Experimental Study and Molecular Simulation of the Effect of Temperature on the Stability of Surfactant Foam
Abstract
:1. Introduction
2. Experimental and Numerical Simulation
2.1. Experimental Temperature and Surfactant Selection
2.2. Measurement of Foam Volume and Half-Life
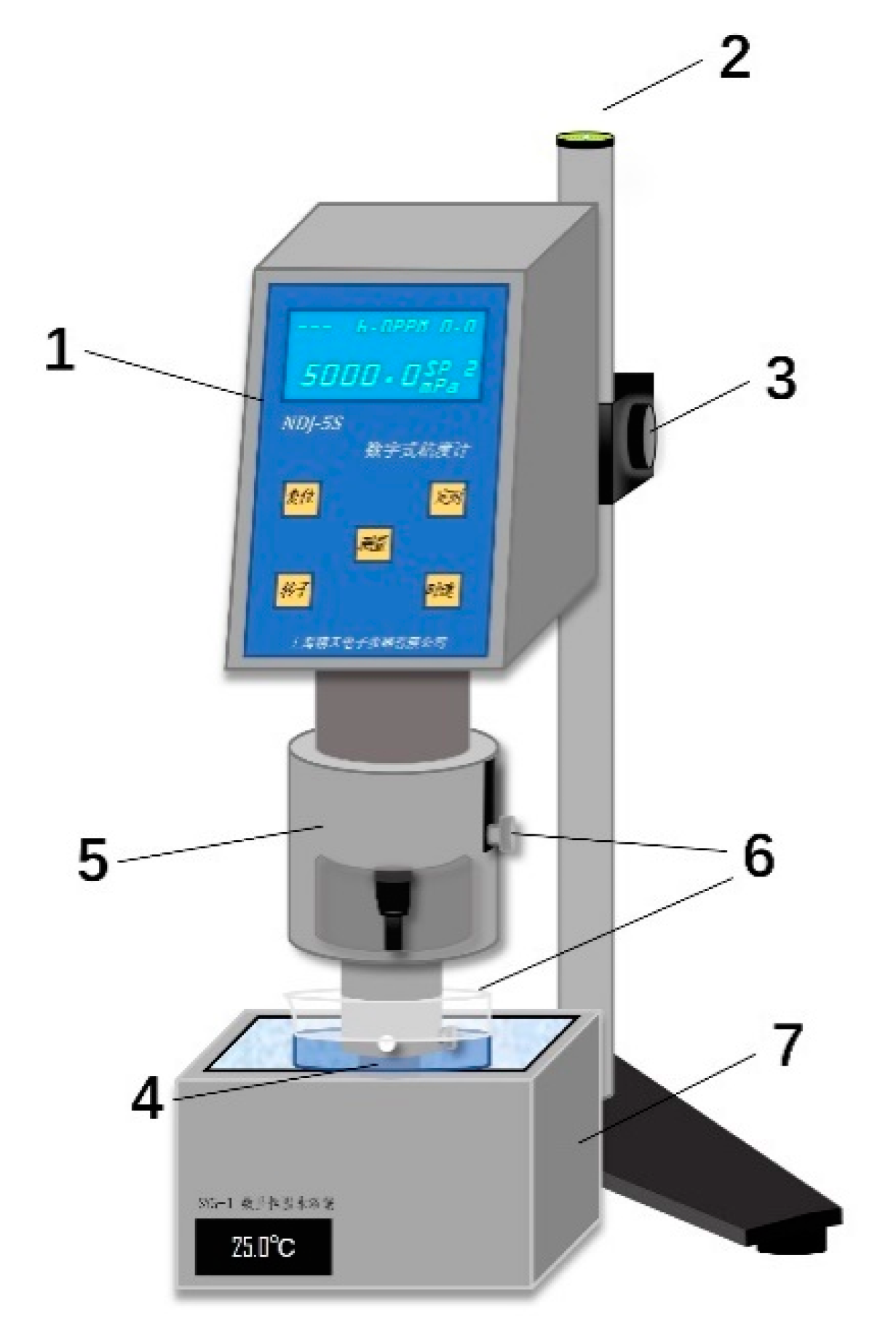
2.3. Viscosity Measurement Test
2.4. Molecular Dynamics Numerical Simulation
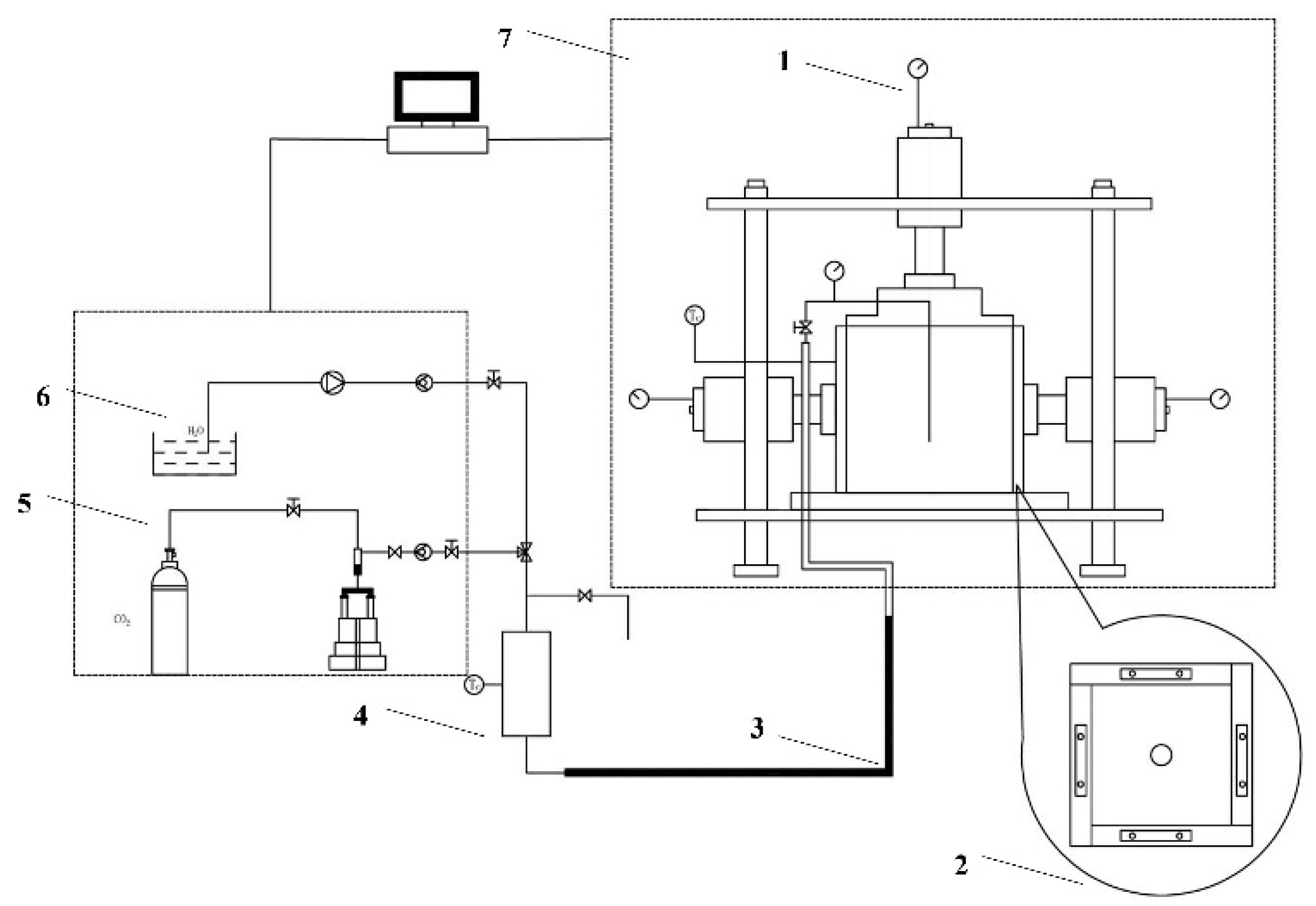
2.5. Foam Fracturing Similar Simulation Experiment
3. Results and Analysis
3.1. CMC Value Range Analysis
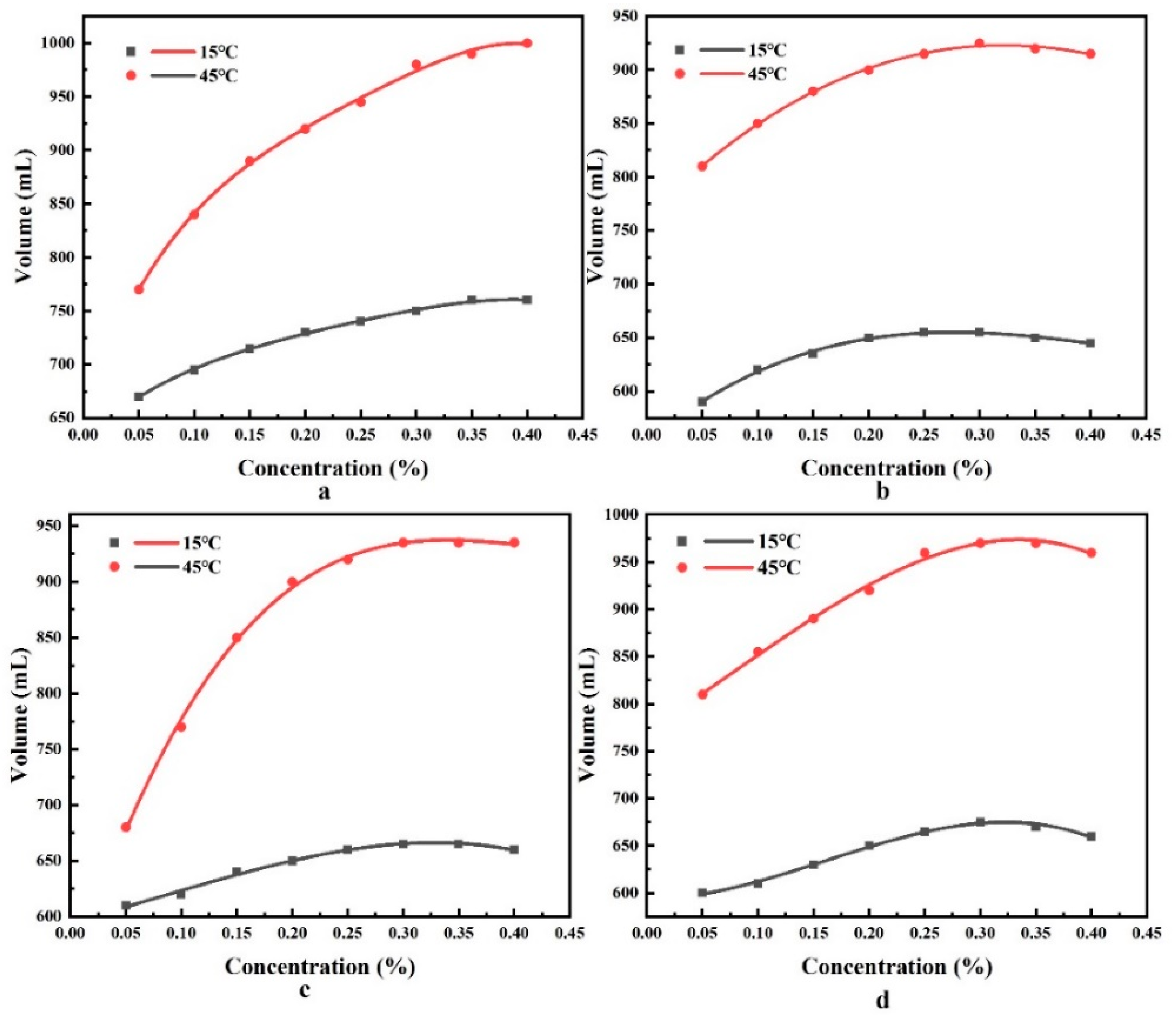
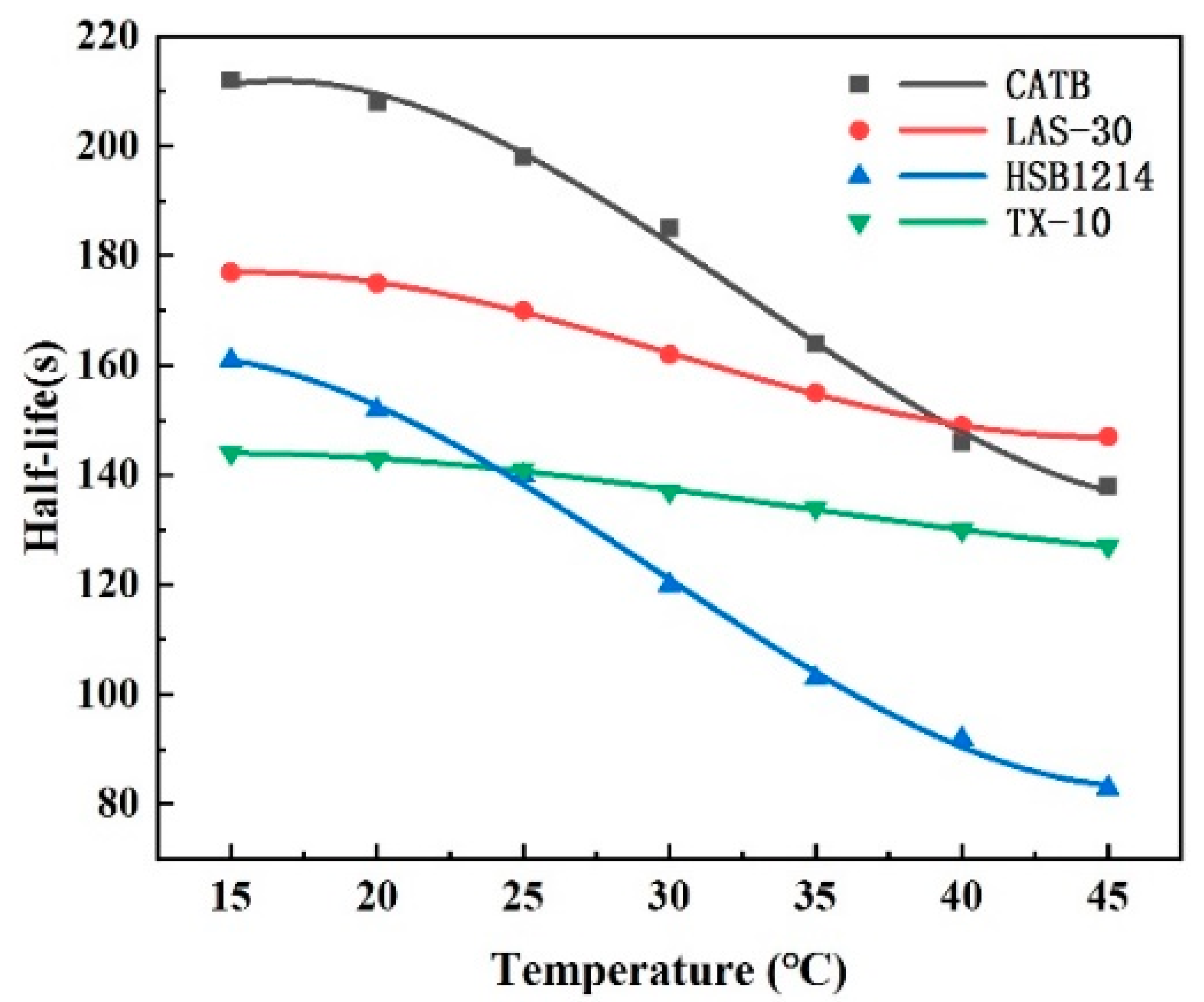
3.2. Foam Half-Life Experimental Results and Analysis
3.3. Viscosity Test Results and Analysis
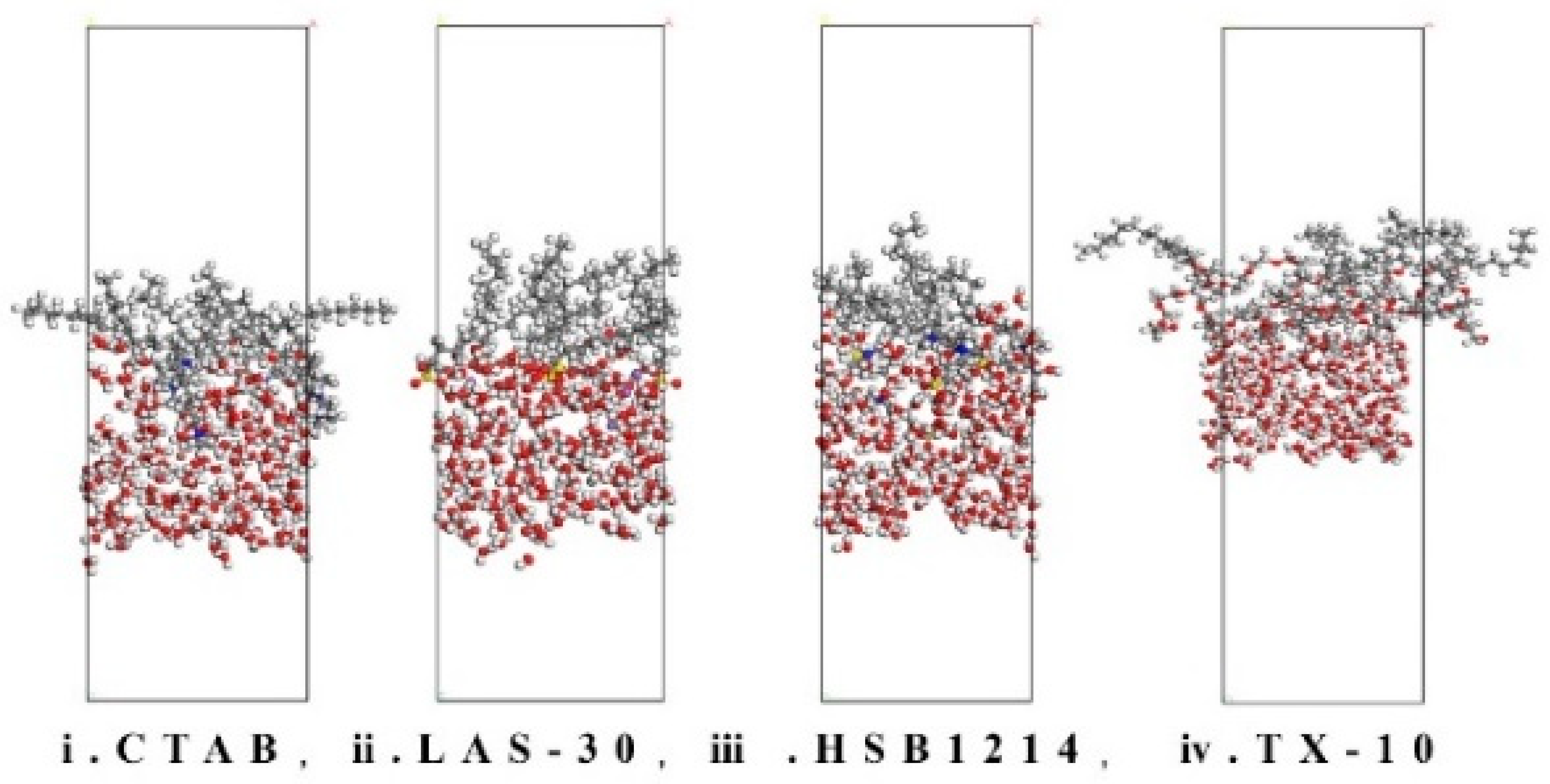
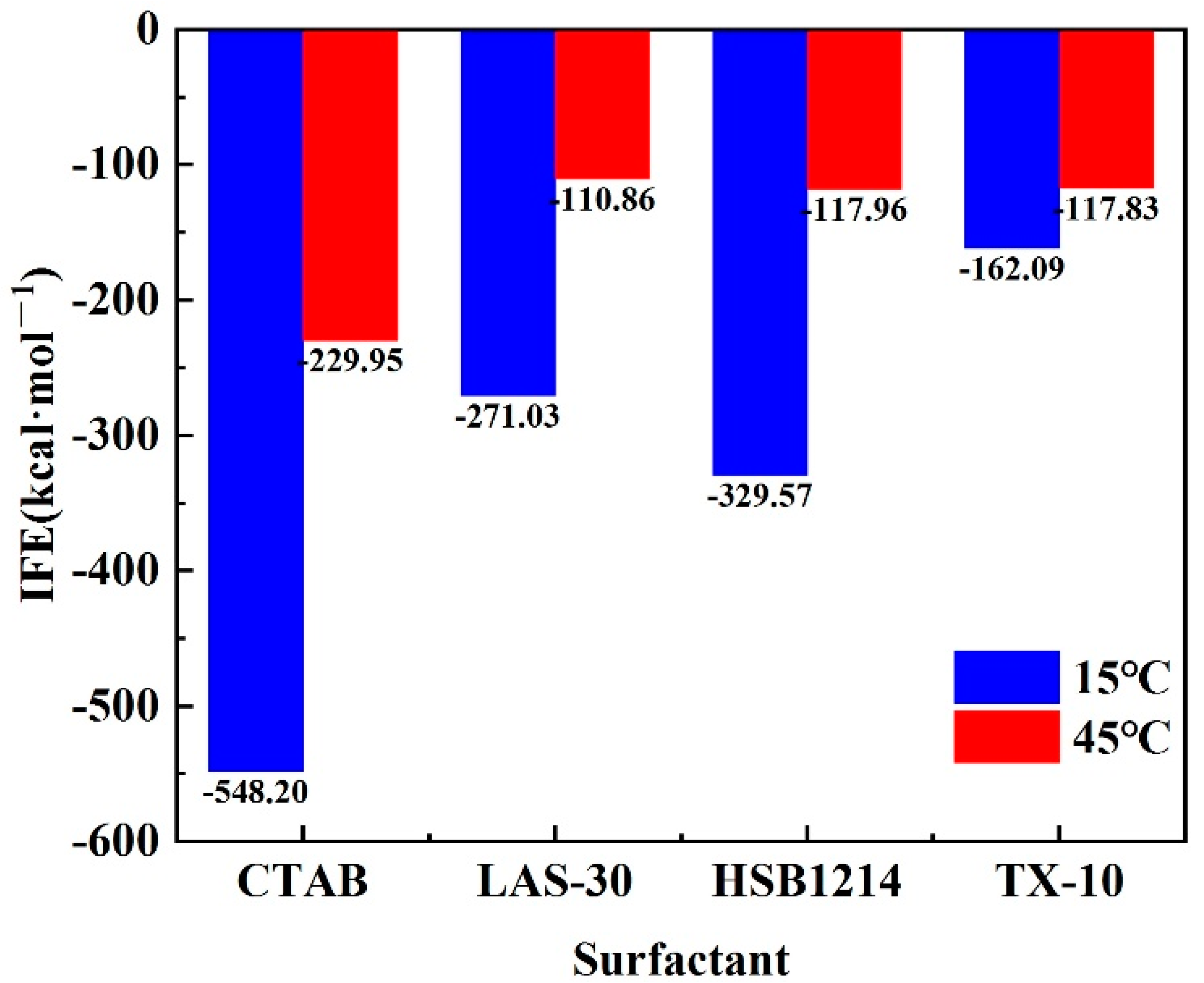
3.4. Analysis of Interface Formation Energy Characteristics
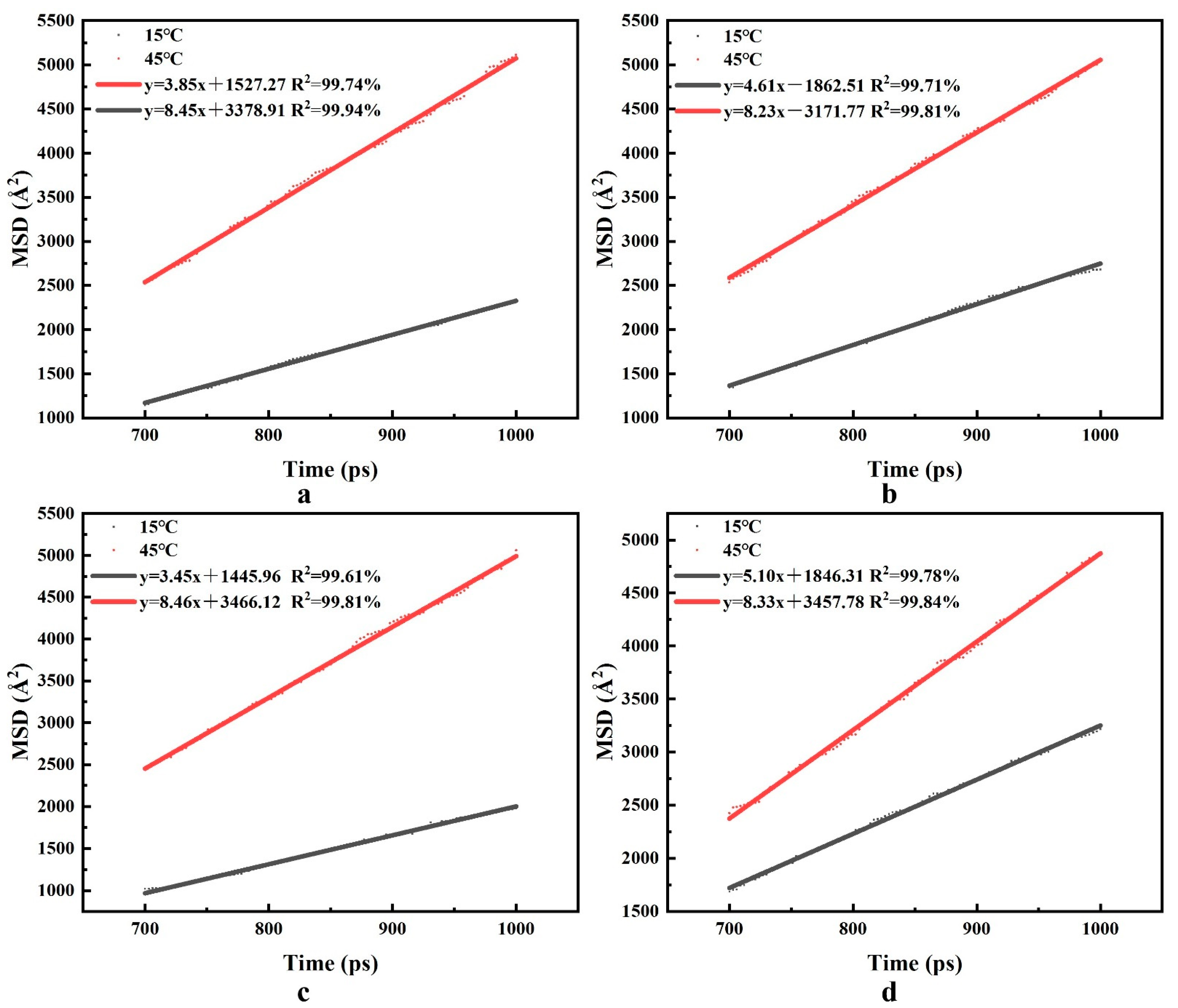
3.5. Analysis of Diffusion Characteristics of Water Molecules
3.6. Cracking Pressure Results and Analysis
4. Conclusions
- Temperature is negatively correlated with half-life and liquid viscosity. With the increase in temperature, the foam half-life of CTAB, LAS-30, HSB1214, and TX-10 decreased by 74 s, 30 s, 78 s, and 17 s, respectively, and the liquid viscosity of CTAB, LAS-30, HSB1214, and TX-10, decreased by 6.45 mPa·s, 7.24 mPa·s, 5.39 mPa·s, and 5.59 mPa·s, respectively. With the increase in temperature, the film’s liquid separation speed becomes faster, the water-holding capacity becomes weaker, the foam bursts faster, and the half-life is shortened. At the same time, viscosity decreases, elasticity decreases, strength decreases, and foam stability decreases.
- There is a positive correlation between temperature and interface energy. With the temperature rise, the IFE of CTAB, LAS-30, HSB1214, and TX-10 increases by 318.25 kcal·mol−1, 160.17 kcal·mol−1, 211.61 kcal·mol−1, and 44.26 kcal·mol−1, respectively. With the increase in temperature, the interface state changes from low to high energy, IFE increases, liquid film-forming ability weakens, interface thinning rate increases, and the foam system accelerates instability.
- There is a positive correlation between temperature and the self-diffusion coefficient of water molecules. With the temperature increase, Dwater of CTAB, LAS-30, HSB1214, and TX-10 increased by 0.77 × 10−5 cm2·s−1, 0.60 × 10−5 cm2·s−1, 0.83 × 10−5 cm2·s−1, and 0.54 × 10−5 cm2·s−1, respectively. With the increase in temperature, the thermal movement rate of water molecules becomes faster, hydrogen bonds break faster, the attraction and binding capacity of the liquid membrane interface to bound water molecules become weaker, the water holding capacity of the foam liquid membrane decreases, and the foam system becomes unstable.
- There is a positive correlation between temperature and MCP. With a temperature increase from 15 to 45 °C, MCP of CTAB, LAS-30, HSB1214, and TX-10 increases by 0.89 Mpa, 2.33 Mpa, 1.88 Mpa, and 0.85 Mpa, respectively. Under same stress conditions, temperature increase results in a reduction of foam stability, while enhancement of MCP weakens the seam-making ability of surfactant foam, which in turn affects the efficiency CO2 foam fracturing construction.
- In future field applications, considerations should be made towards the effects of ground temperature on fracturing operations and attention should also be paid towards lowering the pipe temperature which is especially the case for high-temperature CBM reservoirs. For low-temperature CBM reservoirs, the coal seam should be heated to ensure optimal fracturing conditions, maximize the sequestration of CH4 by CO2, and optimize the production of CBM. Furthermore, of the four surfactants, TX-10(nonionic) has the best resistance to temperature, therefore it should be selected for use (and possibly in unision with other Ionic surfactants) in CBM fracturing operations with large temperature ranges as it helps operations in terms of resistance to temperature effects and improves fracturing efficacy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mallick, N.; Prabu, V. Energy analysis on Coalbed Methane (CBM) coupled power systems. J. CO2 Util. 2017, 19, 16–27. [Google Scholar] [CrossRef]
- Dong, P.; Puerto, M.; Jian, G.; Ma, K.; Mateen, K.; Ren, G. Exploring Low-IFT Foam EOR in Fractured Carbonates: Success and Particular Challenges of Sub-10-md Limestone. SPE J. 2020, 25, 867–882. [Google Scholar] [CrossRef]
- Alexander, S.; Barron, A.R.; Denkov, N.; Grassia, P.; Kiani, S.; Sagisaka, M. Foam generation and stability: Role of the surfactant structure and asphaltene aggregates. Ind. Eng. Chem. Res. 2021, 61, 372–381. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, S.; Nie, X.; Zhang, Y.; Dong, K.; Wang, J. Experimental and Molecular Simulation Research on the Effect of Metal Ions on the Stability of SDS Foam. Energy Fuels 2022, 36, 521–526. [Google Scholar] [CrossRef]
- Abdelaal, A.; Aljawad, M.S.; Alyousef, Z.; Almajid, M.M.A. review of foam-based fracturing fluids applications: From lab studies to field implementations. J. Nat. Gas Sci. Eng. 2021, 95, 104236. [Google Scholar] [CrossRef]
- Ju, S.; Huang, Q.; Wang, G.; Li, J.; Wang, E.; Qin, C.; Qiao, J. Rheological and morphological characteristics of foam fluid using hydroxypropyl guar and surfactant. J. Pet. Sci. Eng. 2022, 211, 110124. [Google Scholar] [CrossRef]
- Ahmed, S.; Elraies, K.A.; Hashmet, M.R.; Alnarabiji, M.S. Empirical modeling of the viscosity of supercritical carbon dioxide foam fracturing fluid under different downhole conditions. Energies 2018, 11, 782. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhaolong, G.; Jinlong, Z.; Zhiyu, T. Viscoelastic surfactant fracturing fluid for underground hydraulic fracturing in soft coal seams. J. Pet. Sci. Eng. 2018, 169, 646–653. [Google Scholar] [CrossRef]
- Chandra, M.S.; Horvath-Szabo, G.; Bulova, M. Influence of temperature, concentration, and gas/liquid ratios on the gas-mobility reduction of foams in porous media. J. Dispers. Sci. Technol. 2012, 33, 1560–1568. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Cheremisin, A.N.; Barifcani, A.; Iglauer, S.; Phan, C. Molecular insights in the temperature effect on adsorption of cationic surfactants at liquid/liquid interfaces. J. Mol. Liq. 2020, 299, 112104. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Yan, M.; Zhang, L.; Liu, Z. The effect of surfactants on carbon xerogel structure and CO2 capture. J. Non-Cryst. Solids 2018, 499, 101–106. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhang, L.; Gong, Q.T.; Zhang, L.; Luo, L.; Li, Z.Q.; Yu, J.Y. Study on foaming properties and dynamic surface tension of sodium branched-alkyl benzene sulfonates. J. Dispers. Sci. Technol. 2009, 30, 137–143. [Google Scholar] [CrossRef]
- Smith, G.A.; Huggett, A.; Jones, C.; Ortego, G. Surface activity and performance properties of gemini salts of linear alkylbenzene sulfonate in aqueous solution. J. Surfactants Deterg. 2012, 24, 563–574. [Google Scholar] [CrossRef]
- Ranjani, I.S.; Ramamurthy, K. Relative assessment of density and stability of foam produced with four synthetic surfactants. Mater. Struct. 2010, 43, 1317–1325. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Zheng, C.; Wang, D.; Zhan, H. Effect of temperature on foaming ability and foam stability of typical surfactants used for foaming agent. J. Surfactants Deterg. 2017, 20, 615–622. [Google Scholar] [CrossRef]
- Cong, Z.; Li, Y.; Pan, Y.; Liu, B.; Shi, Y.; Wei, J.; Li, W. Study on CO2 foam fracturing model and fracture propagation simulation. Energy 2022, 238, 121778. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, K.; Song, X.; Li, G.; Zhu, Z.; Sun, B. A unified model to predict flowing pressure and temperature distributions in horizontal wellbores for different energized fracturing fluids. SPE J. 2019, 24, 834–856. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Chen, W.; Xu, J.; Wang, Z. Calculation model of unsteady temperature–pressure fields in wellbores and fractures of supercritical CO2 fracturing. Fuel 2019, 253, 1168–1183. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.; Zhao, W.; Cai, H.; Zhao, J.; Liu, Y. Study on coal spontaneous combustion at low-medium temperature in the same coal seam with different buried depths and protolith temperatures. Int. J. Coal Prep. Util. 2021, 42, 3451–3463. [Google Scholar] [CrossRef]
- Fuguet, E.; Ràfols, C.; Rosés, M.; Bosch, E. Critical micelle concentration of surfactants in aqueous buffered and unbuffered systems. Anal. Chim. Acta 2005, 548, 95–100. [Google Scholar] [CrossRef]
- Wasan, D.T.; Gupta, L.; Vora, M.K. Interfacial shear viscosity at fluid-fluid interfaces. AIChE J. 1971, 17, 1287–1295. [Google Scholar] [CrossRef]
- Wereszczynski, J.; McCammon, J.A. Statistical mechanics and molecular dynamics in evaluating thermodynamic properties of biomolecular recognition. Q. Rev. Biophys. 2012, 45, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Crooks, R.; Cooper-White, J.; Boger, D.V. The role of dynamic surface tension and elasticity on the dynamics of drop impact. Chem. Eng. Sci. 2001, 56, 5575–5592. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Chen, H.; Wang, P.; Xie, Z.; Yao, Y.; Zhang, J. Effect of surfactant headgroups on the oil/water interface: An interfacial tension measurement and simulation study. J. Mol. Struct. 2003, 1052, 50–56. [Google Scholar] [CrossRef]
- Varshney, V.; Patnaik, S.S.; Roy, A.K.; Farmer, B.L. A molecular dynamics study of epoxy-based networks: Cross-linking procedure and prediction of molecular and material properties. Macromolecules 2008, 41, 6837–6842. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Konouz, E. Prediction of critical micelle concentration of some anionic surfactants using multiple regression techniques: A quantitative structure-activity relationship study. J. Surfactants Deterg. 2000, 3, 47–52. [Google Scholar] [CrossRef]
- Gao, S.; Kang, Z.; Yuan, R.; Liu, N.; Zhu, P.; Wang, B. Molecular dynamics study of nonylphenol-substituted dodecyl sulfonate at air/water interface: Role of steric effect of surfactant headgroups. J. Mol. Struct. 2019, 1192, 35–41. [Google Scholar] [CrossRef]
- Grebenkov, D.S. Probability distribution of the time-averaged mean-square displacement of a Gaussian process. Phys. Rev. E 2011, 84, 031124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symeonidis, V.; Karniadakis, G.E.; Caswell, B. A Seamless Approach to Multiscale Complex Fluid Simulation. Comput. Sci. Eng. 2005, 7, 39–46. [Google Scholar] [CrossRef]
- Chen, L.J.; Lin, S.Y.; Huang, C.C.; Chen, E.M. Temperature dependence of critical micelle concentration of polyoxyethylenated non-ionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 1998, 135, 175–181. [Google Scholar] [CrossRef]
- Tennouga, L.; Mansri, A.; Medjahed, K.; Chetouani, A.; Warad, I. The micelle formation of cationic and anionic surfactants in aqueous medium: Determination of CMC and thermodynamic parameters at different temperatures. J. Mater. Environ. Sci. 2015, 6, 2711–2716. [Google Scholar]






| Surfactant | Chemical Name | Ion Type |
|---|---|---|
| CTAB | Cetyl Trimethyl Ammonium Bromide | Cationic |
| LAS-30 | Sodium Lauryl Benzene Sulfonate | Anionic |
| HSB1214 | Alkyl C12–14Hydroxypropyl Sulfobetaine | Amphoteric |
| TX-10 | Polyoxy Ethylene Nonyl Phenyl Ether | Nonionic |
| Surfactant | Chemical Formula | Basic Properties | Manufacturer Information | Purities |
|---|---|---|---|---|
| CTAB | C19H42NBr | White to Light Yellow, Solid or Gelatinous Liquid. Easily Soluble in Water, Ethanol, Slightly Soluble in Acetone, Almost Insoluble in Ether and Benzene. | China Shanghai Aladdin Biochemical Technology Co., Ltd. | 99.00% |
| LAS-30 | C18H29O3SNa | Easily Soluble in Water, Non-toxic, Excellent Foaming, Good Detergency, Emulsification, and Certain Permeability. | Candace Chemical (Hubei, China) Co., LTD | 60.00% |
| HSB1214 | C18H12O4NS C20H12O4NS | Good Emulsification, Dispersion, and Antistatic Properties, with Sterilization, Mold Inhibition, and Viscoelasticity, etc. | China Shandong Urso Chemical Technology Co. | 45.00% |
| TX-10 | C33H60O10 | Excellent Penetration, Emulsification, Dispersion, and Washing Properties. | 99.00% |
| Surfactant | Temperature (°C) | Etotal (kcal·mol−1) | Ewater (kcal·mol−1) | Esurfactant (kcal·mol−1) |
|---|---|---|---|---|
| CTAB | 15 | −7643.91 | −5543.63 | 128.14 |
| 45 | −4431.82 | −4388.55 | 221.29 | |
| LAS-30 | 15 | −12,540.53 | −6345.55 | −967.97 |
| 45 | −10,620.41 | −5824.65 | −848.29 | |
| HSB1214 | 15 | −9769.82 | −6357.82 | −352.83 |
| 45 | −7788.23 | −5526.05 | −334.48 | |
| TX-10 | 15 | −7289.55 | −7628.31 | 229.84 |
| 45 | −4972.55 | −6792.55 | 481.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Liu, S.; Dong, Z.; Dong, K.; Zhang, Y.; Wang, J. Experimental Study and Molecular Simulation of the Effect of Temperature on the Stability of Surfactant Foam. Processes 2023, 11, 801. https://doi.org/10.3390/pr11030801
Nie X, Liu S, Dong Z, Dong K, Zhang Y, Wang J. Experimental Study and Molecular Simulation of the Effect of Temperature on the Stability of Surfactant Foam. Processes. 2023; 11(3):801. https://doi.org/10.3390/pr11030801
Chicago/Turabian StyleNie, Xin, Shuo Liu, Zhiyu Dong, Kaili Dong, Yulong Zhang, and Junfeng Wang. 2023. "Experimental Study and Molecular Simulation of the Effect of Temperature on the Stability of Surfactant Foam" Processes 11, no. 3: 801. https://doi.org/10.3390/pr11030801





