Feasibility Analysis for the Direct Hydration of 1-Octene in a Catalytic Distillation Process Using Residual Curve Maps
Abstract
1. Introduction
2. Theoretical Model
2.1. The Kinetic Model
2.1.1. Selection of the Cosolvent
2.1.2. Materials and Methods
2.1.3. Experimental Procedure

2.1.4. The Effect of Diffusion on the Reaction
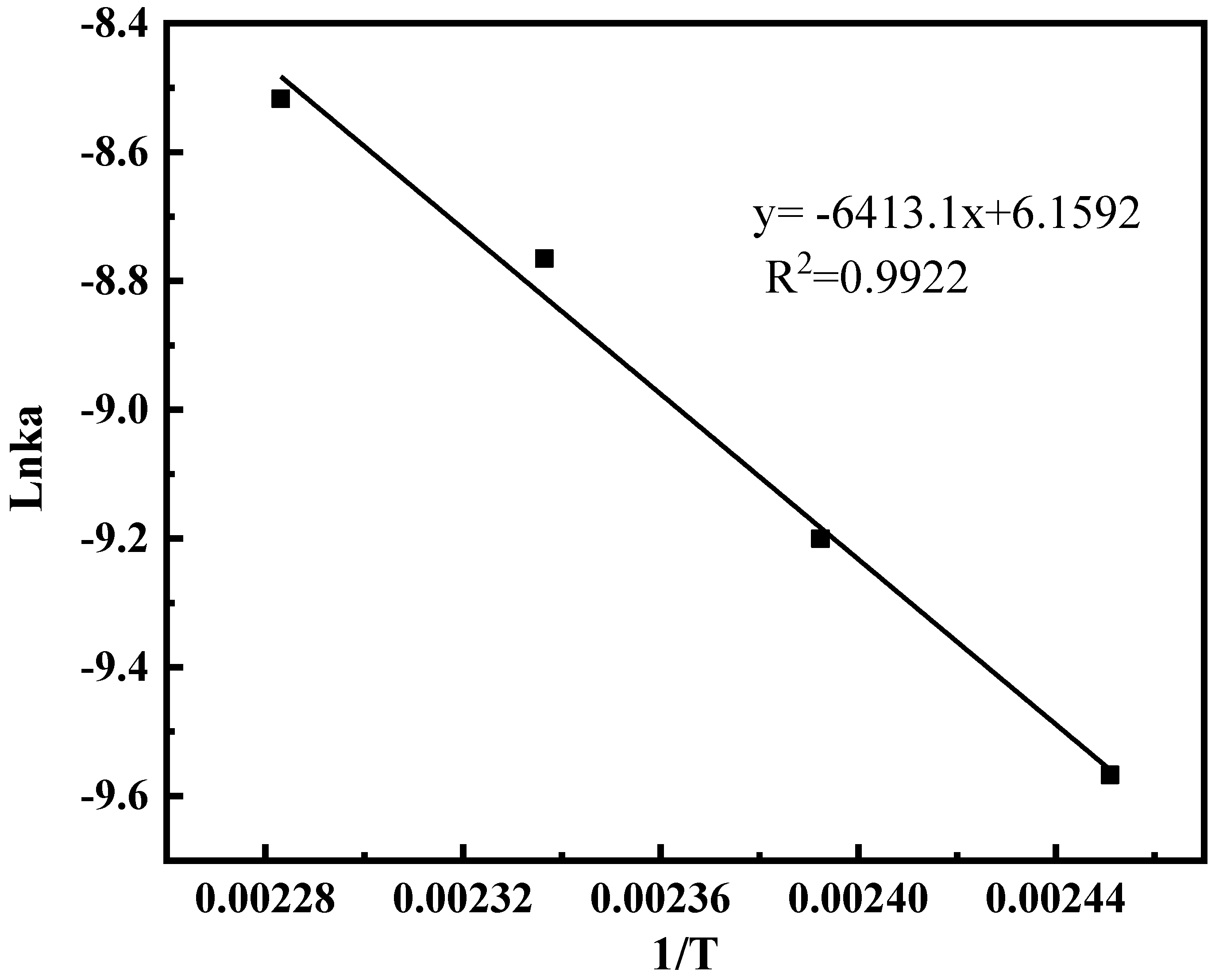
2.1.5. The Effect of Temperature on the Reaction
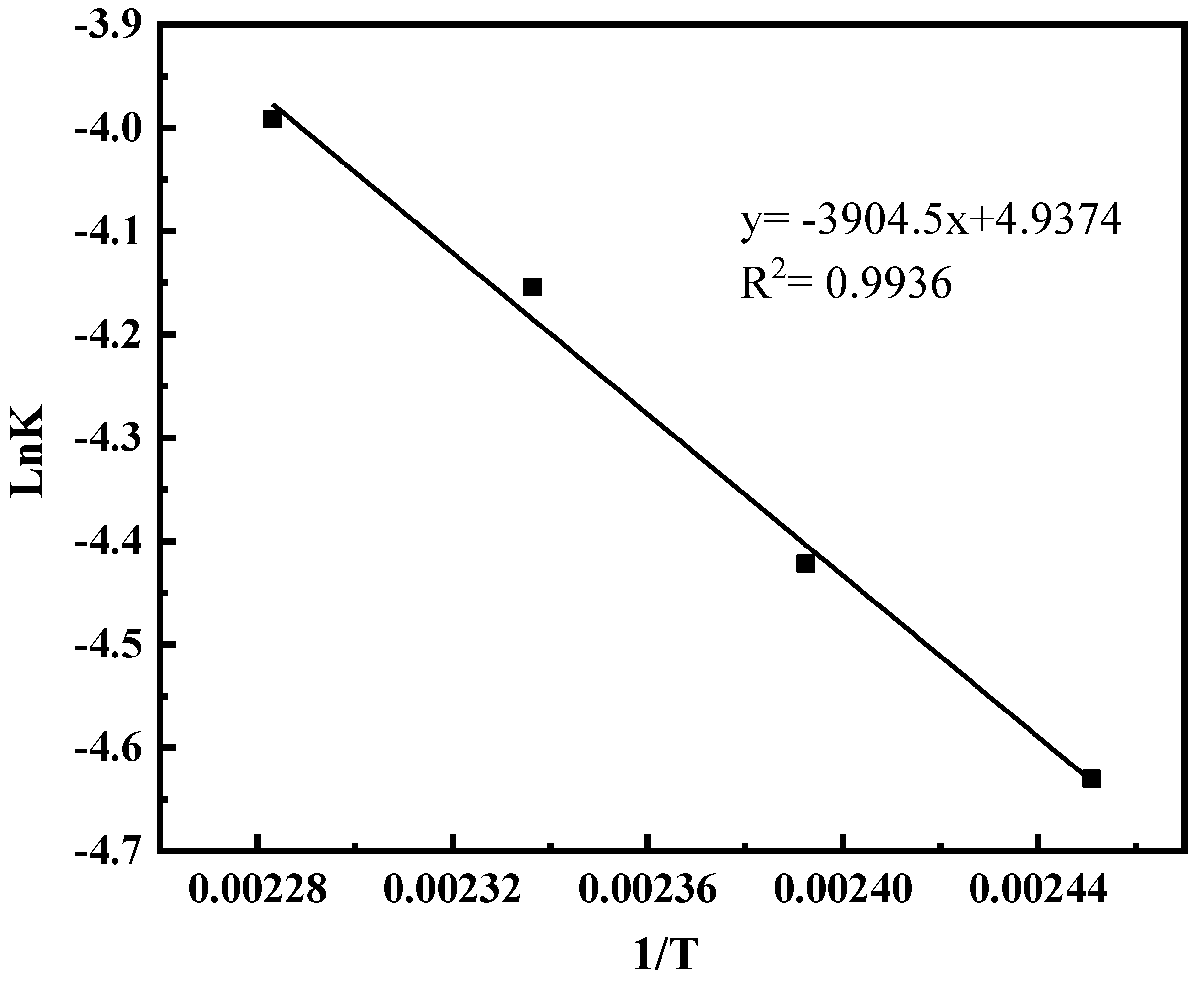
2.1.6. The Effect of Catalyst Content on the Reaction
2.1.7. Model Parameter Identification and Reliability Verification
2.2. The Non-Random Two-Liquid (NRTL) Model Equation
2.3. The Residual Curve Model
3. Residual Curve Analysis
3.1. The Non-Reactive Residual Curves
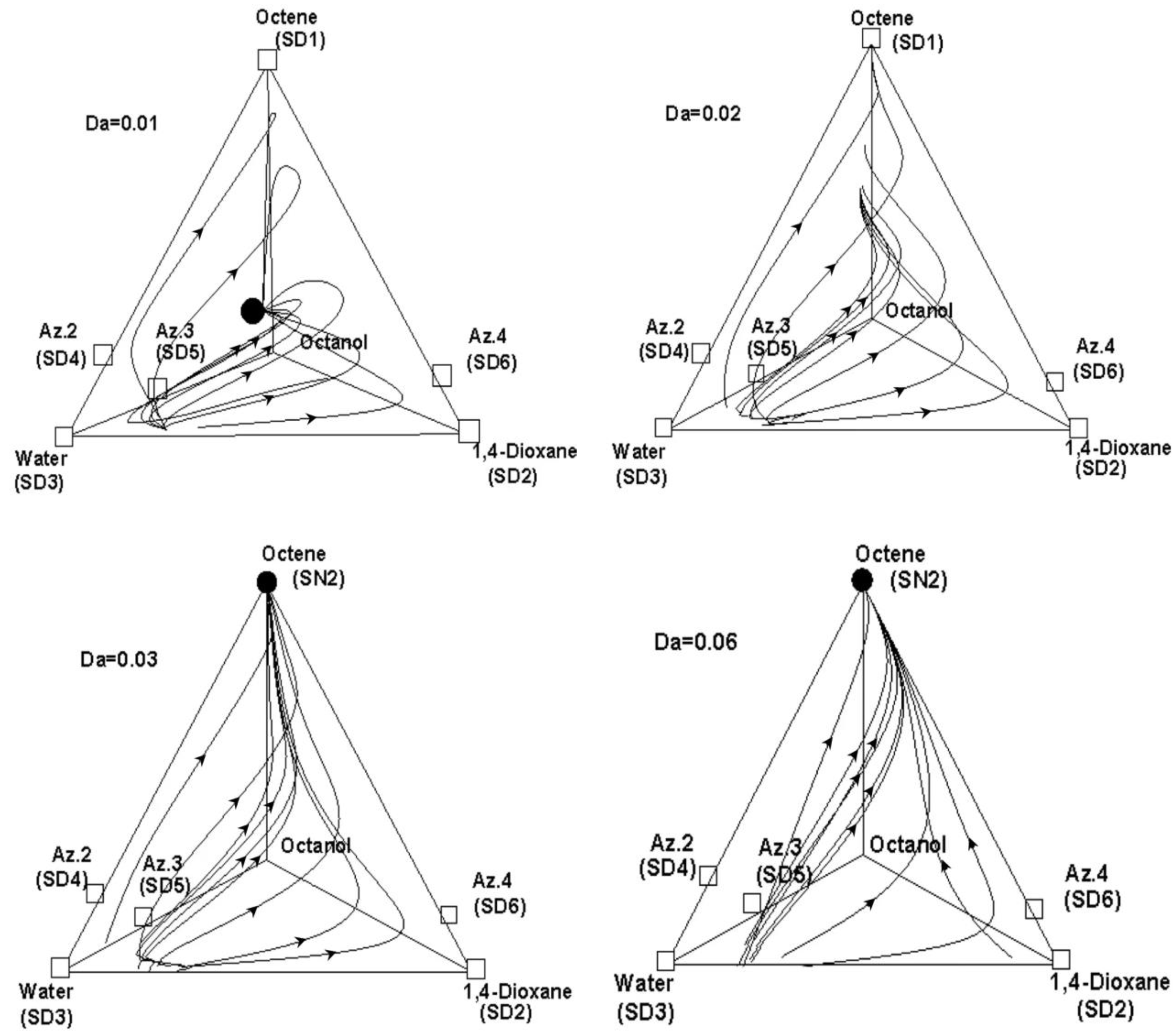
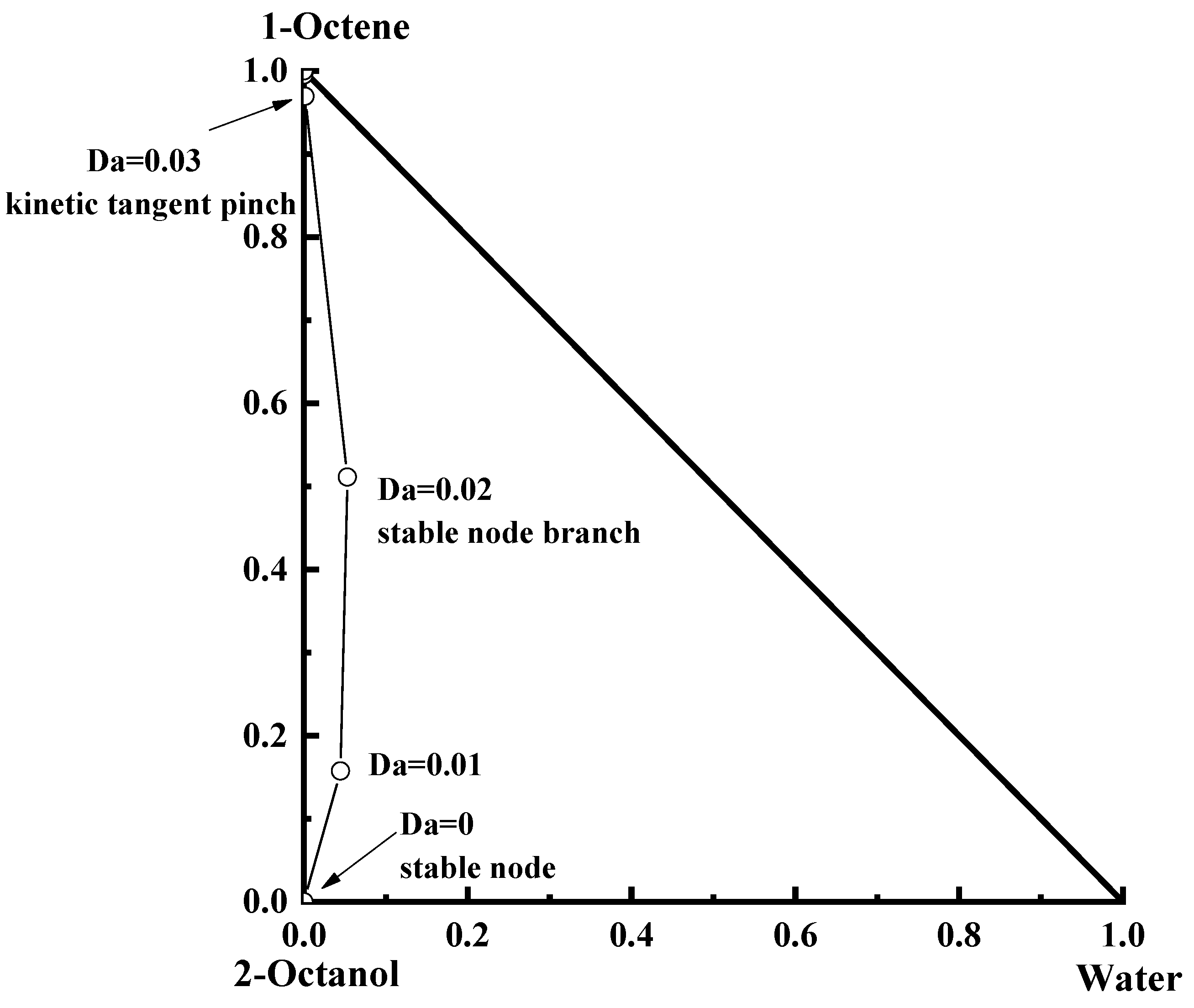
3.2. The Reactive Residual Curves
4. The Conceptual Design Based on the Residual Curve Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lv, C. Research and Development of Chloroisooctane-Isooctanol Separation Technology; TJU: Tianjin, China; Philadelphia, PA, USA, 2013. [Google Scholar]
- Liu, S. Study on the Process of Condensation-Hydrogenation of Secoctanone to Advanced Alcohols; UPC: Qingdao, China, 2010. [Google Scholar]
- Yu, S. Study on the Improvement of the Preparation Process of Sebacic Acid; NCU: Taiyuan, China; Scottsdale, AZ, USA, 2020. [Google Scholar]
- Panneman, H.J.; Beenackers, A. Solvent effects on the hydration of cyclohexene catalyzed by a strong acid ion-exchange resin.1.Solubility of Cyclohexene in Aqueous SulfolaneMixtures. Ind. Eng. Chem. Res. 1992, 31, 1227–1231. [Google Scholar] [CrossRef]
- Panneman, H.J.; Beenackers, A. Solvent effects on the hydration of cyclohexene catalyzed by a strong acid ion-exchange resin.2. Effect of sulfolane on the Reaction Kinetics. Ind. Eng. Chem. Res. 1992, 31, 1425–1433. [Google Scholar]
- Panneman, H.J.; Beenackers, A. Solvent effects on the hydration of cyclohexene catalyzed by a strong acid ion-exchange resin.3. Effect of sulfolane on the equilibrium conversion. Ind. Eng. Chem. Res. 1992, 31, 1433–1440. [Google Scholar]
- Shan, X.; Cheng, Z.; Li, Y. Solvent Effects on Hydration of Cyclohexene over H-ZSM-5 Catalyst. J. Chem. Eng. Data 2011, 56, 4310–4316. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Taylor, R. A nonequilibrium stage model of multicomponent separation processes. Part I: Model description and method of solution. AIChE J. 1985, 31, 449–456. [Google Scholar] [CrossRef]
- Kong, Z.Y.; Sánchez-Ramírez, E.; Yang, A.; Shen, W.; Segovia-Hernández, J.G.; Sunarso, J. Process intensification from conventional to advanced distillations: Past, present, and future. Chem. Eng. Res. Des. 2022, 188, 378–392. [Google Scholar] [CrossRef]
- Van Dongen, D.B.; Doherty, M.F. Design and synthesis of homogeneous azeotropic distillations. 1. Problem formulation for a single column. Ind. Eng. Chem. Fundam. 1985, 24, 454–463. [Google Scholar] [CrossRef]
- Barbosa, D.; Doherty, M.F. A New Set of Composition Variables for the Representation of Reactive-Phase Diagrams. Proc. R. Soc. A-Math. Phys. Sci. 1987, 413, 459–464. [Google Scholar]
- Barbosa, D.; Doherty, M.F. The simple distillation of homogeneous reactive mixtures. Chem. Eng. Sci. 1988, 43, 541–550. [Google Scholar] [CrossRef]
- Barbosa, D.; Doherty, M.F. Design and minimum-reflux calculations for single-feed multicomponent reactive distillation columns. Chem. Eng. Sci. 1988, 43, 1523–1537. [Google Scholar] [CrossRef]
- Barbosa, D.; Doherty, M.F. Design and minimum-reflux calculations for double-feed multicomponent reactive distillation columns. Chem. Eng. Sci. 1988, 43, 2377–2389. [Google Scholar] [CrossRef]
- Thiel, C.; Kai, S.; Ulrich, H. Residue curve maps for heterogeneously catalysed reactive distillation of fuel ethers MTBE and TAME. Chem. Eng. Sci. 1997, 52, 993–1005. [Google Scholar] [CrossRef]
- Thiel, C.; Kai, S.; Ulrich, H. Synthesis of ETBE: Residue Curve Maps for the Heterogeneously Catalysed Reactive Distillation Process. Chem. Eng. J. 1997, 66, 181–191. [Google Scholar] [CrossRef]
- Venimadhavan, G.; Buzad, G.; Doherty, M.F.; Malone, M.F. Effect of kinetics on residue curve maps for reactive distillation. AIChE J. 1994, 40, 1814–1824. [Google Scholar] [CrossRef]
- Zheng, H.; Tian, H.; Zou, W.; Huang, Z.; Wang, X.; Qiu, T.; Zhao, S.; Wu, Y. Residue curve maps of ethyl acetate synthesis reaction. J. Cent. South. Univ. 2013, 20, 50–55. [Google Scholar] [CrossRef]
- Binous, H. Residue curve map for homogeneous reactive quaternary mixtures. Comput. Appl. Eng. Educ. 2007, 15, 73–77. [Google Scholar] [CrossRef]
- Claudia, G.; Miguel, V.; Aruturo, J. A Fast Method to Calculate Residue Curve Maps. Ind. Eng. Chem. Res. 2006, 45, 4429–4432. [Google Scholar]
- Raphaële, T.; Xuan, M.; Meyer, M.; Xavier, J. Feasibility analysis, synthesis, and design of reactive distillation processes: A focus on double-feed processes. AIChE J. 2012, 58, 2346–2356. [Google Scholar]
- Steyer, F.; Qi, Z.; Kai, S. Synthesis of cylohexanol by three-phase reactive distillation: Influence of kinetics on phase equilibria. Chem. Eng. Sci. 2002, 57, 1511–1520. [Google Scholar] [CrossRef]
- Khaledi, R.; Bishnoi, P.R. A Method for Modeling Two- and Three-Phase Reactive Distillation Columns. Ind. Eng. Chem. Res. 2006, 45, 6007–6020. [Google Scholar] [CrossRef]
- Steyer, F.; Sundmacher, K. Cyclohexanol Production via Esterification of Cyclohexene with Formic Acid and Subsequent Hy-dration of the EsterReaction Kinetics. Ind. Eng. Chem. Res. 2007, 46, 1099–1104. [Google Scholar] [CrossRef]
- Steyer, F.; Freund, H.; Sundmacher, K. A Novel Reactive Distillation Process for the Indirect Hydration of Cyclohexene to Cy-clohexanol Using a Reactive Entrainer. Ind. Eng. Chem. Res. 2008, 47, 9581–9587. [Google Scholar] [CrossRef]
- Zheng, H.; Lin, M.; Qiu, T.T.; Shen, Y.; Tian, H.; Zhao, S. Simulation study of direct hydration of cyclohexene to cyclohexanol using isophorone as cosolvent. Chem. Eng. Res. Des. 2017, 117, 346–354. [Google Scholar] [CrossRef]
- Frey, T.; Stichlmair, J. Reactive Azeotropes in Kinetically Controlled Reactive Distillation. Chem. Eng. Res. Des. 1999, 77, 613–618. [Google Scholar] [CrossRef]
- Qiu, T.; Kuang, C.-H.; Li, C.-G.; Zhang, X.-W.; Wang, X.-D. Study on Feasibility of Reactive Distillation Process for the Direct Hydration of Cyclohexene to Cyclohexanol Using a Cosolvent. Ind. Eng. Chem. Res. 2013, 52, 8139–8148. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Sha, Y.; Lin, H.; Zhou, D. Evaluation of Reactive Distillation and Side Reactor Configuration for Direct Hydration of Cyclohexene to Cyclohexanol. Ind. Eng. Chem. Res. 2014, 53, 1461–1469. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; He, L.; Sui, H.; Yin, W. Ionic Liquid-Assisted Solvent Extraction for Unconventional Oil Recovery: Computational Simulation and Experimental Tests. Energy Fuels 2016, 30, 7074–7081. [Google Scholar] [CrossRef]
- Zhang, H.; Mahajani, S.; Sharma, M.; Sridhar, T. Hydration of cyclohexane with solid acid catalysts. Chem. Eng. Sci. 2002, 57, 315–322. [Google Scholar] [CrossRef]
- Cheng, L. Solvent Manual; Chem Ind Pre.: Beijing, China, 2007; pp. 1–1309. [Google Scholar]
- Qi, Z.; Kolah, A.; Sundmacher, K. Residue curve maps for reactive distillation systems with liquid-phase splitting. Chem. Eng. Sci. 2002, 57, 163–178. [Google Scholar] [CrossRef]
- Qi, Z.; Flockerzi, D.; Sundmacher, K. Singular points of reactive distillation systems. AIChE J. 2004, 50, 2866–2876. [Google Scholar] [CrossRef]
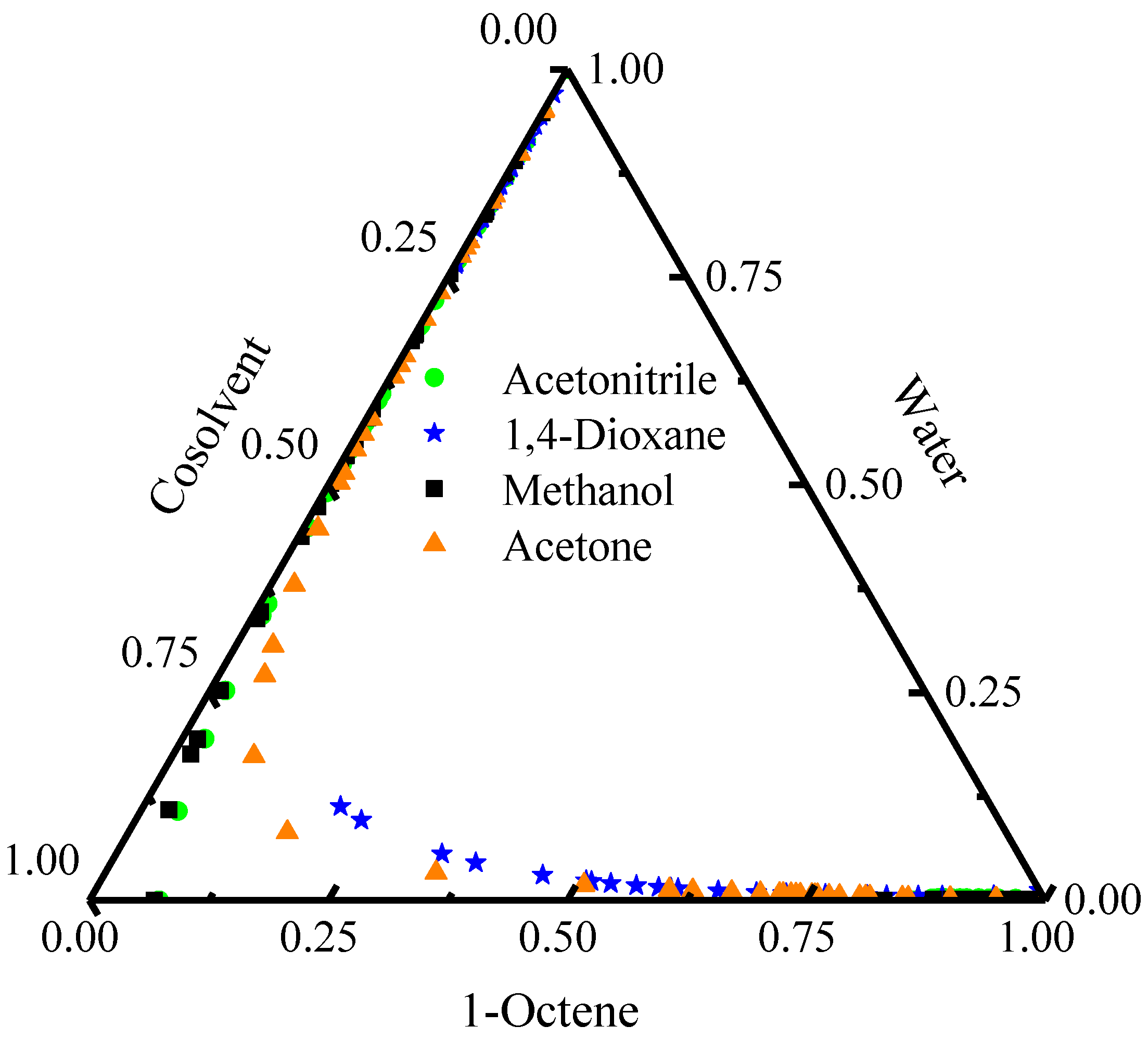

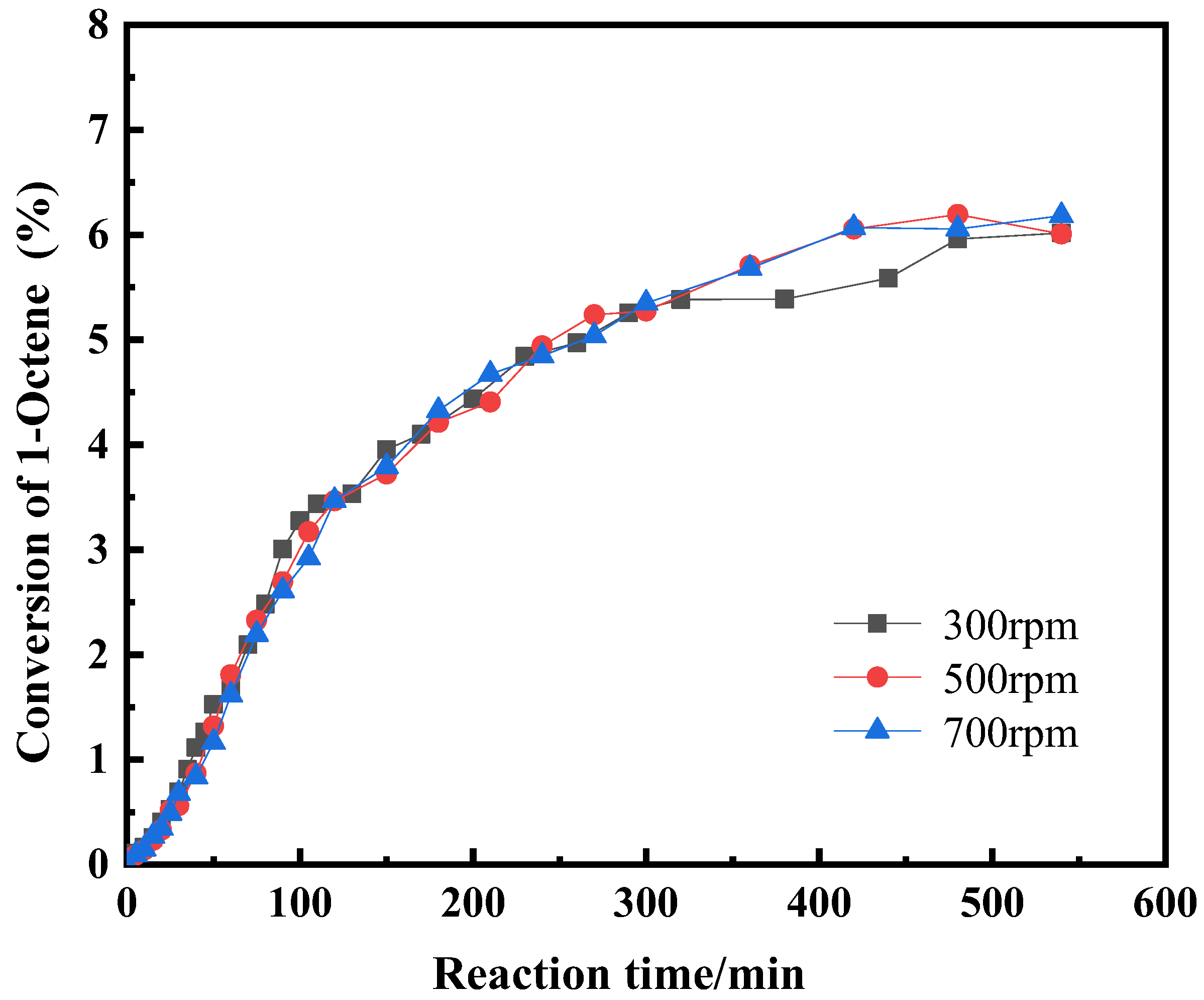
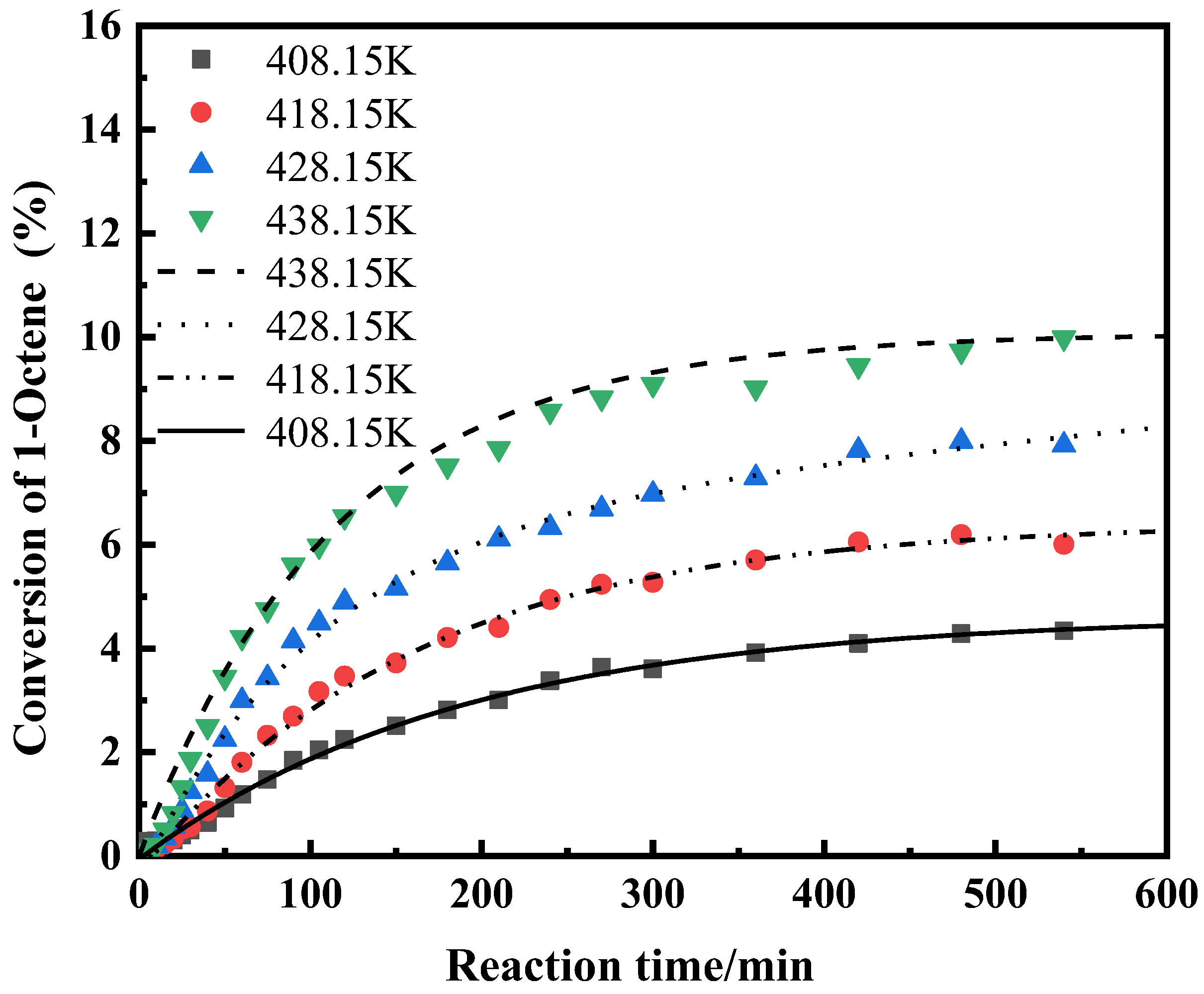
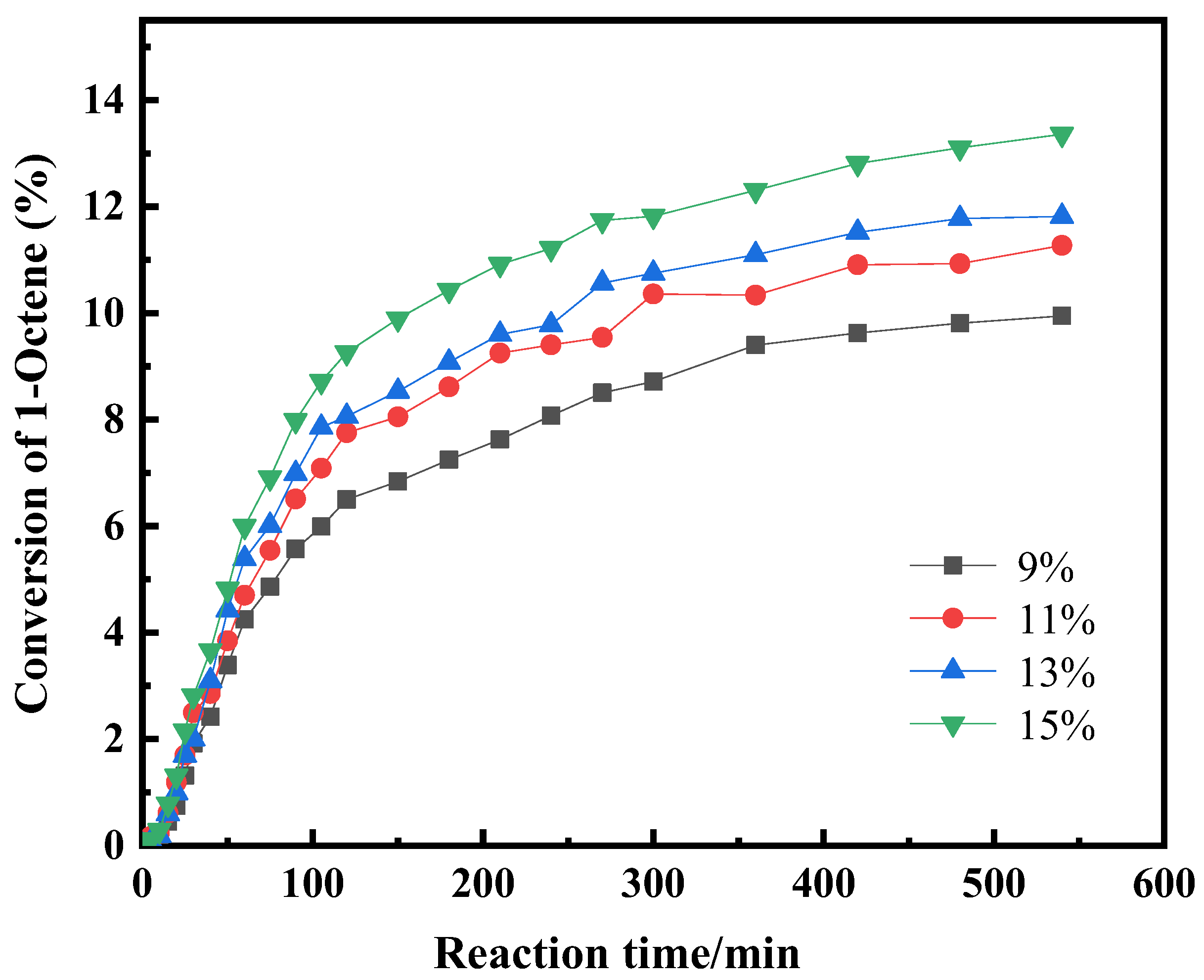

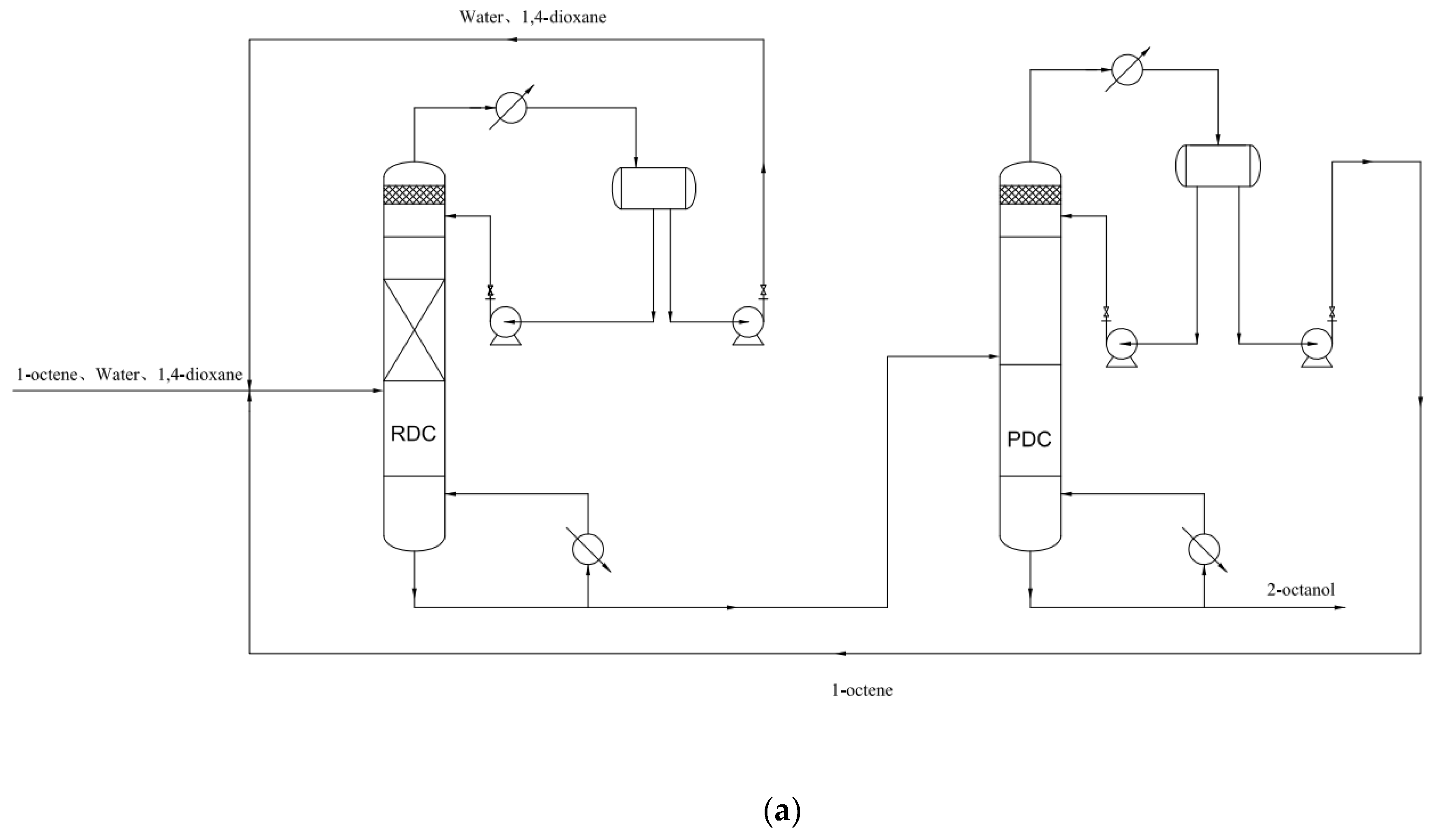
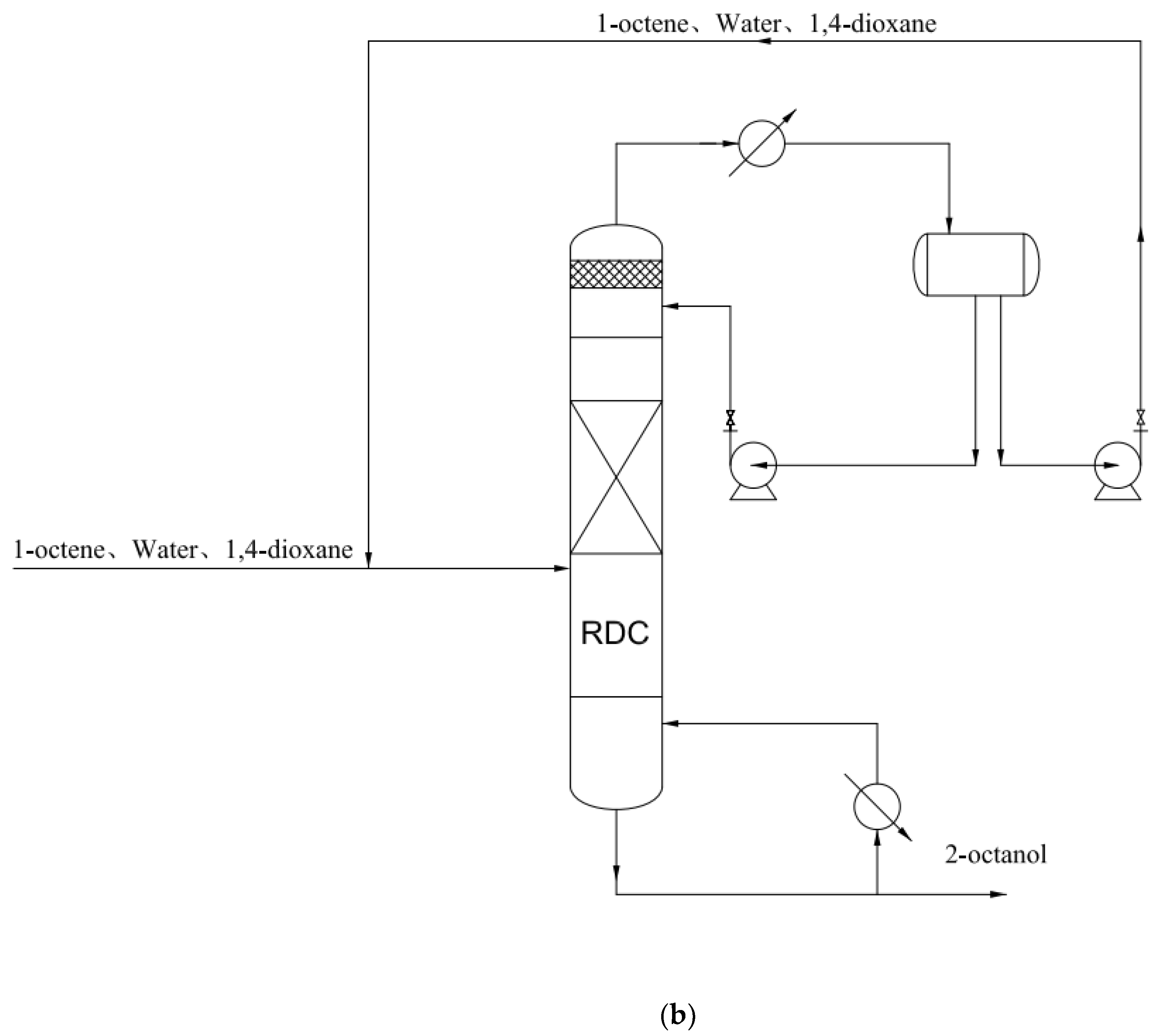
| Component i | ene | H2O | ene | ene | H2O | nol |
| Component j | H2O | nol | nol | ane | ane | ane |
| T | K | K | K | K | K | K |
| 0 | 0 | 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 0 | |
| 994.113 | 4404.598 | 494.851 | −357.970 | 2359.867 | −582.155 | |
| 4637.743 | −303.495 | −138.528 | 727.074 | −78.838 | 692.938 |
| Azeotrope | Mass Composition/% | Literature Value [32] | Calculated Value | Absolute Difference |
|---|---|---|---|---|
| Water-1,4-dioxane | Water | 18.4 | 19.54 | 1.14 |
| 1, 4-dioxane | 81.6 | 80.46 | 1.14 | |
| T/°C | 87.8 | 87.88 | 0.08 | |
| 1-octene-water | 1-octene | No literature was found | 76.95 | |
| water | 23.05 | |||
| T/°C | 88.41 | |||
| 2-octanol-water | 2-octanol | 27 | 22.25 | 4.75 |
| water | 73 | 77.75 | 4.75 | |
| T/°C | 98 | 99.03 | 1.03 | |
| 1-octene-1, 4-dioxane | 1-octene | No literature was found | 20.20 | |
| 1, 4-dioxane | 79.80 | |||
| T/°C | 99.98 |
| Molar Fraction | Component | Experimental Value | Calculated Value | Absolute Error |
|---|---|---|---|---|
| 1-octene | 0.000171 | 4.19 × 10−4 | 2.48 × 10−4 | |
| Aqueous phase | Water | 0.835050 | 0.946819 | 0.111769 |
| 1,4-dioxane | 0.164780 | 0.052762 | 0.112018 | |
| 1-octene | 0.527150 | 0.666472 | 0.139322 | |
| Oil phase | Water | 0.020290 | 0.132541 | 0.112251 |
| 1,4-dioxane | 0.452560 | 0.200987 | 0.251573 | |
| 1-octene | 0.000280 | 0.000861 | 5.81 × 10−4 | |
| Aqueous phase | Water | 0.810710 | 0.923842 | 0.113132 |
| 1,4-dioxane | 0.189010 | 0.075298 | 0.113712 | |
| 1-octene | 0.442740 | 0.557079 | 0.114339 | |
| Oil phase | Water | 0.046320 | 0.177738 | 0.131418 |
| 1,4-dioxane | 0.510940 | 0.265182 | 0.245758 | |
| 1-octene | 0.000425 | 0.001474 | 1.05 × 10−3 | |
| Aqueous phase | Water | 0.790290 | 0.902422 | 0.112132 |
| 1,4-dioxane | 0.209290 | 0.096104 | 0.113186 | |
| 1-octene | 0.379780 | 0.474092 | 0.094312 | |
| Oil phase | Water | 0.068210 | 0.211312 | 0.143102 |
| 1,4-dioxane | 0.552010 | 0.314596 | 0.237414 |
| Calculation Formula |
|---|
| The units of ,, and are Pa, and the units of temperature T are K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Li, J.; Cao, M.; Chen, X. Feasibility Analysis for the Direct Hydration of 1-Octene in a Catalytic Distillation Process Using Residual Curve Maps. Processes 2023, 11, 777. https://doi.org/10.3390/pr11030777
Tian H, Li J, Cao M, Chen X. Feasibility Analysis for the Direct Hydration of 1-Octene in a Catalytic Distillation Process Using Residual Curve Maps. Processes. 2023; 11(3):777. https://doi.org/10.3390/pr11030777
Chicago/Turabian StyleTian, Hui, Jianshu Li, Min Cao, and Xiaoping Chen. 2023. "Feasibility Analysis for the Direct Hydration of 1-Octene in a Catalytic Distillation Process Using Residual Curve Maps" Processes 11, no. 3: 777. https://doi.org/10.3390/pr11030777
APA StyleTian, H., Li, J., Cao, M., & Chen, X. (2023). Feasibility Analysis for the Direct Hydration of 1-Octene in a Catalytic Distillation Process Using Residual Curve Maps. Processes, 11(3), 777. https://doi.org/10.3390/pr11030777






