Characterization of a Gliding Arc Igniter from an Equilibrium Stage to a Non–Equilibrium Stage Using a Coupled 3D–0D Approach
Abstract
:1. Introduction
2. Experimental and Modeling Approaches
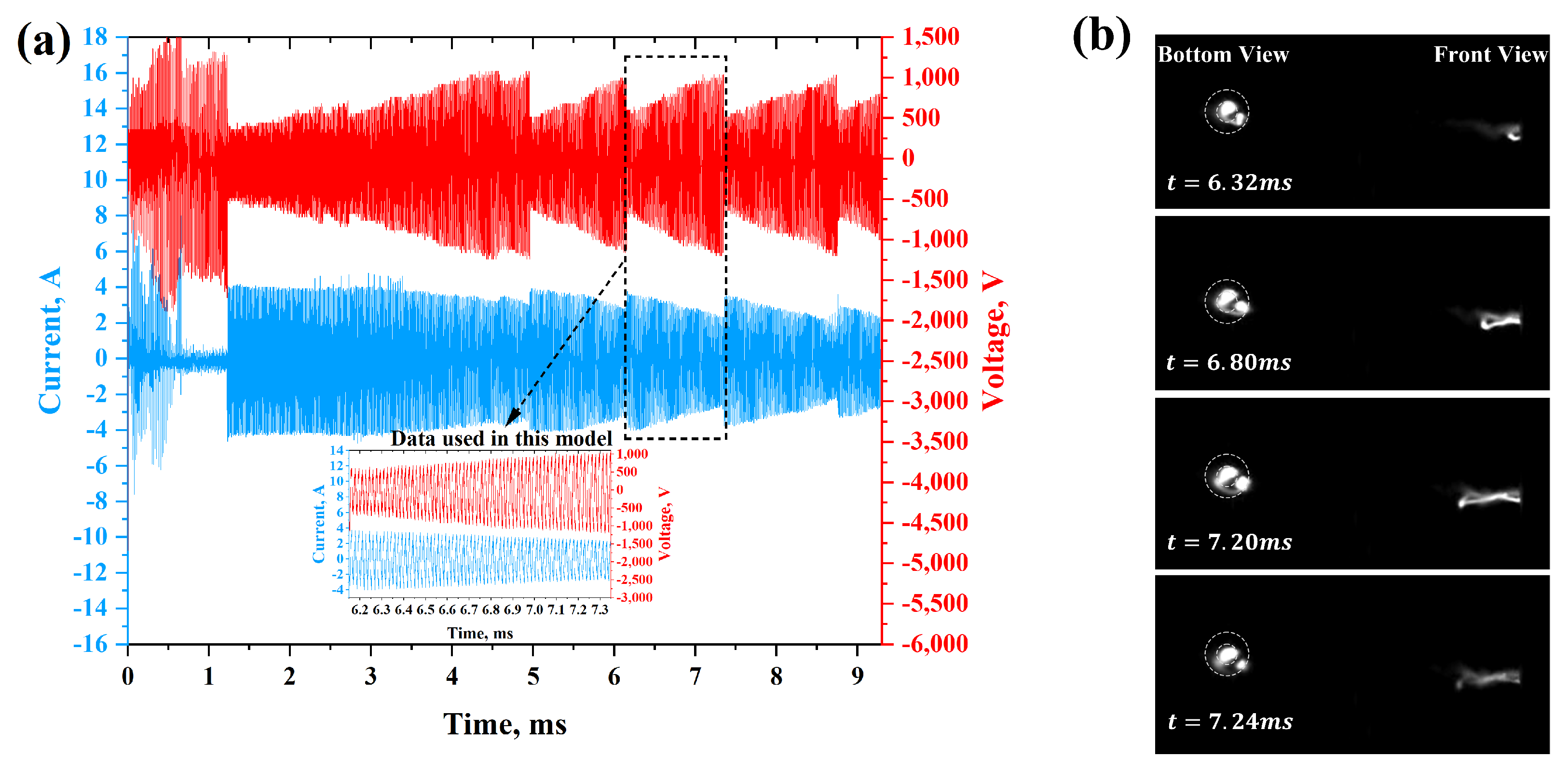
2.1. Brief Description of the Experiment
2.2. The 3D Gas Dynamics Model
2.3. The Global Chemistry Model
- (i)
- electron attachment reactions
- (ii)
- and electron–impact detachment and associative detachment reactions
- (iii)
- Ion–ion recombination of and with positive species
- (iv)
- Electron impact excitation of molecule
- (v)
- and quenching reactions with neutral species.
2.4. The Coupling Strategy
2.5. Base Case Validation
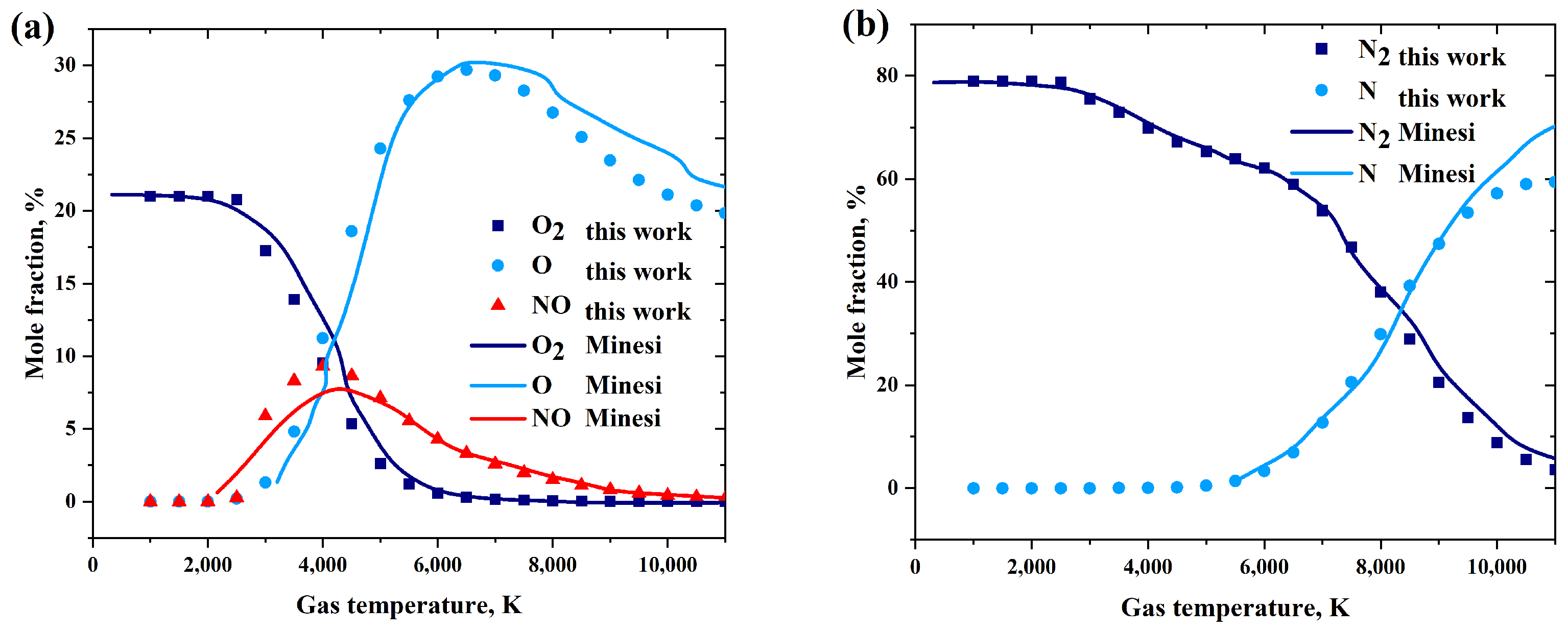
2.5.1. The Chemistry Mechanism
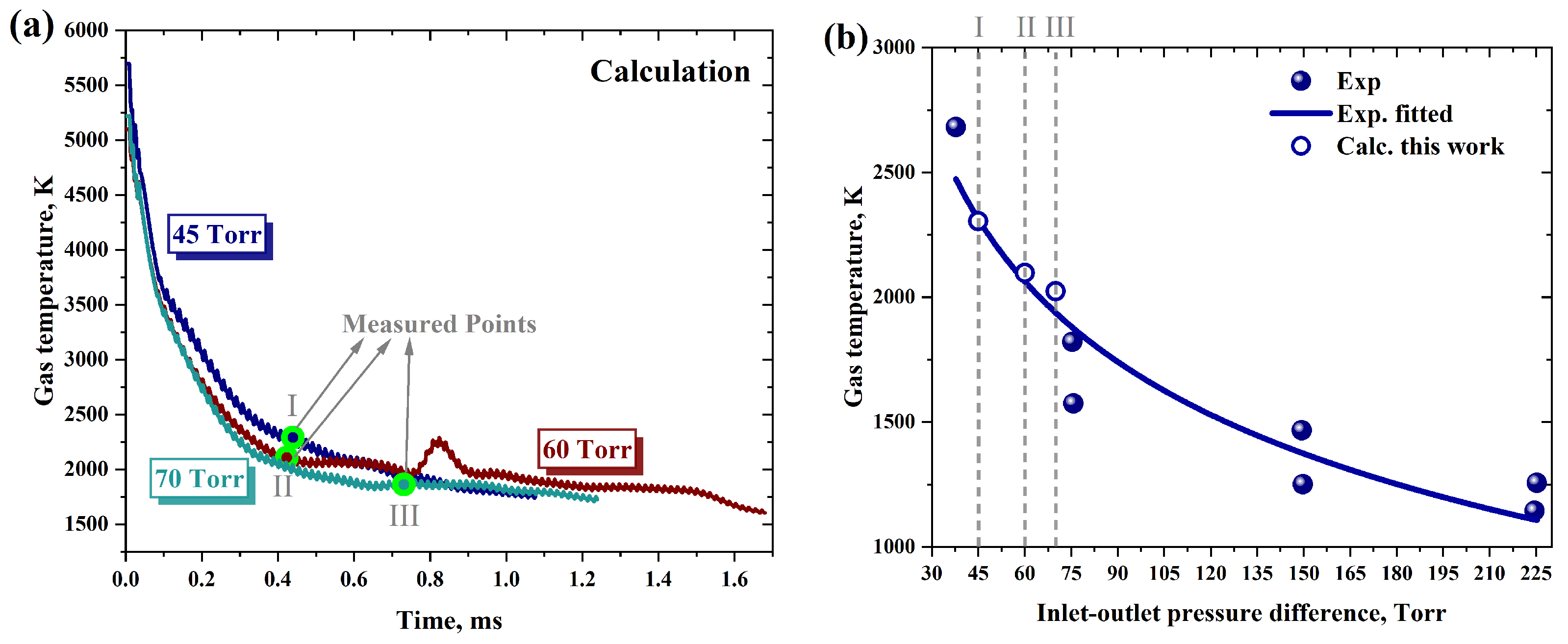
2.5.2. The Outlet Temperature
3. Results and Discussion
3.1. The Structure of the Flow in/out of the Gliding Arc Igniter
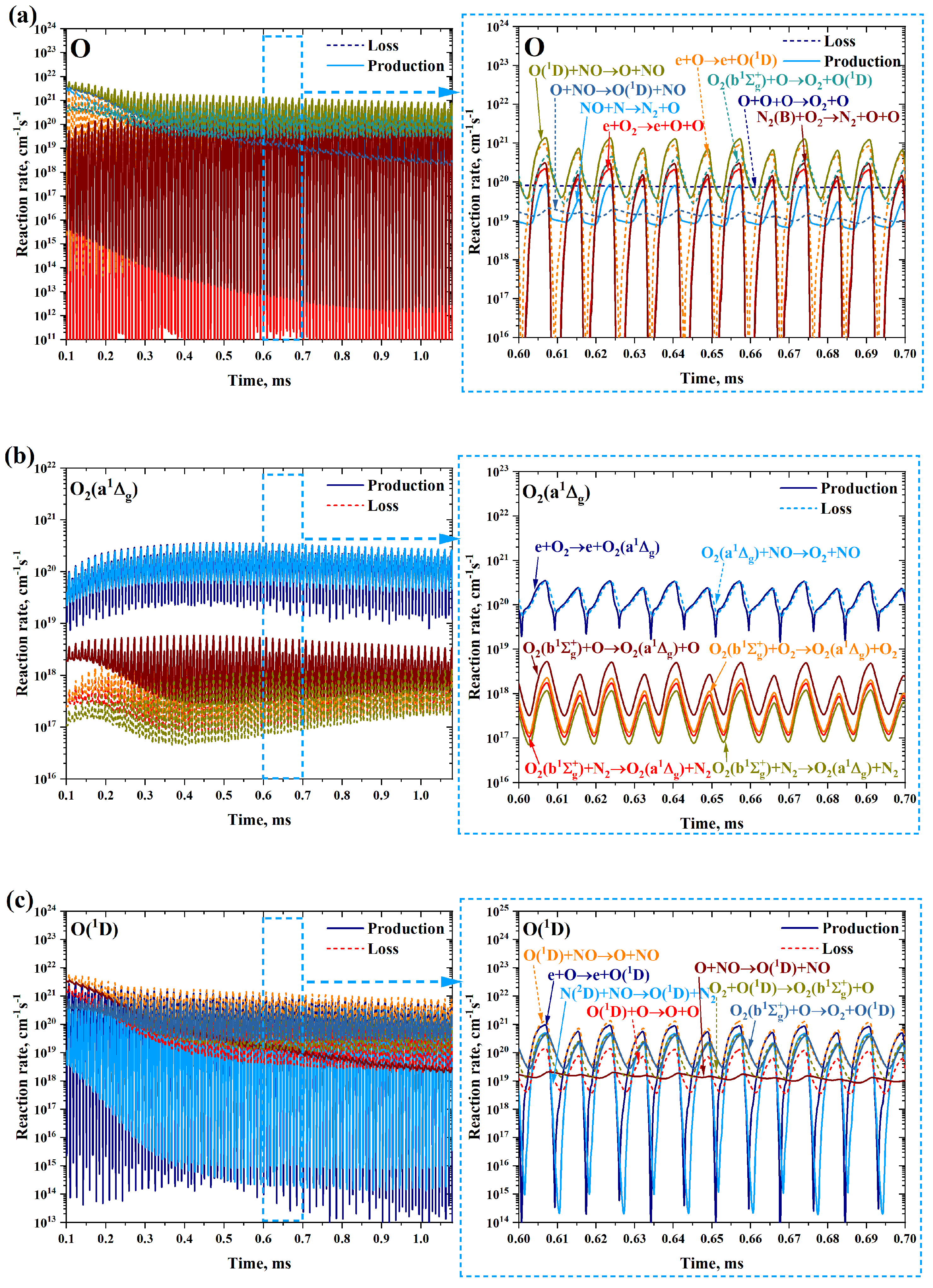
3.2. Transition from Equilibrium to Non-Equilibrium
3.3. Extinction of the Non-Equilibrium Gliding Arc
3.4. A Test Case of Gliding Arc-Assisted Ignition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ju, Y.; Lefkowitz, J.K.; Reuter, C.B.; Won, S.H.; Yang, X.; Yang, S.; Sun, W.; Jiang, Z.; Chen, Q. Plasma assisted low temperature combustion. Plasma Chem. Plasma Process. 2016, 36, 85–105. [Google Scholar] [CrossRef] [Green Version]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Barjasteh, A.; Dehghani, Z.; Lamichhane, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent progress in applications of non-thermal plasma for water purification, bio-sterilization, and decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, T.H.; Choi, S. Hydrophilic surface modification of polytetrafluoroethylene film with gliding arc plasma. Appl. Sci. Converg. Technol. 2019, 28, 101–106. [Google Scholar] [CrossRef]
- Macedo, M.J.; Silva, G.S.; Feitor, M.C.; Costa, T.H.; Ito, E.N.; Melo, J.D. Surface modification of kapok fibers by cold plasma surface treatment. J. Mater. Res. Technol. 2020, 9, 2467–2476. [Google Scholar] [CrossRef]
- Wang, W.; Berthelot, A.; Kolev, S.; Tu, X.; Bogaerts, A. CO2 conversion in a gliding arc plasma: 1D cylindrical discharge model. Plasma Sources Sci. Technol. 2016, 25, 065012. [Google Scholar] [CrossRef]
- Trenchev, G.; Kolev, S.; Wang, W.; Ramakers, M.; Bogaerts, A. CO2 conversion in a gliding arc plasmatron: Multidimensional modeling for improved efficiency. J. Phys. Chem. C 2017, 121, 24470–24479. [Google Scholar] [CrossRef]
- Van Alphen, S.; Slaets, J.; Ceulemans, S.; Aghaei, M.; Snyders, R.; Bogaerts, A. Effect of N2 on CO2-CH4 conversion in a gliding arc plasmatron: Can this major component in industrial emissions improve the energy efficiency? J. CO2 Util. 2021, 54, 101767. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Li, X.; Kong, X.; Xu, R.; Tay, K.; Tu, X. Plasma-assisted CO2 conversion in a gliding arc discharge: Improving performance by optimizing the reactor design. J. CO2 Util. 2019, 29, 296–303. [Google Scholar] [CrossRef]
- Song, F.; Wu, Y.; Yang, X.K.; Zhou, J.P.; Chen, X. Gliding arc plasma adjusting pre-combustion cracking products. Def. Technol. 2022, 18, 2198–2202. [Google Scholar] [CrossRef]
- Lin, B.; Wu, Y.; Zhu, Y.; Song, F.; Bian, D. Experimental investigation of gliding arc plasma fuel injector for ignition and extinction performance improvement. Appl. Energy 2019, 235, 1017–1026. [Google Scholar] [CrossRef]
- Huang, S.; Wu, Y.; Zhang, K.; Jin, D.; Zhang, Z.; Li, Y. Experimental investigation of spray characteristics of gliding arc plasma airblast fuel injector. Fuel 2021, 293, 120382. [Google Scholar] [CrossRef]
- Kai, Z.; Feilong, S.; Di, J.; Shida, X.; Jiulun, S.; Shengfang, H. Experimental investigation on the cracking of pre-combustion cracking gas with gliding arc discharge plasma. Int. J. Hydrogen Energy 2021, 46, 9019–9029. [Google Scholar] [CrossRef]
- Wang, W.Z.; Jia, M.; Feng, R.; Zhu, J.J. Experimental investigation on the gliding arc plasma supported combustion in the scramjet combustor. Acta Astronaut. 2020, 177, 133–141. [Google Scholar] [CrossRef]
- Liming, H.; Yi, C.; Jun, D.; Jianping, L.; Li, F.; Pengfei, L. Experimental study of rotating gliding arc discharge plasma-assisted combustion in an aero-engine combustion chamber. Chin. J. Aeronaut. 2019, 32, 337–346. [Google Scholar]
- Feng, R.; Huang, Y.; Zhu, J.; Wang, Z.; Sun, M.; Wang, H.; Cai, Z. Ignition and combustion enhancement in a cavity-based supersonic combustor by a multi-channel gliding arc plasma. Exp. Therm. Fluid Sci. 2021, 120, 110248. [Google Scholar] [CrossRef]
- Feng, R.; Li, J.; Wu, Y.; Jia, M.; Jin, D. Ignition and blow-off process assisted by the rotating gliding arc plasma in a swirl combustor. Aerosp. Sci. Technol. 2020, 99, 105752. [Google Scholar] [CrossRef]
- Wu, W.W.; Ni, G.H.; Lin, Q.F.; Guo, Q.J.; Meng, Y.D. Experimental investigation of premixed methane–air combustion assisted by alternating-current rotating gliding arc. IEEE Trans. Plasma Sci. 2015, 43, 3979–3985. [Google Scholar] [CrossRef]
- Ombrello, T.; Ju, Y.; Fridman, A. Kinetic ignition enhancement of diffusion flames by nonequilibrium magnetic gliding arc plasma. AIAA J. 2008, 46, 2424–2433. [Google Scholar] [CrossRef]
- Pinto, A.; Sbampato, M.; Sagás, J.; Lacava, P. Gliding arc discharge for emission control in swirl fuel-lean non-premixed combustion. Combust. Sci. Technol. 2021, 195, 1235–1250. [Google Scholar] [CrossRef]
- Pellerin, S.; Richard, F.; Chapelle, J.; Cormier, J.; Musiol, K. Heat string model of bi-dimensional dc Glidarc. J. Phys. D Appl. Phys. 2000, 33, 2407. [Google Scholar] [CrossRef]
- Richard, F.; Cormier, J.; Pellerin, S.; Chapelle, J. Physical study of a gliding arc discharge. J. Appl. Phys. 1996, 79, 2245–2250. [Google Scholar] [CrossRef]
- Kolev, S.; Bogaerts, A. A 2D model for a gliding arc discharge. Plasma Sources Sci. Technol. 2014, 24, 015025. [Google Scholar] [CrossRef]
- Sun, S.; Kolev, S.; Wang, H.; Bogaerts, A. Coupled gas flow-plasma model for a gliding arc: Investigations of the back-breakdown phenomenon and its effect on the gliding arc characteristics. Plasma Sources Sci. Technol. 2016, 26, 015003. [Google Scholar] [CrossRef]
- Wang, W.; Mei, D.; Tu, X.; Bogaerts, A. Gliding arc plasma for CO2 conversion: Better insights by a combined experimental and modelling approach. Chem. Eng. J. 2017, 330, 11–25. [Google Scholar] [CrossRef]
- Bourlet, A.; Labaune, J.; Tholin, F.; Vincent, A.; Pechereau, F.; Laux, C.O.; Vincent-Randonnier, A.; Laux, C.O.; Vincent, A.; Pechereau, F.; et al. Numerical model of restrikes in DC gliding arc discharges. In Proceedings of the AIAA SCITECH 2022 Forum, Virtual, 3–7 January 2022; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2022; pp. 1–10. [Google Scholar] [CrossRef]
- Cheng, X.; Song, H.; Huang, S.; Zhu, Y.; Zhang, Z.; Li, Z.; Jia, M. Discharge and jet characteristics of gliding arc plasma igniter driven by pressure difference. Plasma Sci. Technol. 2022, 24, 115502. [Google Scholar] [CrossRef]
- Available online: http://www.comsol.com (accessed on 5 January 2022).
- Pancheshnyi, S.; Eismann, B.; Hagelaar, G.J.M.; Pitchford, L.C. Computer Code ZDPlasKin; University of Toulouse, LAPLACE, CNRS-UPS-INP: Toulouse, France, 2008; Available online: http://www.zdplaskin.laplace.univ-tlse.fr (accessed on 5 April 2022).
- Hagelaar, G.; Pitchford, L.C. Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci. Technol. 2005, 14, 722. [Google Scholar] [CrossRef]
- Minesi, N. Thermal Spark Formation and Plasma-Assisted Combustion by Nanosecond Repetitive Discharges. Ph.D. Thesis, Université Paris-Saclay, Gif-sur-Yvette, France, 2020. [Google Scholar]
- Rong, M. Gas Discharge Plasma Database. Available online: http://www.plasma-data.net/index (accessed on 5 April 2022).
- Mikhailov, V.; Lebedev, V.; Mukhin, A. Experimental development of a plasma spark plug for GTE altitude start. Russ. Aeronaut. (Iz VUZ) 2010, 53, 235–241. [Google Scholar] [CrossRef]
- Doyle, S.; Xu, K. Use of thermocouples and argon line broadening for gas temperature measurement in a radio frequency atmospheric microplasma jet. Rev. Sci. Instrum. 2017, 88, 023114. [Google Scholar] [CrossRef] [Green Version]
- Rousso, A.; Mao, X.; Chen, Q.; Ju, Y. Kinetic studies and mechanism development of plasma assisted pentane combustion. Proc. Combust. Inst. 2019, 37, 5595–5603. [Google Scholar] [CrossRef]









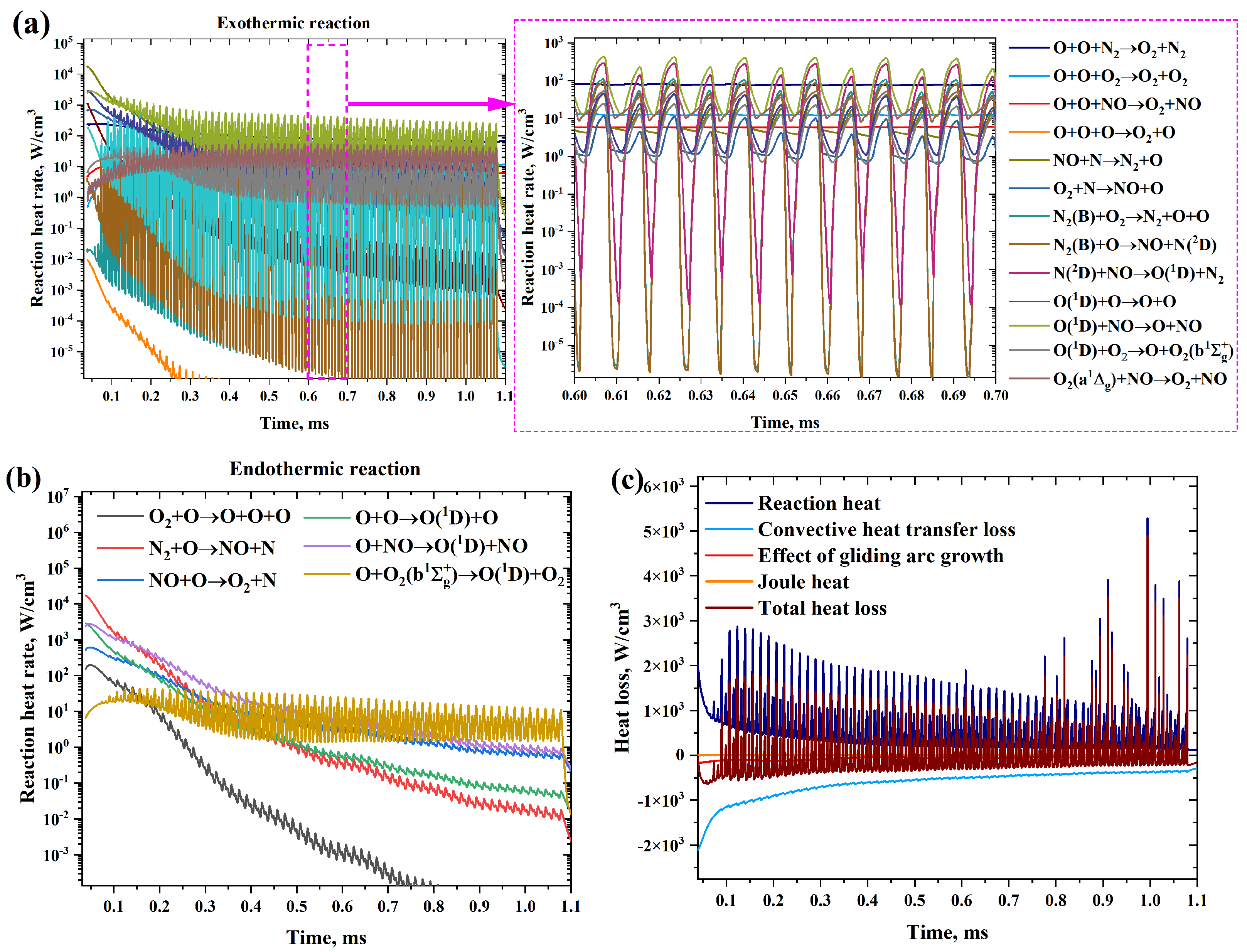
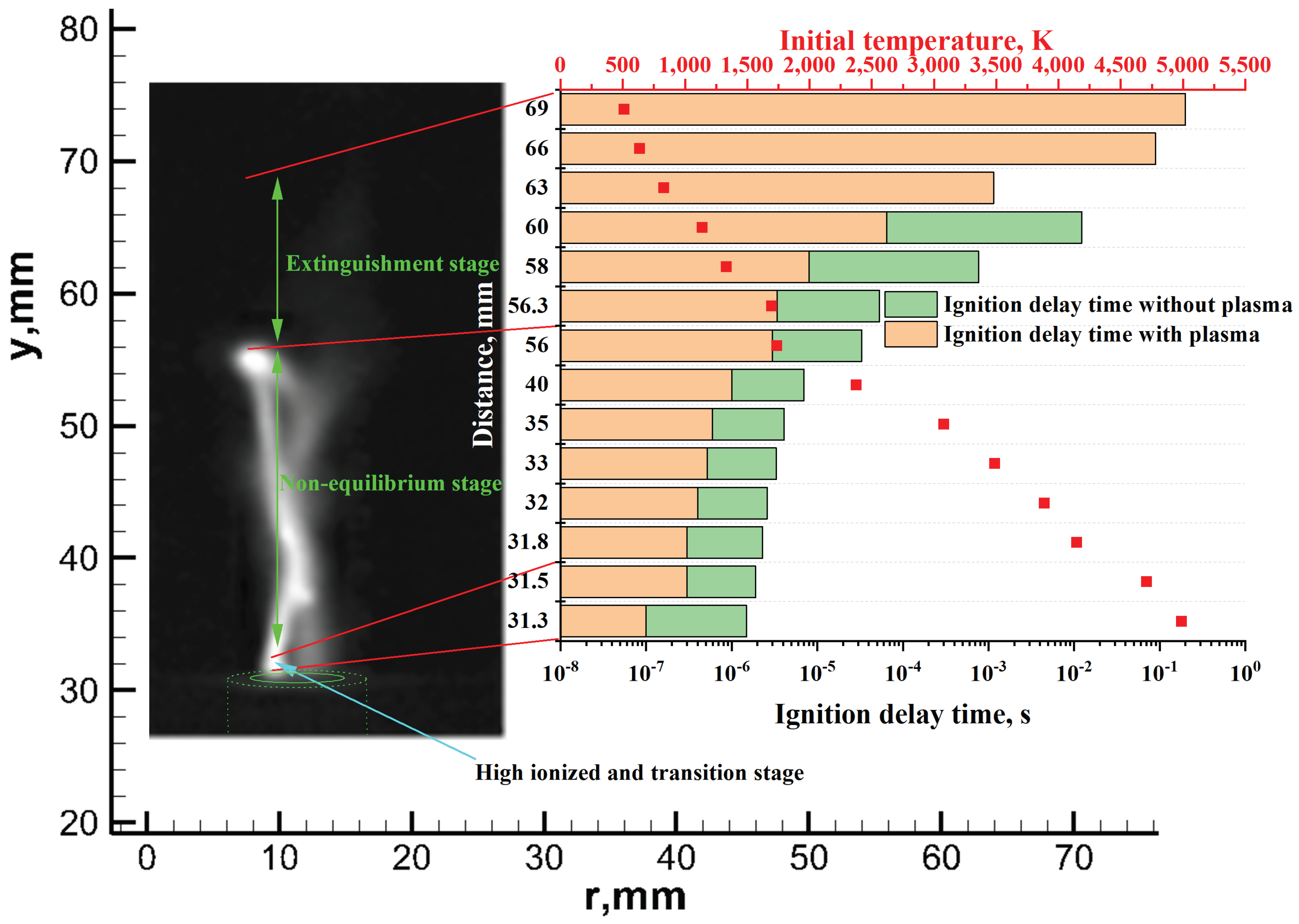
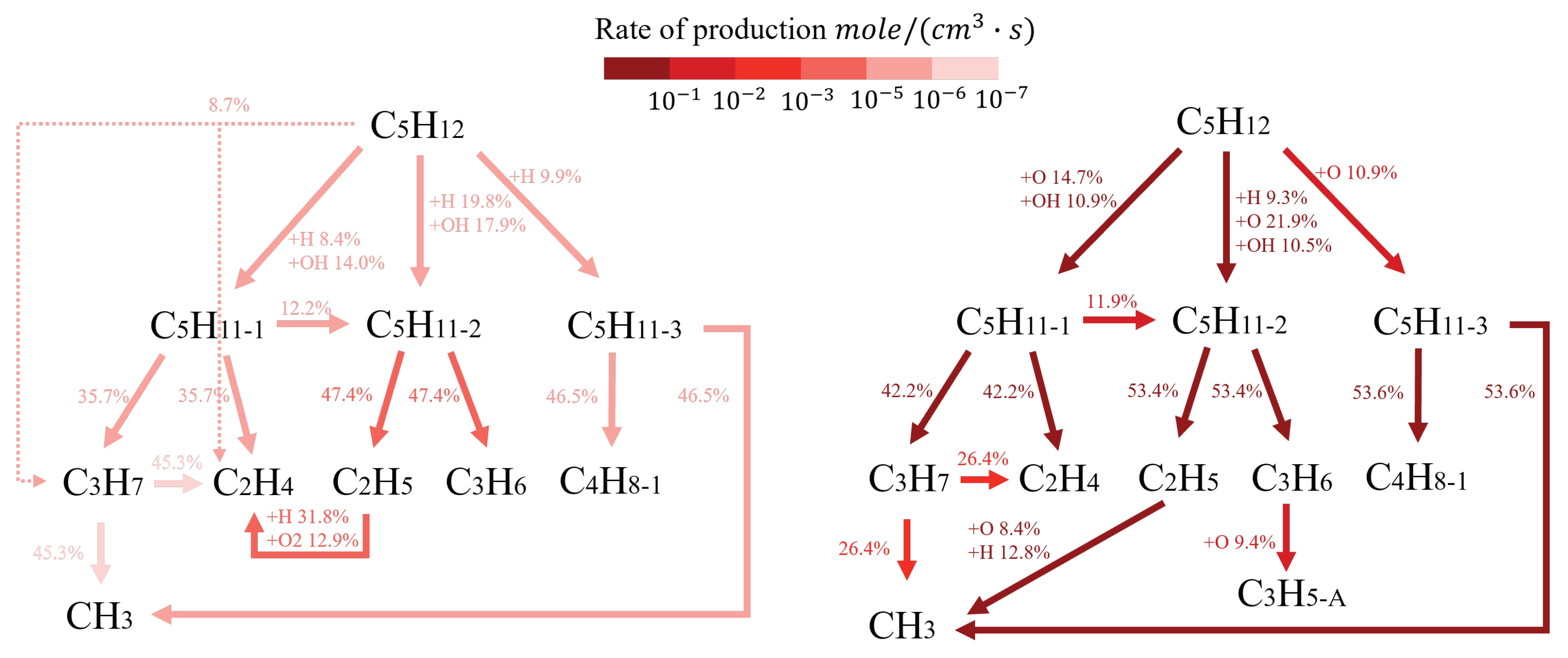

| Boundary | Boundary Condition | Comments |
|---|---|---|
| Wall | With the unity vector normal to the walls | |
| Outlet | With the pressure at the outlet (1 atm) | |
| Inlet | With the pressure at the inlet (1 atm + 45 Torr) |
| Model | Variable | Case I | Case II | Case III |
|---|---|---|---|---|
| The 3D gas dynamics model | Inlet gage pressure | 45 Torr | 60 Torr | 75 Torr |
| Outlet gage pressure | 0 Torr | 0 Torr | 0 Torr | |
| The global chemistry model | Gas temperature | 5700 K | 5100 K | 5220 K |
| Mole fraction of N2 | 0.79 | 0.79 | 0.79 | |
| Mole fraction of O2 | 0.21 | 0.21 | 0.21 | |
| Power | From experiment | |||
| Air velocity | From the 3D gas dynamics model | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhu, Y.; Pan, D.; Cheng, X. Characterization of a Gliding Arc Igniter from an Equilibrium Stage to a Non–Equilibrium Stage Using a Coupled 3D–0D Approach. Processes 2023, 11, 873. https://doi.org/10.3390/pr11030873
Li Z, Zhu Y, Pan D, Cheng X. Characterization of a Gliding Arc Igniter from an Equilibrium Stage to a Non–Equilibrium Stage Using a Coupled 3D–0D Approach. Processes. 2023; 11(3):873. https://doi.org/10.3390/pr11030873
Chicago/Turabian StyleLi, Zhenyang, Yifei Zhu, Di Pan, and Xinyao Cheng. 2023. "Characterization of a Gliding Arc Igniter from an Equilibrium Stage to a Non–Equilibrium Stage Using a Coupled 3D–0D Approach" Processes 11, no. 3: 873. https://doi.org/10.3390/pr11030873





