From Waste Biomass to Hard Carbon Anodes: Predicting the Relationship between Biomass Processing Parameters and Performance of Hard Carbons in Sodium-Ion Batteries
Abstract
:1. Introduction
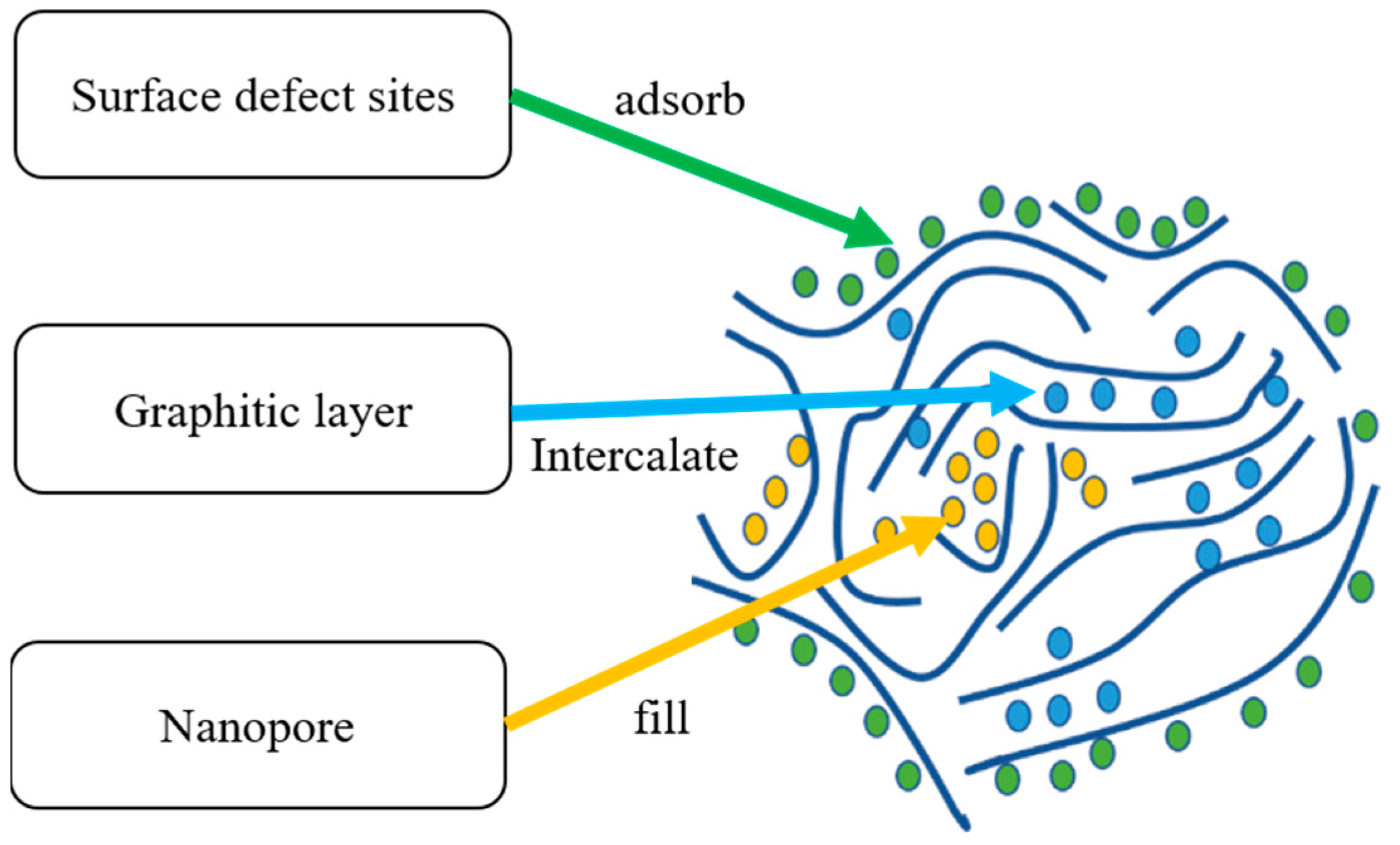
2. Sodium Storage Mechanism in HCs
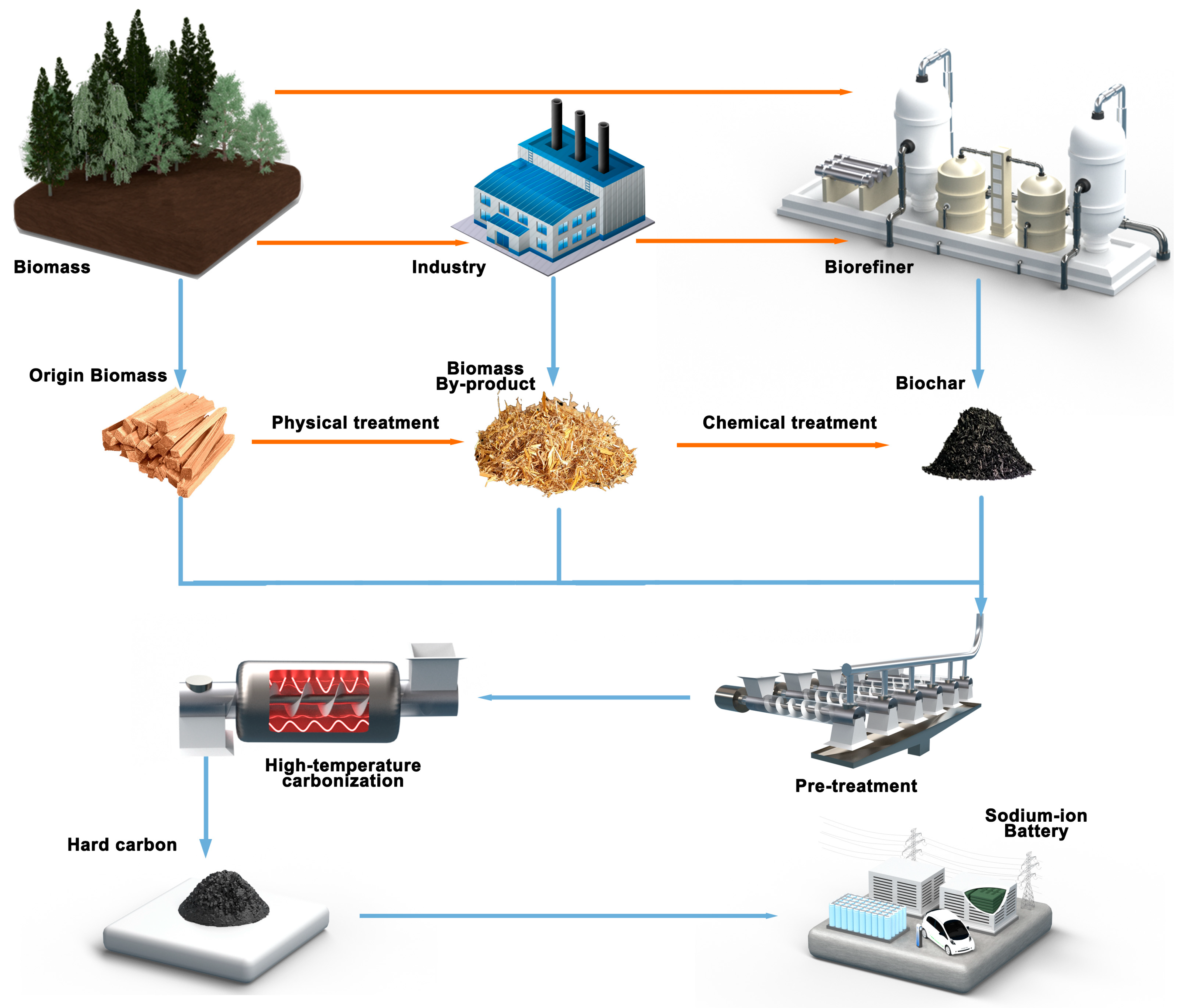
3. HC Synthesis Process
3.1. Biomass Precursor
3.1.1. Origin Biomass
3.1.2. Biomass By-Product
3.1.3. Biochar
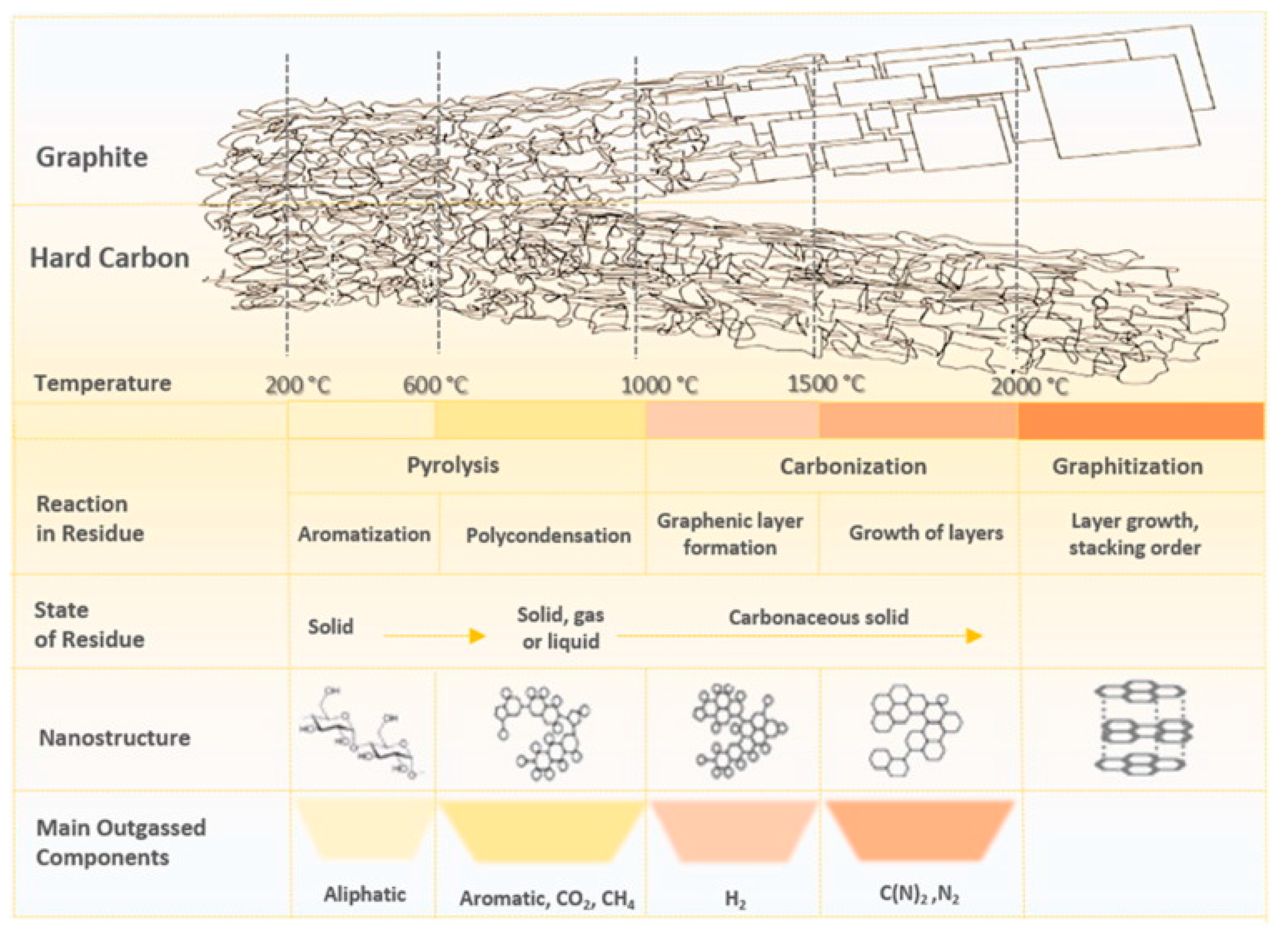
3.2. HC Production Process
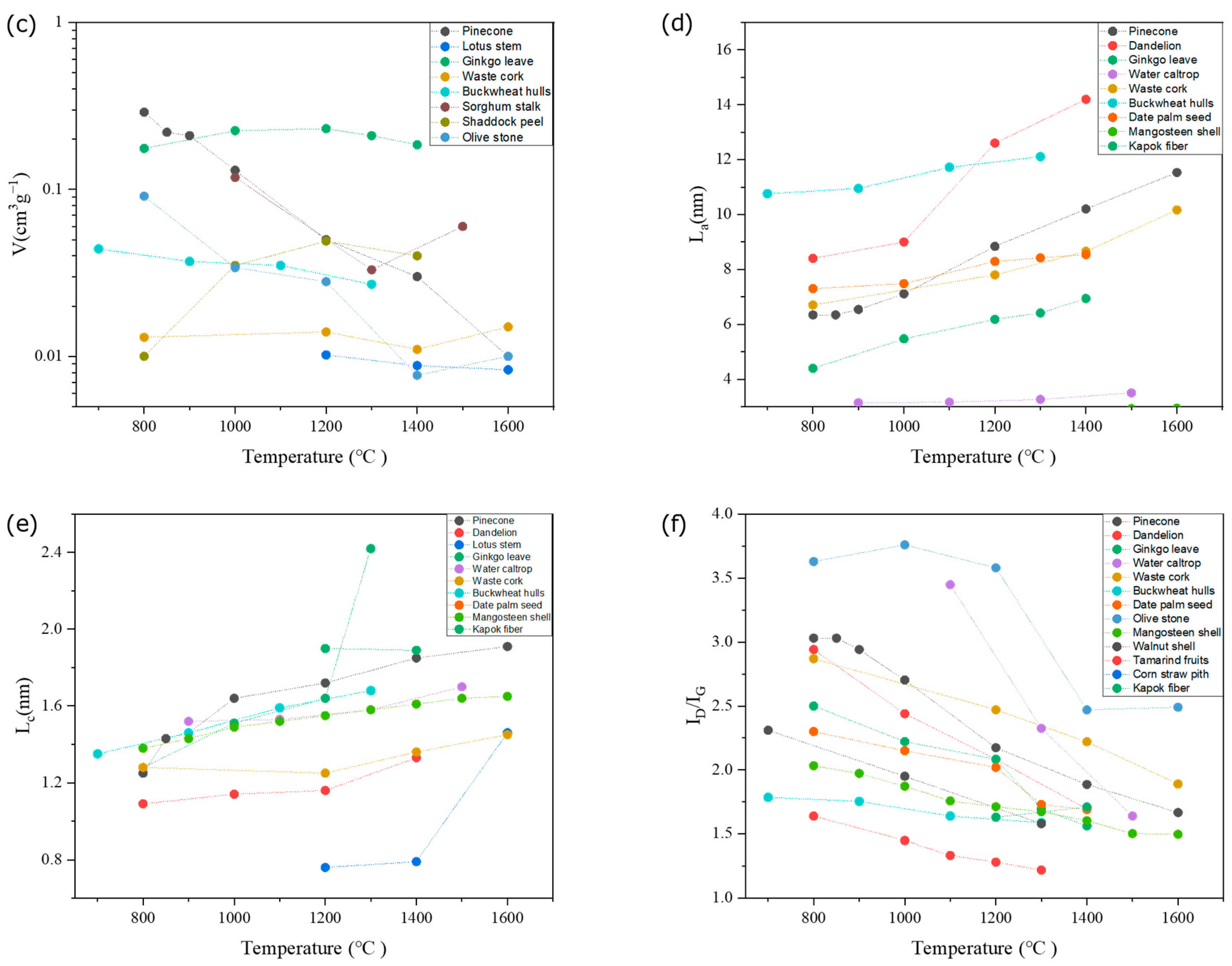
4. Influence of Biomass Processing Parameter on the HC Structure
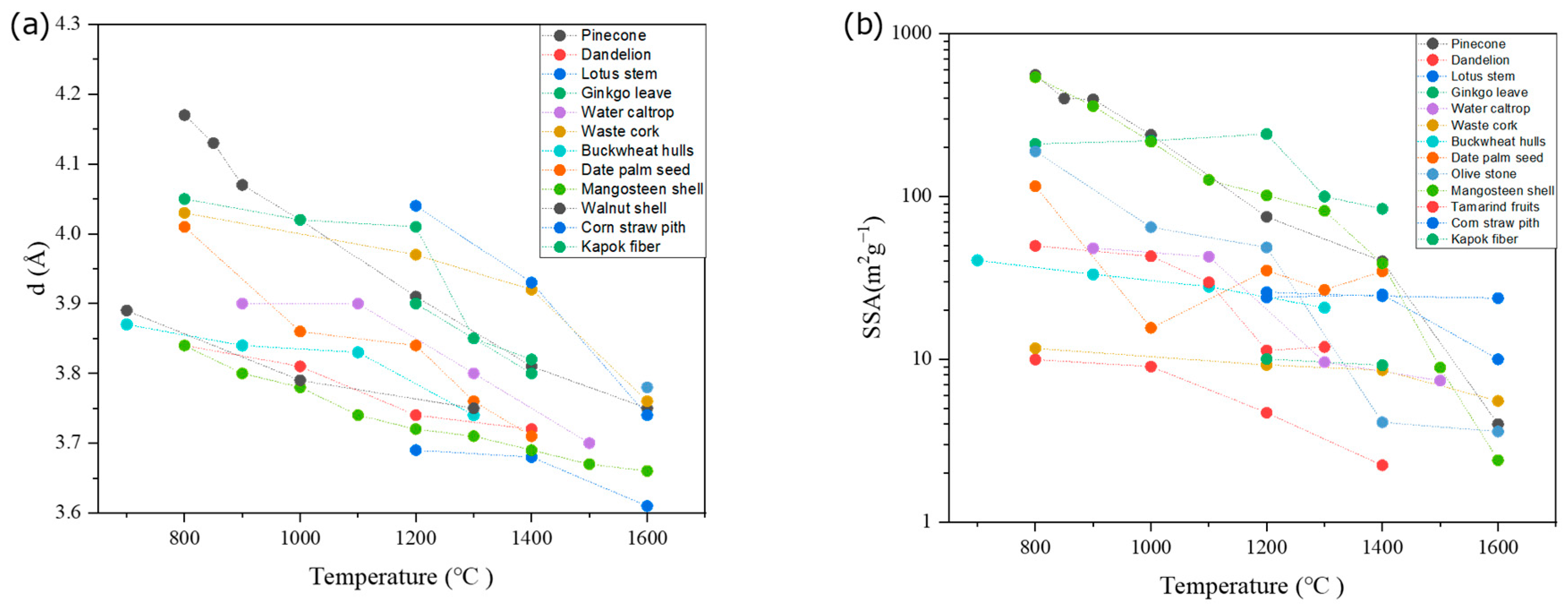
4.1. Influence of Biomass Precursor
4.2. Influence of Processing Parameters
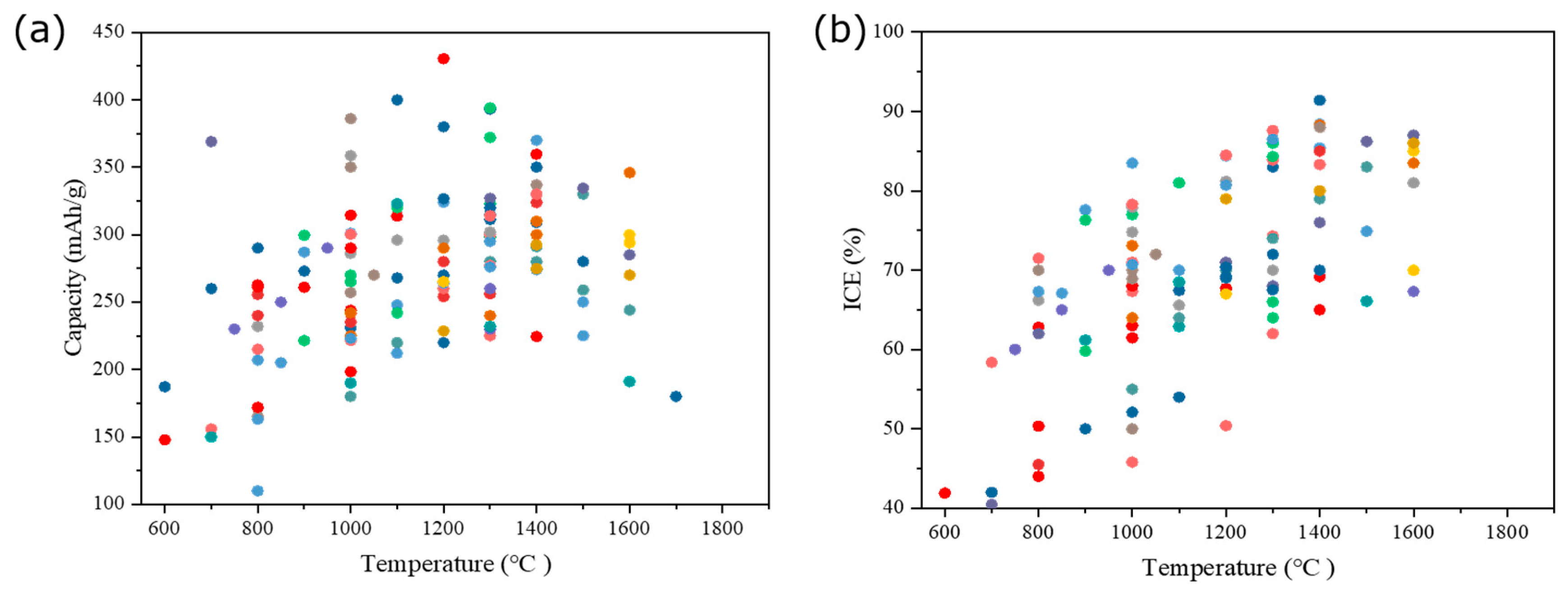
5. Relationship between Structure and Electrochemical Performance of HCs
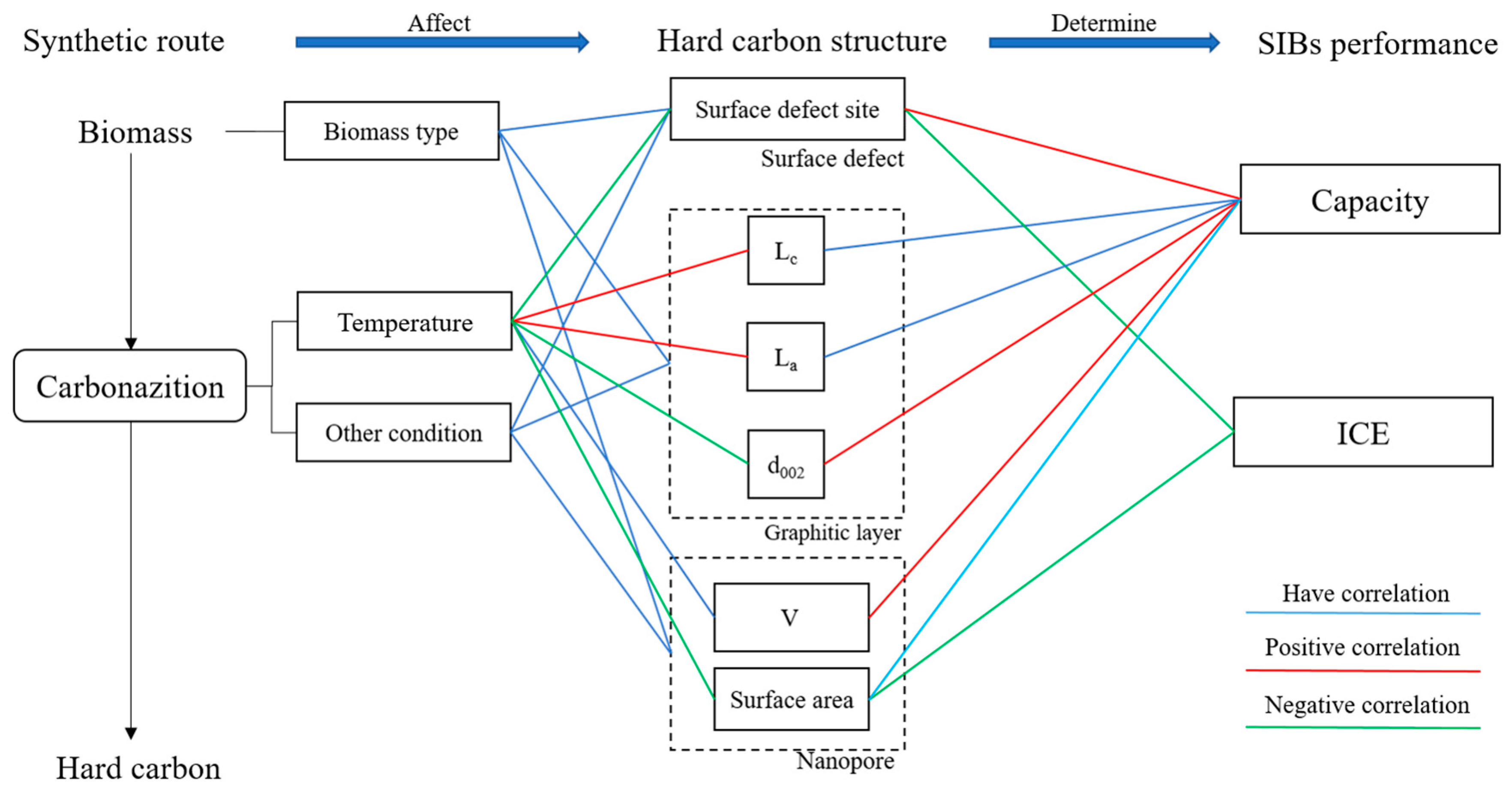
6. Proposed Relationship between Processing Parameters and the HC Performance
7. Summary and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| SIBs | sodium-ion batteries |
| LIBs | lithium-ion batteries |
| HCs | hard carbons |
| SSA | specific surface area |
| d002 | graphene lattice layer spacing |
| ICE | initial coulombic efficiency |
| La | the crystallite width along the a-axis |
| Lc | the crystallite width along the c-axis |
| SEI | solid electrolyte interphase |
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-ion and Na-ion batteries as “beyond Li-ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Yudhistira, R.; Khatiwada, D.; Sanchez, F. A comparative life cycle assessment of lithium-ion and lead-acid batteries for grid energy storage. J. Clean. Prod. 2022, 358, 131999. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research–global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Vargas, P. Lithium and the Foreseeable Future. Bachelor’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2018. [Google Scholar]
- Liu, D.; Gao, X.; An, H.; Qi, Y.; Sun, X.; Wang, Z.; Chen, Z.; An, F.; Jia, N. Supply and demand response trends of lithium resources driven by the demand of emerging renewable energy technologies in China. Resour. Conserv. Recycl. 2019, 145, 311–321. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Liu, W.; Zhang, C.; Li, S.; Li, J. High-performance sodium-ion batteries with a hard carbon anode: Transition from the half-cell to full-cell perspective. Nanoscale 2019, 11, 22196–22205. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.; Xia, Q.; Hu, Z.; Zhao, X.S. A review on biomass-derived hard carbon materials for sodium-ion batteries. Mater. Adv. 2021, 2, 5881–5905. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H. Sodium-ion battery materials and electrochemical properties reviewed. Adv. Energy Mater. 2018, 8, 1800079. [Google Scholar] [CrossRef]
- Faradion. Available online: https://faradion.co.uk/technology-benefits/strong-performance/ (accessed on 8 February 2023).
- Natrium. Available online: http://www.natriumenergy.cn/ (accessed on 8 February 2023).
- CATL. Available online: https://www.catl.com/ (accessed on 8 February 2023).
- Metal price. Available online: https://www.metal.com/ (accessed on 27 February 2023).
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.; Ikuhara, Y. Why is sodium-intercalated graphite unstable? RSC Adv. 2017, 7, 36550–36554. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, Y.; Han, Y.; Zhang, Z.; Xu, J.; Du, Y.; Bao, J.; Zhou, X. Facile synthesis of SnSe2 nanoparticles supported on graphite nanosheets for improved sodium storage and hydrogen evolution. J. Power Sources 2019, 436, 226860. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.L.; Liu, Y.; Dou, S.X. The cathode choice for commercialization of sodium-ion batteries: Layered transition metal oxides versus Prussian blue analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, M.; Zhang, D.; Yuan, T.; Sun, W.; Xu, B.; Yan, M. Transition metal oxides for high performance sodium ion battery anodes. Nano Energy 2014, 5, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Lu, Y.; Qi, X.; Wang, Y.; Mu, L.; Li, Y.; Ma, Q.; Li, J.; Hu, Y.-S. Superior electrochemical performance of sodium-ion full-cell using poplar wood derived hard carbon anode. Energy Storage Mater. 2019, 18, 269–279. [Google Scholar] [CrossRef]
- Tin powder. Available online: https://www.alibaba.com/product-detail/Sn-Sn-Tin-Powder-Tin-Powder_62359699945.html?spm=a2700.galleryofferlist.normal_offer.d_title.23203583obX2GZ&s=p (accessed on 27 February 2023).
- Liu, Y.; Zhang, N.; Jiao, L.; Tao, Z.; Chen, J. Ultrasmall Sn nanoparticles embedded in carbon as high-performance anode for sodium-ion batteries. Adv. Funct. Mater. 2015, 25, 214–220. [Google Scholar] [CrossRef]
- Dong, S.; Lv, N.; Wu, Y.; Zhang, Y.; Zhu, G.; Dong, X. Titanates for sodium-ion storage. Nano Today 2022, 42, 101349. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, H.; Yang, X.; Chen, J.; Jing, M.; Wu, Z.; Jia, X.; Ji, X. Sodium titanate cuboid as advanced anode material for sodium ion batteries. J. Power Sources 2016, 305, 200–208. [Google Scholar] [CrossRef]
- Titanate price. Available online: https://www.sigmaaldrich.com/SE/en/search/sodium-titanate?focus=products&page=1&perpage=30&sort=relevance&term=sodium%20titanate&type=product (accessed on 27 February 2023).
- Doeff, M.M.; Cabana, J.; Shirpour, M. Titanate anodes for sodium ion batteries. J. Inorg. Organomet. Polym. Mater. 2014, 24, 5–14. [Google Scholar] [CrossRef]
- Xu, J.; Ding, W.; Zhao, W.; Zhao, W.; Hong, Z.; Huang, F. In situ growth enabling ideal graphene encapsulation upon mesocrystalline MTiO3 (M = Ni, Co, Fe) nanorods for stable lithium storage. ACS Energy Lett. 2017, 2, 659–663. [Google Scholar] [CrossRef]
- BTR. Available online: https://www.btrchina.com/NegativeProducts/info.aspx?itemid=822 (accessed on 27 February 2023).
- BSG. Available online: http://www.cdbsg.com/index/news/detail.html?id=125&cid=15&pid=3 (accessed on 27 February 2023).
- JFE. Available online: https://www.jfe-chem.com/en/product/battery/carbon/ (accessed on 27 February 2023).
- Alvira, D.; Antorán, D.; Manyà, J.J. Plant-derived hard carbon as anode for sodium-ion batteries: A comprehensive review to guide interdisciplinary research. Chem. Eng. J. 2022, 447, 137468. [Google Scholar] [CrossRef]
- Dou, X.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Au, H.; Alptekin, H.; Jensen, A.C.; Olsson, E.; O’Keefe, C.A.; Smith, T.; Crespo-Ribadeneyra, M.; Headen, T.F.; Grey, C.P.; Cai, Q. A revised mechanistic model for sodium insertion in hard carbons. Energy Environ. Sci. 2020, 13, 3469–3479. [Google Scholar] [CrossRef]
- Asfaw, H.D.; Tai, C.-W.; Valvo, M.; Younesi, R. Facile synthesis of hard carbon microspheres from polyphenols for sodium-ion batteries: Insight into local structure and interfacial kinetics. Mater. Today Energy 2020, 18, 100505. [Google Scholar] [CrossRef]
- Asfaw, H.D.; Gond, R.; Kotronia, A.; Tai, C.-W.; Younesi, R. Bio-derived hard carbon nanosheets with high rate sodium-ion storage characteristics. Sustain. Mater. Technol. 2022, 32, e00407. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Fang, Y.; Ai, X.; Zhong, F.; Yang, H.; Cao, Y. Understanding of the sodium storage mechanism in hard carbon anodes. Carbon Energy 2022, 4, 1133–1150. [Google Scholar] [CrossRef]
- Cheng, X.-Q.; Li, H.-J.; Zhao, Z.-X.; Wang, Y.-Z.; Wang, X.-M. The use of in-situ Raman spectroscopy in investigating carbon materials as anodes of alkali metal-ion batteries. New Carbon Mater. 2021, 36, 93–105. [Google Scholar] [CrossRef]
- Stevens, D.; Dahn, J. High capacity anode materials for rechargeable sodium-ion batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Xie, F.; Xu, Z.; Guo, Z.; Titirici, M.-M. Hard carbons for sodium-ion batteries and beyond. Prog. Energy 2020, 2, 042002. [Google Scholar] [CrossRef]
- Moon, H.; Innocenti, A.; Liu, H.; Zhang, H.; Weil, M.; Zarrabeitia, M.; Passerini, S. Bio-waste-derived-hard carbon anodes through a sustainable and cost-effective synthesis process for Sodium-ion batteries. ChemSusChem 2022, 16, e202201713. [Google Scholar] [CrossRef] [PubMed]
- Senthil, C.; Park, J.W.; Shaji, N.; Sim, G.S.; Lee, C.W. Biomass seaweed-derived nitrogen self-doped porous carbon anodes for sodium-ion batteries: Insights into the structure and electrochemical activity. J. Energy Chem. 2022, 64, 286–295. [Google Scholar] [CrossRef]
- Tan, M.; Zhang, W.; Fan, C.; Li, L.; Chen, H.; Li, R.; Luo, T.; Han, S. Boric Acid–Catalyzed Hard Carbon Microfiber Derived from Cotton as a High-Performance Anode for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1801164. [Google Scholar] [CrossRef]
- Prabakar, S.R.; Han, S.C.; Park, C.; Bhairuba, I.A.; Reece, M.J.; Sohn, K.-S.; Pyo, M. Spontaneous formation of interwoven porous channels in hard-wood-based hard-carbon for high-performance anodes in potassium-ion batteries. J. Electrochem. Soc. 2017, 164, A2012. [Google Scholar] [CrossRef]
- Lumber. Available online: https://markets.businessinsider.com/commodities/lumber-price (accessed on 24 February 2023).
- Bagasse. Available online: https://pellets-wood.com/sell-b534_0.html (accessed on 24 February 2023).
- Rath, P.C.; Patra, J.; Huang, H.T.; Bresser, D.; Wu, T.Y.; Chang, J.K. Carbonaceous Anodes Derived from Sugarcane Bagasse for Sodium-Ion Batteries. ChemSusChem 2019, 12, 2302–2309. [Google Scholar] [CrossRef]
- Chen, M.; Luo, F.; Liao, Y.; Liu, C.; Xu, D.; Wang, Z.; Liu, Q.; Wang, D.; Ye, Y.; Li, S. Hard carbon derived for lignin with robust and low-potential sodium ion storage. J. Electroanal. Chem. 2022, 919, 116526. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Tan, H.; Zhang, B. Advanced lignin-derived hard carbon for Na-ion batteries and a comparison with Li and K ion storage. Carbon 2020, 157, 316–323. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin derived carbon materials: Current status and future trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Sawdust. Available online: https://www.alibaba.com/showroom/wood-sawdust-price.html (accessed on 24 February 2023).
- Erdogan, V. Hazelnut production in Turkey: Current situation, problems and future prospects. In Proceedings of the IX International Congress on Hazelnut 1226, Atakum, Turkey, 15–18 August 2017; pp. 13–24. [Google Scholar]
- Corn. Available online: https://www.statista.com/statistics/254292/global-corn-production-by-country/ (accessed on 24 February 2023).
- Ren, J.; Yu, P.; Xu, X. Straw utilization in China—Status and recommendations. Sustainability 2019, 11, 1762. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Liu, H.; Xu, B. Facile synthesis of high performance hard carbon anode materials for sodium ion batteries. J. Mater. Chem. A 2015, 3, 20560–20566. [Google Scholar] [CrossRef]
- Zhang, T.; Mao, J.; Liu, X.; Xuan, M.; Bi, K.; Zhang, X.L.; Hu, J.; Fan, J.; Chen, S.; Shao, G. Pinecone biomass-derived hard carbon anodes for high-performance sodium-ion batteries. RSC Adv. 2017, 7, 41504–41511. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Jin, Y.; Sun, S.; Huang, Y.; Peng, J.; Luo, J.; Zhang, Q.; Qiu, Y.; Fang, C.; Han, J. Low-cost and high-performance hard carbon anode materials for sodium-ion batteries. ACS Omega 2017, 2, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, Q.; Chen, W.; Wan, M.; Li, X.; Wang, L.; Xue, L.; Zhang, W. High capacity hard carbon derived from lotus stem as anode for sodium ion batteries. J. Power Sources 2018, 378, 331–337. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Meng, Q.; Jensen, A.C.S.; Zhang, Q.; Zhang, Q.; Tong, Y.; Qi, Y.; Gu, L.; Titirici, M.M.; et al. Regulating pore structure of hierarchical porous waste cork-derived hard carbon anode for enhanced Na storage performance. Adv. Energy Mater. 2019, 9, 1902852. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Maity, S.K. Opportunities, recent trends and challenges of integrated biorefinery: Part I. Renew. Sustain. Energy Rev. 2015, 43, 1427–1445. [Google Scholar] [CrossRef] [Green Version]
- Alvin, S.; Yoon, D.; Chandra, C.; Susanti, R.F.; Chang, W.; Ryu, C.; Kim, J. Extended flat voltage profile of hard carbon synthesized using a two-step carbonization approach as an anode in sodium ion batteries. J. Power Sources 2019, 430, 157–168. [Google Scholar] [CrossRef]
- Biochar. Available online: https://www.alibaba.com/countrysearch/CN/biochar.html?fsb=y&IndexArea=product_en&CatId=&SearchText=biochar&isGalleryList=G (accessed on 27 February 2023).
- Rios, C.D.M.S.; Simone, V.; Simonin, L.; Martinet, S.; Dupont, C. Biochars from various biomass types as precursors for hard carbon anodes in sodium-ion batteries. Biomass Bioenergy 2018, 117, 32–37. [Google Scholar] [CrossRef]
- Mitchual, S.J.; Frimpong-Mensah, K.; Darkwa, N.A. Evaluation of fuel properties of six tropical hardwood timber species for briquettes. J. Sustain. Bioenergy Syst. 2014, 4, 44225. [Google Scholar] [CrossRef] [Green Version]
- Miranda, N.T.; Motta, I.L.; Maciel Filho, R.; Maciel, M.R.W. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Grieco, E.; Baldi, G. Analysis and modelling of wood pyrolysis. Chem. Eng. Sci. 2011, 66, 650–660. [Google Scholar] [CrossRef]
- Sørmo, E.; Silvani, L.; Thune, G.; Gerber, H.; Schmidt, H.P.; Smebye, A.B.; Cornelissen, G. Waste timber pyrolysis in a medium-scale unit: Emission budgets and biochar quality. Sci. Total Environ. 2020, 718, 137335. [Google Scholar] [CrossRef] [PubMed]
- Garcìa-Pèrez, M.; Chaala, A.; Roy, C. Vacuum pyrolysis of sugarcane bagasse. J. Anal. Appl. Pyrolysis 2002, 65, 111–136. [Google Scholar] [CrossRef]
- Hard carbon. Available online: https://www.alibaba.com/countrysearch/CN/hard-carbon.html (accessed on 24 February 2023).
- Xiang, J.; Lv, W.; Mu, C.; Zhao, J.; Wang, B. Activated hard carbon from orange peel for lithium/sodium ion battery anode with long cycle life. J. Alloys Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Qi, X.; Lin, T.; Zhang, S.; Xu, J.; Zhang, H.; Xu, F.; Huang, F. Nitrogen doped hierarchical porous hard carbon derived from a facial Ti-peroxy-initiating in-situ polymerization and its application in electrochemical capacitors. Microporous Mesoporous Mater. 2020, 294, 109884. [Google Scholar] [CrossRef]
- Li, Z.; Bommier, C.; Chong, Z.S.; Jian, Z.; Surta, T.W.; Wang, X.; Xing, Z.; Neuefeind, J.C.; Stickle, W.F.; Dolgos, M. Mechanism of Na-ion storage in hard carbon anodes revealed by heteroatom doping. Adv. Energy Mater. 2017, 7, 1602894. [Google Scholar] [CrossRef]
- Dewar, D.; Glushenkov, A.M. Optimisation of sodium-based energy storage cells using pre-sodiation: A perspective on the emerging field. Energy Environ. Sci. 2021, 14, 1380–1401. [Google Scholar] [CrossRef]
- Qi, H.; Xu, J.; Sun, P.; Qi, X.; Xiao, Y.; Zhao, W.; Joshi, R.; Huang, F. Tailoring Conductive 3D Porous Hard Carbon for Supercapacitors. Energy Technol. 2022, 10, 2101103. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Winter, M.; Placke, T.; Ramirez-Rico, J. Correlation of structure and performance of hard carbons as anodes for sodium ion batteries. Chem. Mater. 2019, 31, 7288–7299. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Li, M. Porous hard carbon derived from walnut shell as an anode material for sodium-ion batteries. JOM 2018, 70, 1387–1391. [Google Scholar] [CrossRef]
- Yu, Z.-E.; Lyu, Y.; Wang, Y.; Xu, S.; Cheng, H.; Mu, X.; Chu, J.; Chen, R.; Liu, Y.; Guo, B. Hard carbon micro-nano tubes derived from kapok fiber as anode materials for sodium-ion batteries and the sodium-ion storage mechanism. Chem. Commun. 2020, 56, 778–781. [Google Scholar] [CrossRef]
- Izanzar, I.; Dahbi, M.; Kiso, M.; Doubaji, S.; Komaba, S.; Saadoune, I. Hard carbons issued from date palm as efficient anode materials for sodium-ion batteries. Carbon 2018, 137, 165–173. [Google Scholar] [CrossRef]
- Zhu, Y.-E.; Gu, H.; Chen, Y.-N.; Yang, D.; Wei, J.; Zhou, Z. Hard carbon derived from corn straw piths as anode materials for sodium ion batteries. Ionics 2018, 24, 1075–1081. [Google Scholar] [CrossRef]
- Yu, K.; Zhao, H.; Wang, X.; Zhang, M.; Dong, R.; Li, Y.; Bai, Y.; Xu, H.; Wu, C. Hyperaccumulation route to Ca-rich hard carbon materials with cation self-incorporation and interlayer spacing optimization for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 10544–10553. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Guan, Z.; Liu, Y.; Cao, Y.; Zhu, Q.; Liu, H.; Wang, Z.; Zhang, P.; Xu, B. Extended “Adsorption–Insertion” Model: A New Insight into the Sodium Storage Mechanism of Hard Carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Zhang, B.; de Yuso, A.M.; Réty, B.; Tarascon, J.-M. Valorizing low cost and renewable lignin as hard carbon for Na-ion batteries: Impact of lignin grade. Carbon 2019, 153, 634–647. [Google Scholar] [CrossRef]
- Beda, A.; Le Meins, J.-M.; Taberna, P.-L.; Simon, P.; Ghimbeu, C.M. Impact of biomass inorganic impurities on hard carbon properties and performance in Na-ion batteries. Sustain. Mater. Technol. 2020, 26, e00227. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Yu, P.; Tang, W.; Wu, F.-F.; Zhang, C.; Luo, H.-Y.; Liu, H.; Wang, Z.-G. Recent progress in plant-derived hard carbon anode materials for sodium-ion batteries: A review. Rare Met. 2020, 39, 1019–1033. [Google Scholar] [CrossRef]
- Wang, P.; Fan, L.; Yan, L.; Shi, Z. Low-cost water caltrop shell-derived hard carbons with high initial coulombic efficiency for sodium-ion battery anodes. J. Alloys Compd. 2019, 775, 1028–1035. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Zhu, Y.; Zhang, Z.; Guang, Z.; Meng, Z.; Liu, P. Nonignorable influence of oxygen in hard carbon for sodium ion storage. ACS Sustain. Chem. Eng. 2020, 8, 1497–1506. [Google Scholar] [CrossRef]
- Wang, C.; Huang, J.; Qi, H.; Cao, L.; Xu, Z.; Cheng, Y.; Zhao, X.; Li, J. Controlling pseudographtic domain dimension of dandelion derived biomass carbon for excellent sodium-ion storage. J. Power Sources 2017, 358, 85–92. [Google Scholar] [CrossRef]
- Luo, W.; Shen, F.; Clement, B. Ultra-thick, low-tortuosity, and mesoporous wood carbon anode for high-performance sodium-ion batteries. ACC Chem. Res. 2016, 49, 231. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, F.; Luo, W.; Dai, J.; Han, X.; Chen, Y.; Yao, Y.; Zhu, H.; Fu, K.; Hitz, E. Carbonized-leaf membrane with anisotropic surfaces for sodium-ion battery. ACS Appl. Mater. Interfaces 2016, 8, 2204–2210. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. A waste biomass derived hard carbon as a high-performance anode material for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 13046–13052. [Google Scholar] [CrossRef]
- Kim, K.; Lim, D.G.; Han, C.W.; Osswald, S.; Ortalan, V.; Youngblood, J.P.; Pol, V.G. Tailored carbon anodes derived from biomass for sodium-ion storage. ACS Sustain. Chem. Eng. 2017, 5, 8720–8728. [Google Scholar] [CrossRef]
- Dahbi, M.; Kiso, M.; Kubota, K.; Horiba, T.; Chafik, T.; Hida, K.; Matsuyama, T.; Komaba, S. Synthesis of hard carbon from argan shells for Na-ion batteries. J. Mater. Chem. A 2017, 5, 9917–9928. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, Y.; Wan, J.; Henderson, D.; Zhang, X.; Hu, L. High temperature carbonized grass as a high performance sodium ion battery anode. ACS Appl. Mater. Interfaces 2017, 9, 391–397. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Jiang, E.; Amiralian, N.; Annamalai, P.K.; Martin, D.J.; Kumar, N.A.; Zhao, X. Spinifex nanocellulose derived hard carbon anodes for high-performance sodium-ion batteries. Sustain. Energy Fuels 2017, 1, 1090–1097. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, X.; Wang, Q.; Xu, X.; Zhou, X.; Bao, J. Kelp-derived hard carbons as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 5761–5769. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, X.; Liu, X.; Xiao, L.; Cao, Y. A green route to synthesize low-cost and high-performance hard carbon as promising sodium-ion battery anodes from sorghum stalk waste. Green Energy Environ. 2017, 2, 310–315. [Google Scholar] [CrossRef]
- Puthusseri, D.; Ogale, S.; Kumar, A.; Shelke, M.V.; Gawli, Y.; Wahid, M. Nutty Carbon: Morphology Replicating Hard Carbon from Walnut Shell for Na Ion Battery Anode. ACS Omega 2017, 2, 3601–3609. [Google Scholar]
- Wang, Y.; Feng, Z.; Zhu, W.; Gariépy, V.; Gagnon, C.; Provencher, M.; Laul, D.; Veillette, R.; Trudeau, M.L.; Guerfi, A. High capacity and high efficiency maple tree-biomass-derived hard carbon as an anode material for sodium-ion batteries. Materials 2018, 11, 1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Wang, Y.; Lu, Y.; Hu, Y.-S.; Li, J. A high-performance sodium-ion battery enhanced by macadamia shell derived hard carbon anode. Nano Energy 2017, 39, 489–498. [Google Scholar] [CrossRef]
- Dou, X.; Geng, C.; Buchholz, D.; Passerini, S. Research Update: Hard carbon with closed pores from pectin-free apple pomace waste for Na-ion batteries. APL Mater. 2018, 6, 047501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.; Dong, P.; Wu, G.; Xiao, J.; Zeng, X.; Zhang, Y.; Sun, X. Honeycomb-like hard carbon derived from pine pollen as high-performance anode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 42796–42803. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Liu, Y.; Fang, Y.; Zhou, X.; Bao, J. Rice husk-derived hard carbons as high-performance anode materials for sodium-ion batteries. Carbon 2018, 127, 658–666. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, F.; Zhou, Z.; Zeng, X.; Wang, D.; Dong, P.; Zhao, J.; Sun, S.; Zhang, Y.; Li, X. Expanded biomass-derived hard carbon with ultra-stable performance in sodium-ion batteries. J. Mater. Chem. A 2018, 6, 1513–1522. [Google Scholar] [CrossRef]
- Wu, F.; Liu, L.; Yuan, Y.; Li, Y.; Bai, Y.; Li, T.; Lu, J.; Wu, C. Expanding interlayer spacing of hard carbon by natural K+ doping to boost Na-ion storage. ACS Appl. Mater. Interfaces 2018, 10, 27030–27038. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, M.K.; Li, Y.; Qiao, M.; Hu, Y.-S.; Titirici, M.-M.; Lieder, M. Hard carbon derived from rice husk as low cost negative electrodes in Na-ion batteries. J. Energy Chem. 2019, 29, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Panda, M.R.; Dutta, D.P.; Mitra, S. Bio-derived mesoporous disordered carbon: An excellent anode in sodium-ion battery and full-cell lab prototype. Carbon 2019, 143, 402–412. [Google Scholar]
- Wu, F.; Zhang, M.; Bai, Y.; Wang, X.; Dong, R.; Wu, C. Lotus seedpod-derived hard carbon with hierarchical porous structure as stable anode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 12554–12561. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Huang, Y.; Chen, C. Cedarwood Bark-derived hard carbon as an anode for high-performance sodium-ion batteries. Energy Fuels 2020, 34, 11489–11497. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Yi, H.; Ryu, D.-Y.; Chung, D.; Miyawaki, J.; Yoon, S.-H. Enhancement of first cycle coulombic efficiency of hard carbon derived from eucalyptus in a sodium ion battery. Chem. Lett. 2019, 48, 753–755. [Google Scholar] [CrossRef]
- Marino, C.; Cabanero, J.; Povia, M.; Villevieille, C. Biowaste lignin-based carbonaceous materials as anodes for Na-ion batteries. J. Electrochem. Soc. 2018, 165, A1400. [Google Scholar] [CrossRef]


| Li | Na | |
|---|---|---|
| Cation radius (nm) | 0.076 | 0.106 |
| Ionization energy (eV) | 5.39 | 5.14 |
| Electronegativity | 0.98 | 0.93 |
| Standard electrode potential (V) | −3.04 | −2.71 |
| Molar mass (g/mol) | 6.9 | 23.0 |
| The ratio in Earth’s crust | 0.0065% | 2.83% |
| Price of metal (USD/t) [16] | ~42,000 | ~2325 |
| Material | Storage Mechanism | Theoretical Capacity | Example | Reversible Capacity mAh/g | ICE % | Price USD/kg | Refs. |
|---|---|---|---|---|---|---|---|
| Hard carbon | Intercalation, adsorption, filling | - | Hard carbon from poplar wood | 330 | 88.3 | 1.5 | [21] |
| Alloying (Sn, Si, P) | Alloying reaction M + Na+ + xe− ↔NaxM | 300–750 | Sn@C | 493.6 | - | 30 (Sn powder) | [22,23] |
| Titanate | Insertion | 200 | Na2Ti6O13/ Na2Ti3O7 | 173.6 | 64.4 | 2400 (Na2Ti3O7) | [24,25,26,27] |
| Transition metal oxide (CuO, MoO2) | Conversion reaction MOx + 2xNa+ + 2xe− ↔xNa2O + M | 400–900 | Fe2O3 | 323 | 63 | 50 (Fe2O3) | [20,28] |
| Company (Product Name) | D50 (µm) | SSA (m2/g) | Tap Density (g/cm3) | First Reversible Capacity (mAh/g) | ICE (%) | Ref. |
|---|---|---|---|---|---|---|
| BTR (BSHC-300) | 6.0 ± 1.5 | ≤5.0 | 0.8 ± 0.1 | 295 ± 5.0 | ≥88 | [29] |
| BTR (BSHC-260) | 6.0 ± 1.5 | ≤5.0 | 0.9 ± 0.1 | 260 ± 5.0 | ≥88 | [29] |
| BSG (NHC-330) | 9.25 | 3.58 | 0.77 | 332.5 | 90.5 | [30] |
| BSG (YHC-1) | 9.1 | 5.27 | 0.65 | 294.6 | 89.2 | [30] |
| JFE | - | 0.7–5.0 | 1.61(true density) | ≥350 | - | [31] |
| Precursor | Carbonization Temperature | Capacity mAh/g | ICE% | Capacity Retention | Ref. |
|---|---|---|---|---|---|
| Shaddock peel | 1200 °C | 430.5 (at 0.03 A/g) | 67.7 | 97.5% after 200 cycles | [55] |
| Pinecone | 1400 °C | 370 (at 0.03 A/g) | 85.4 | 90.3% after 120 cycles | [56] |
| Mangosteen shell | 1500 °C | 330 (at 0.02 A/g) | 83 | 98% after 100 cycles | [57] |
| Lotus Stem | 1400 °C | 351 (at 0.02 A/g) | 70 | 94% after 450 cycles | [58] |
| Waste cork | 1600 °C | 358 (at 0.03 A/g) | 81 | 71% after 2000 cycles | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Shi, Z.; Han, T.; Yang, H.; Asfaw, H.D.; Gond, R.; Younesi, R.; Jönsson, P.G.; Yang, W. From Waste Biomass to Hard Carbon Anodes: Predicting the Relationship between Biomass Processing Parameters and Performance of Hard Carbons in Sodium-Ion Batteries. Processes 2023, 11, 764. https://doi.org/10.3390/pr11030764
Jin Y, Shi Z, Han T, Yang H, Asfaw HD, Gond R, Younesi R, Jönsson PG, Yang W. From Waste Biomass to Hard Carbon Anodes: Predicting the Relationship between Biomass Processing Parameters and Performance of Hard Carbons in Sodium-Ion Batteries. Processes. 2023; 11(3):764. https://doi.org/10.3390/pr11030764
Chicago/Turabian StyleJin, Yanghao, Ziyi Shi, Tong Han, Hanmin Yang, Habtom Desta Asfaw, Ritambhara Gond, Reza Younesi, Pär G. Jönsson, and Weihong Yang. 2023. "From Waste Biomass to Hard Carbon Anodes: Predicting the Relationship between Biomass Processing Parameters and Performance of Hard Carbons in Sodium-Ion Batteries" Processes 11, no. 3: 764. https://doi.org/10.3390/pr11030764
APA StyleJin, Y., Shi, Z., Han, T., Yang, H., Asfaw, H. D., Gond, R., Younesi, R., Jönsson, P. G., & Yang, W. (2023). From Waste Biomass to Hard Carbon Anodes: Predicting the Relationship between Biomass Processing Parameters and Performance of Hard Carbons in Sodium-Ion Batteries. Processes, 11(3), 764. https://doi.org/10.3390/pr11030764








