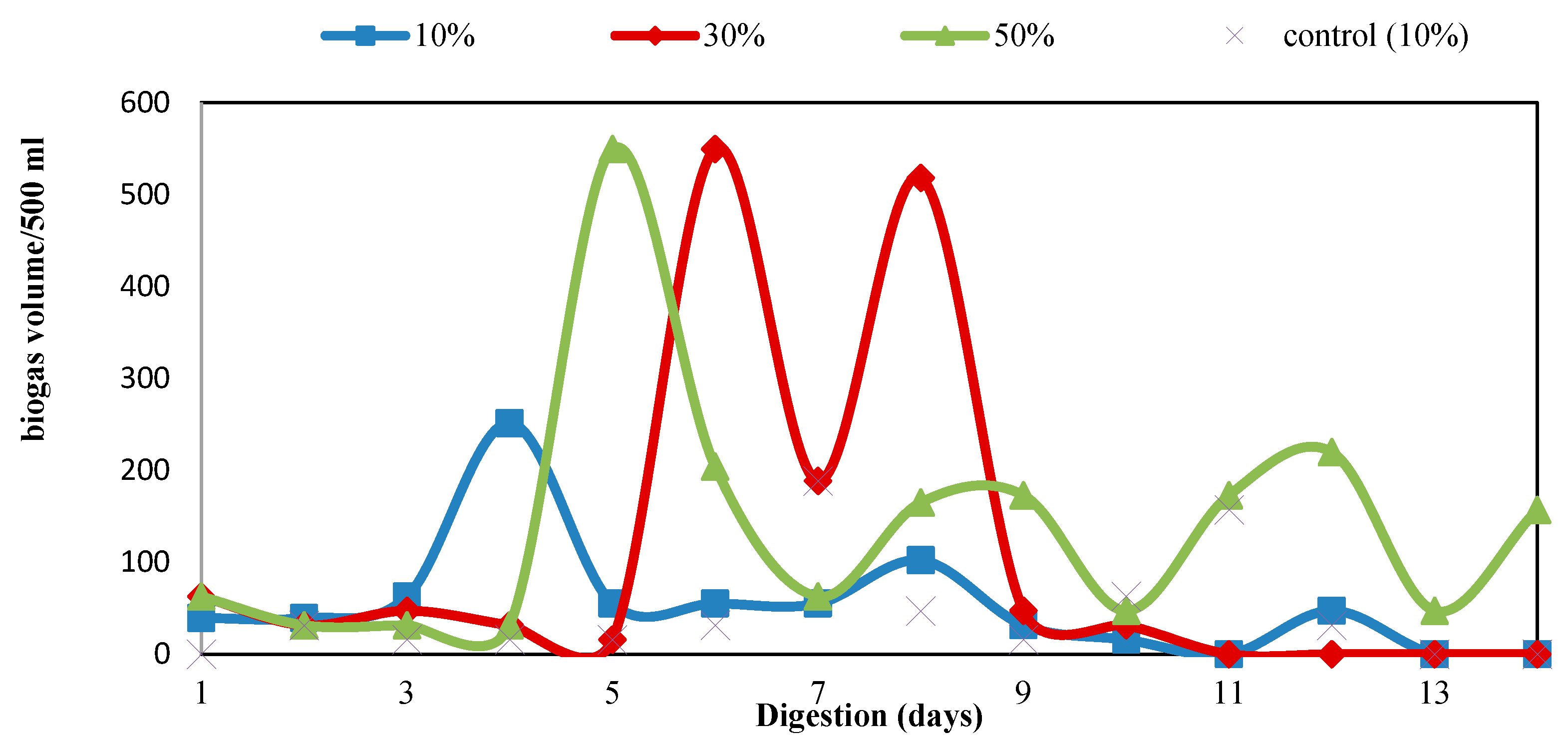





| Parameters | Values |
|---|---|
| TS (g/L) | 100.10 ± 1.35 |
| VS (g/L) | 87.45 ± 1.21 |
| TCOD (mg/L) | 53,800 ± 100 |
| SCOD (mg/L) | 49,600 ± 100 |
| pH | 5.0 ± 0.4 |
| Food Waste | TS (%) | VS (%) | VS/TS Ratio | MC (%) | SCOD (mg/L) | pH |
|---|---|---|---|---|---|---|
| Collected food waste | 36.7 | 9.7 | 0.49 | 72.5 | - | - |
| Prepared sludge | 10 | 9.9 | 0.96 | 90.2 | 6890 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Ali, A.; Alam, M.Z.; Mohamed Abdoul-latif, F.; Jami, M.S.; Gamiye Bouh, I.; Adebayo Bello, I.; Ainane, T. Production of Biogas from Food Waste Using the Anaerobic Digestion Process with Biofilm-Based Pretreatment. Processes 2023, 11, 655. https://doi.org/10.3390/pr11030655
Mohamed Ali A, Alam MZ, Mohamed Abdoul-latif F, Jami MS, Gamiye Bouh I, Adebayo Bello I, Ainane T. Production of Biogas from Food Waste Using the Anaerobic Digestion Process with Biofilm-Based Pretreatment. Processes. 2023; 11(3):655. https://doi.org/10.3390/pr11030655
Chicago/Turabian StyleMohamed Ali, Amina, Md Zahangir Alam, Fatouma Mohamed Abdoul-latif, Mohammed Saedi Jami, Ibrahim Gamiye Bouh, Ibrahim Adebayo Bello, and Tarik Ainane. 2023. "Production of Biogas from Food Waste Using the Anaerobic Digestion Process with Biofilm-Based Pretreatment" Processes 11, no. 3: 655. https://doi.org/10.3390/pr11030655







