Comparison of the Engine Performance of Soybean Oil Biodiesel Emulsions Prepared by Phase Inversion Temperature and Mechanical Homogenization Methods
Abstract
:1. Introduction
2. Experimental Details
2.1. Experimental Materials
2.2. Preparation of Soybean Oil Biodiesel
2.3. Analysis of Emulsion Fuel Properties
2.4. Engine Performance Analysis
3. Results and Discussion
3.1. Fuel Properties of the Test Fuels
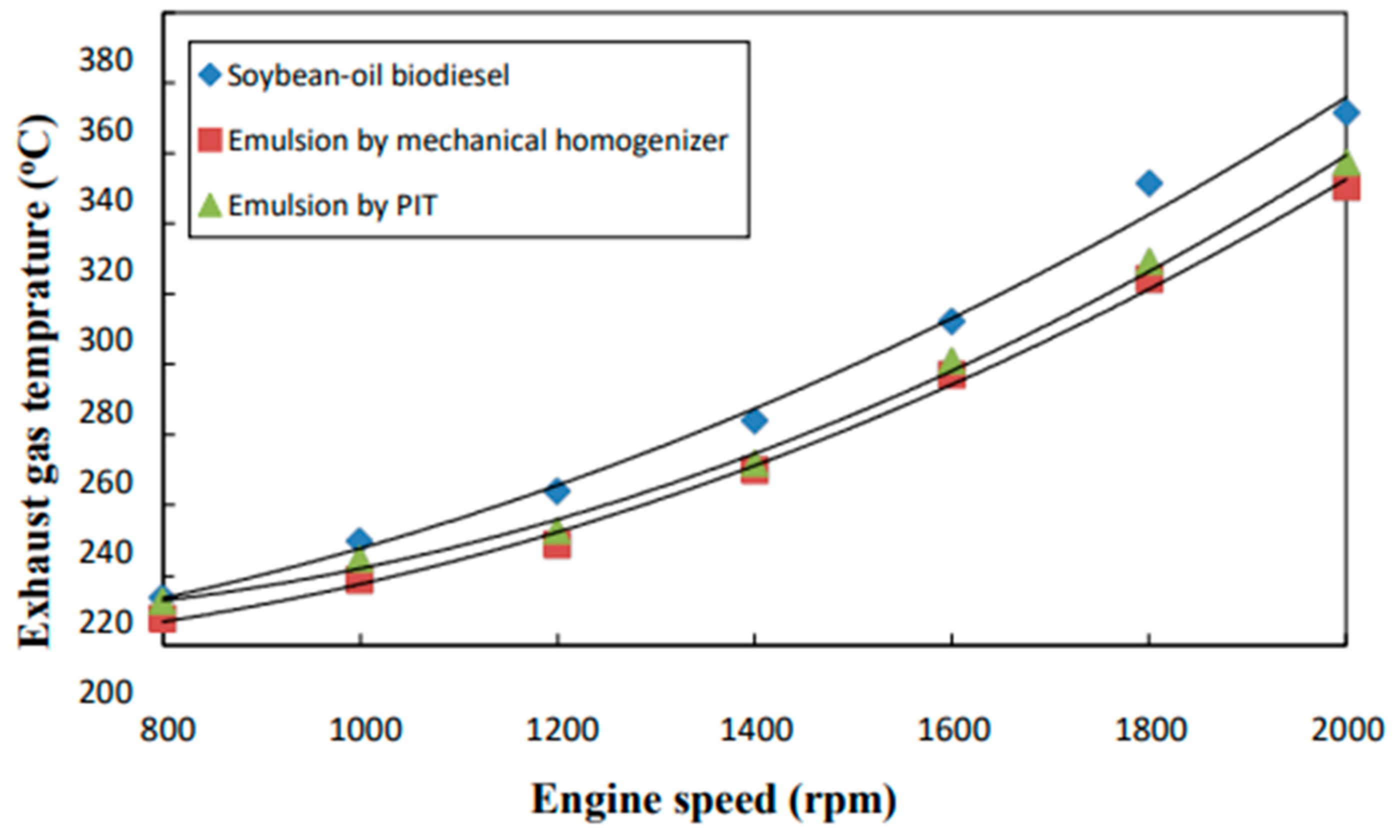
3.2. Exhaust Gas Temperature
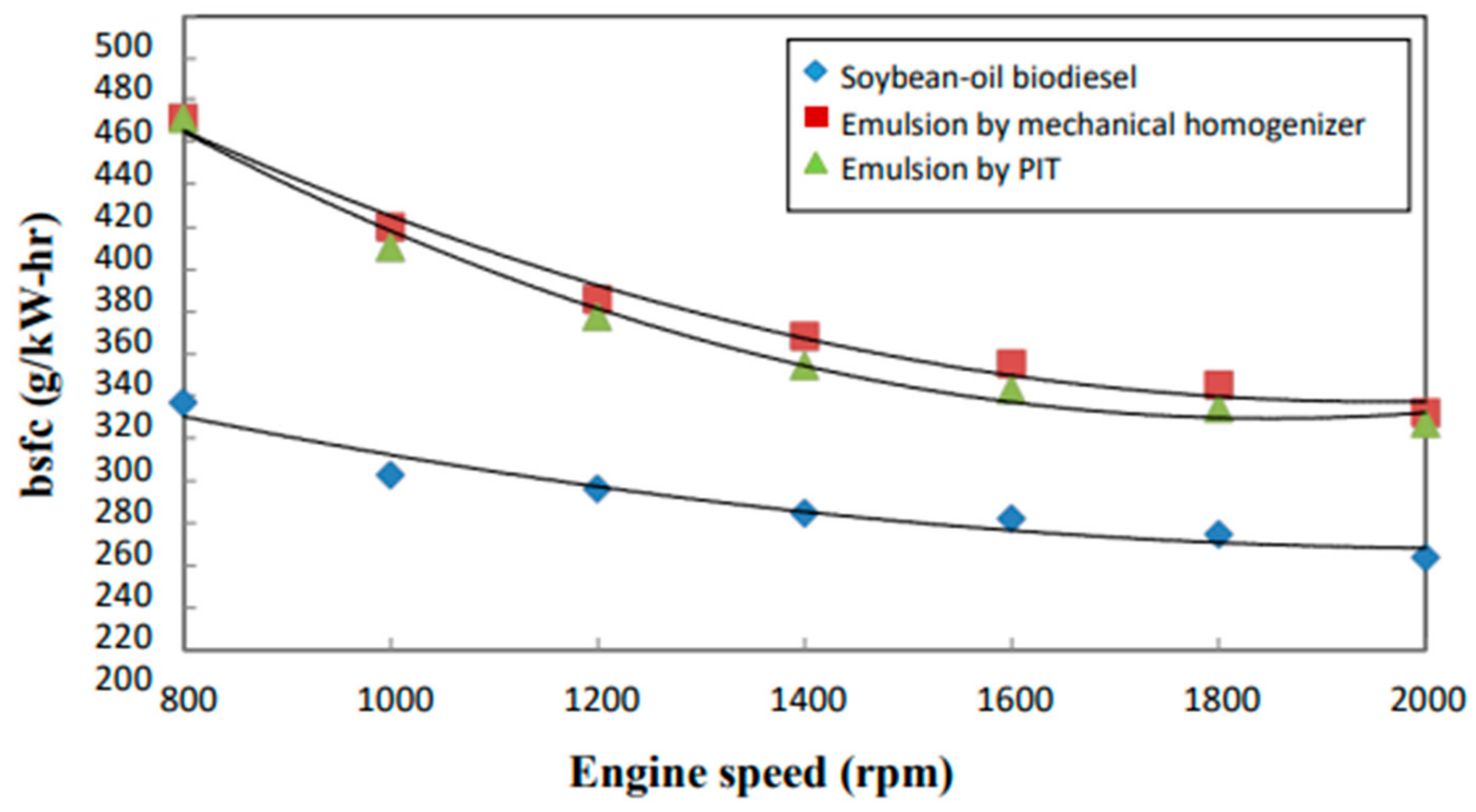
3.3. BSFC
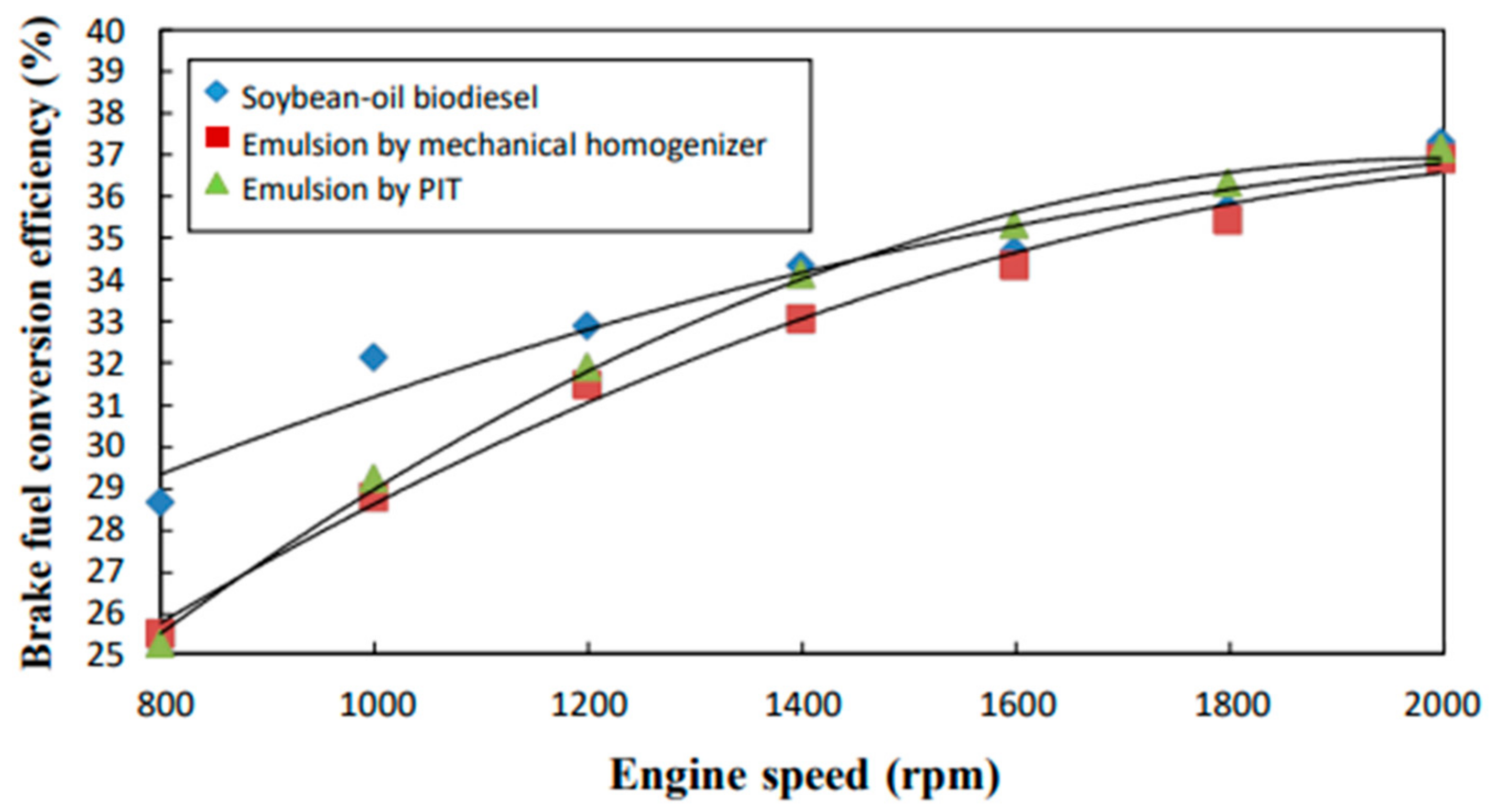
3.4. Brake Fuel Conversion Efficiency
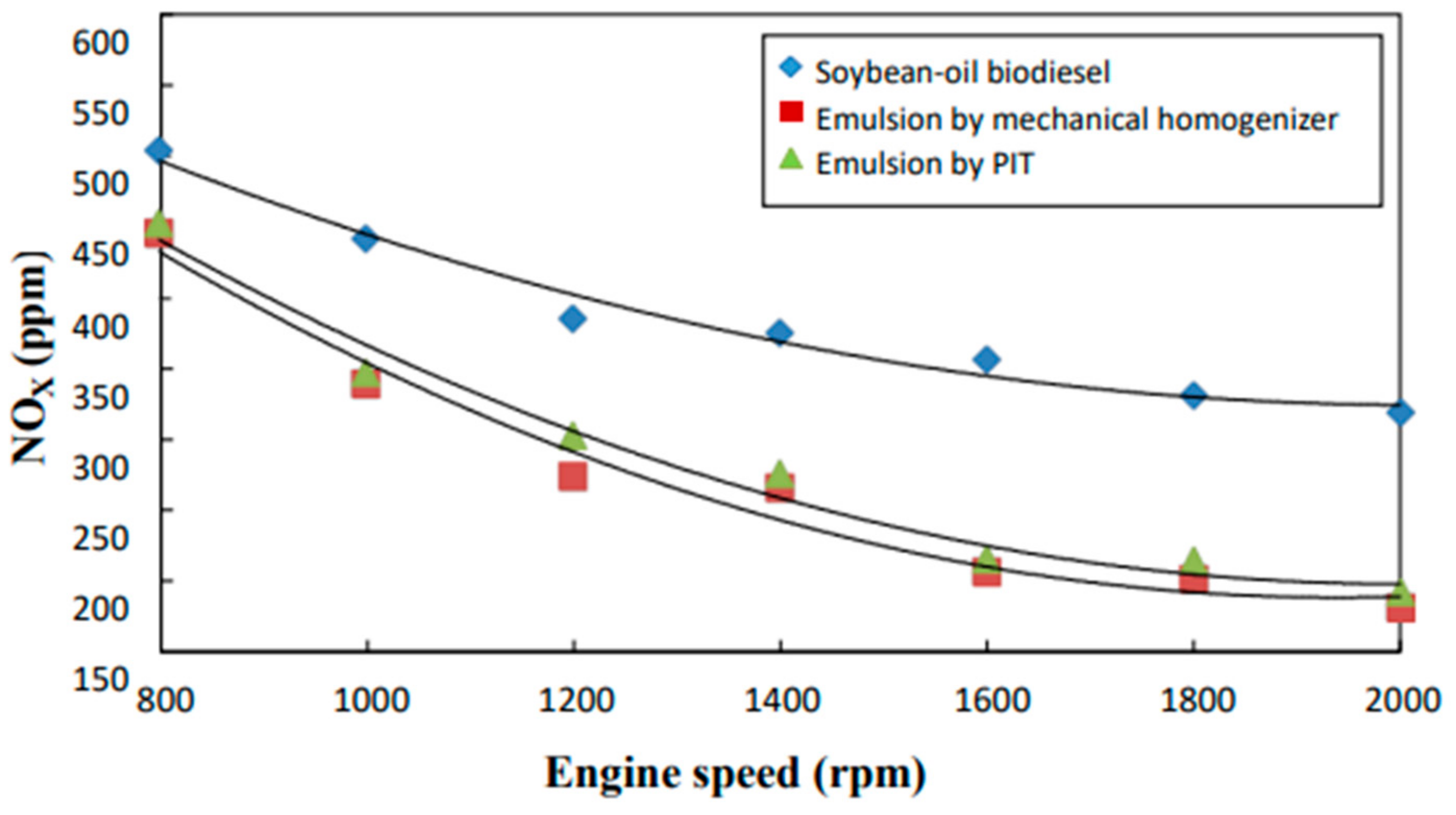
3.5. NOx Emissions
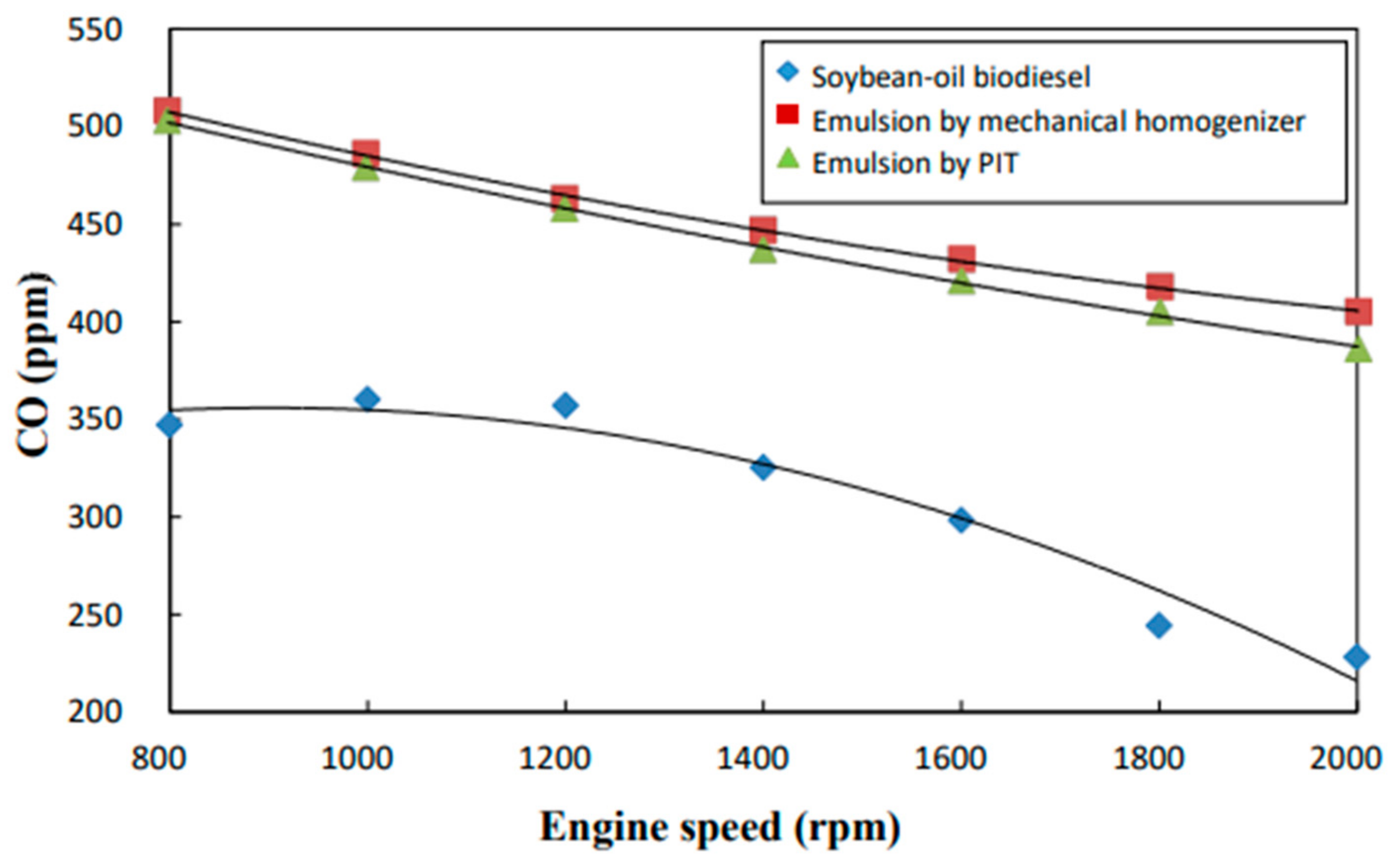
3.6. CO Emissions
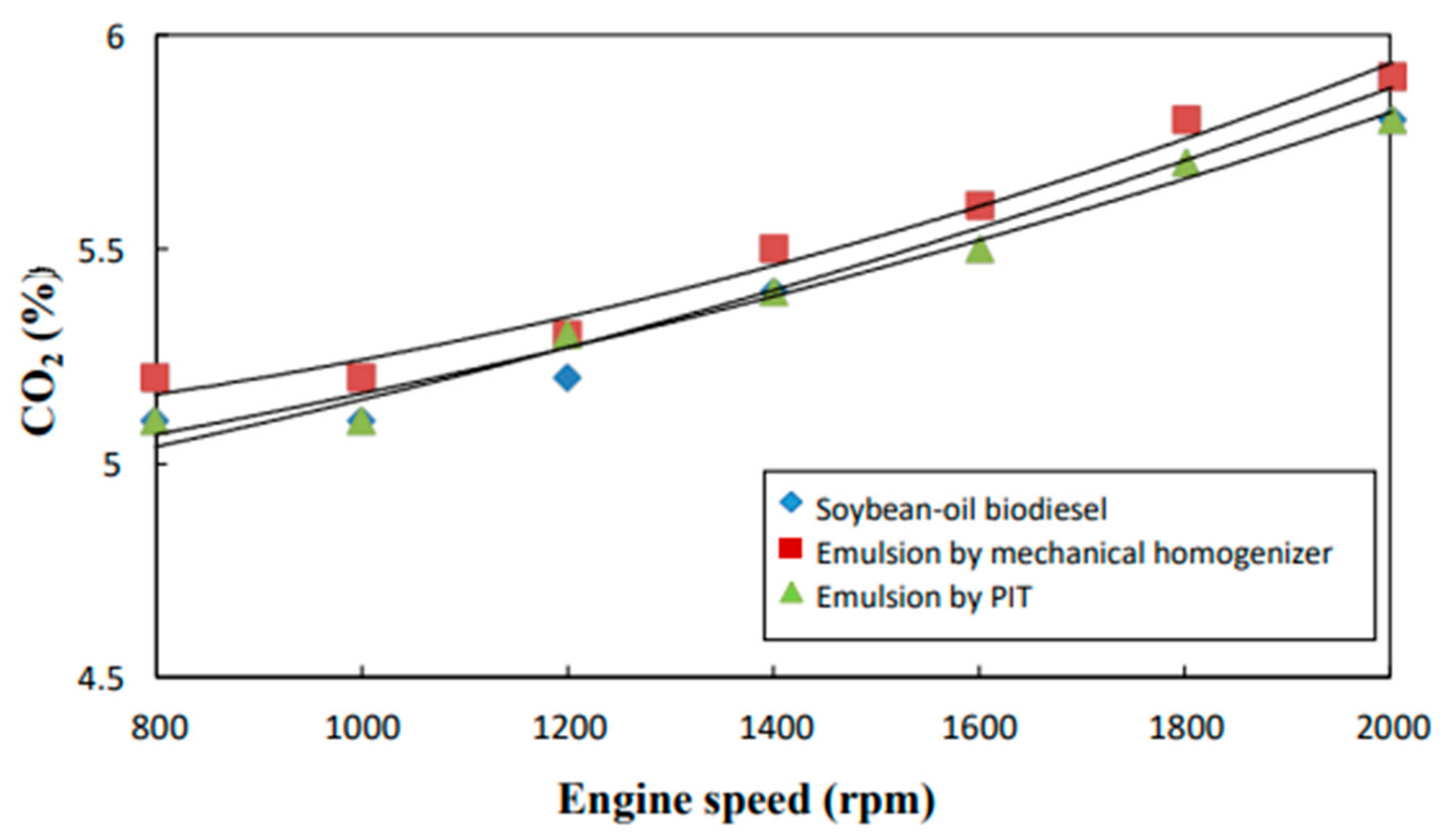
3.7. CO2 Content
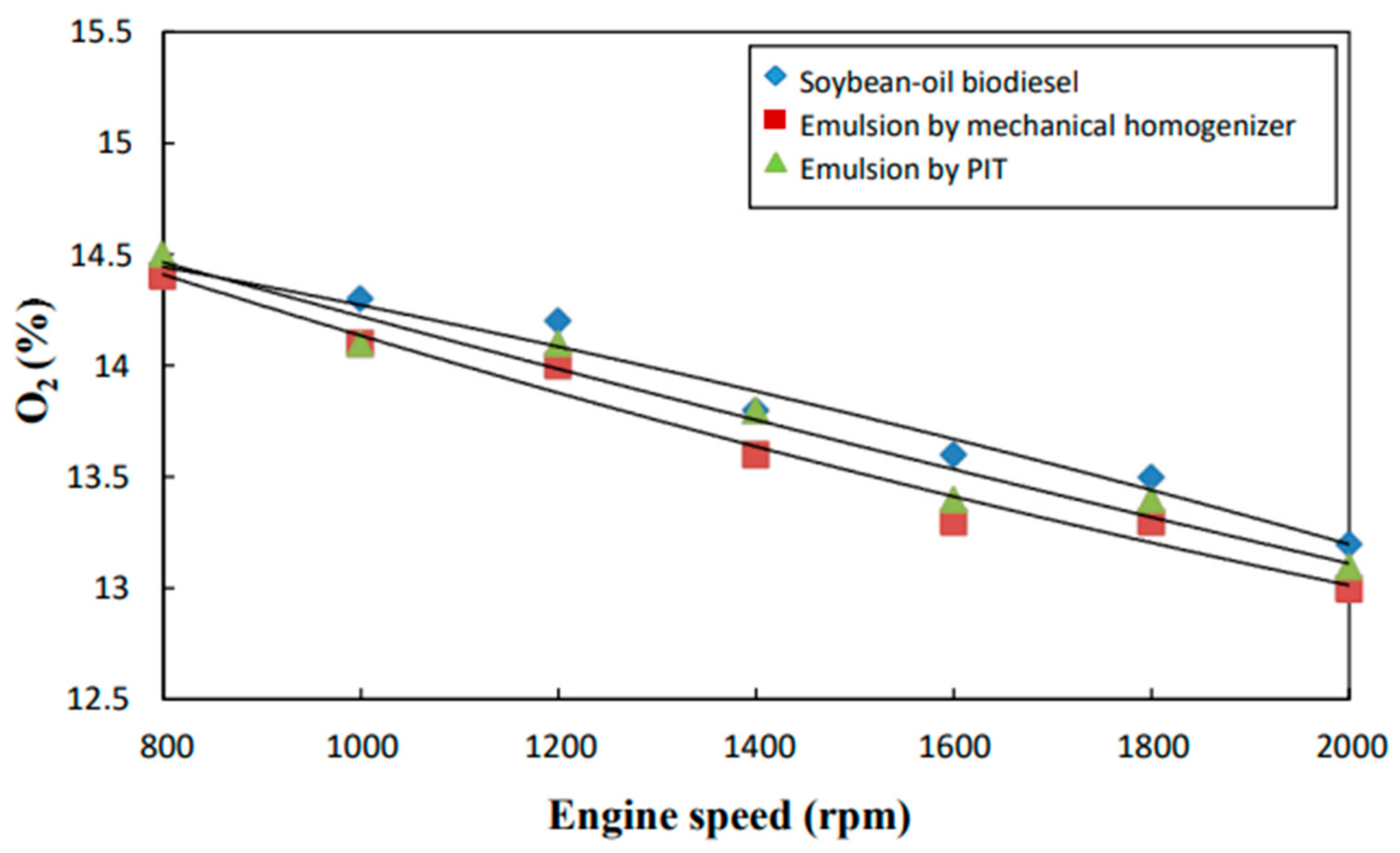
3.8. O2 Content
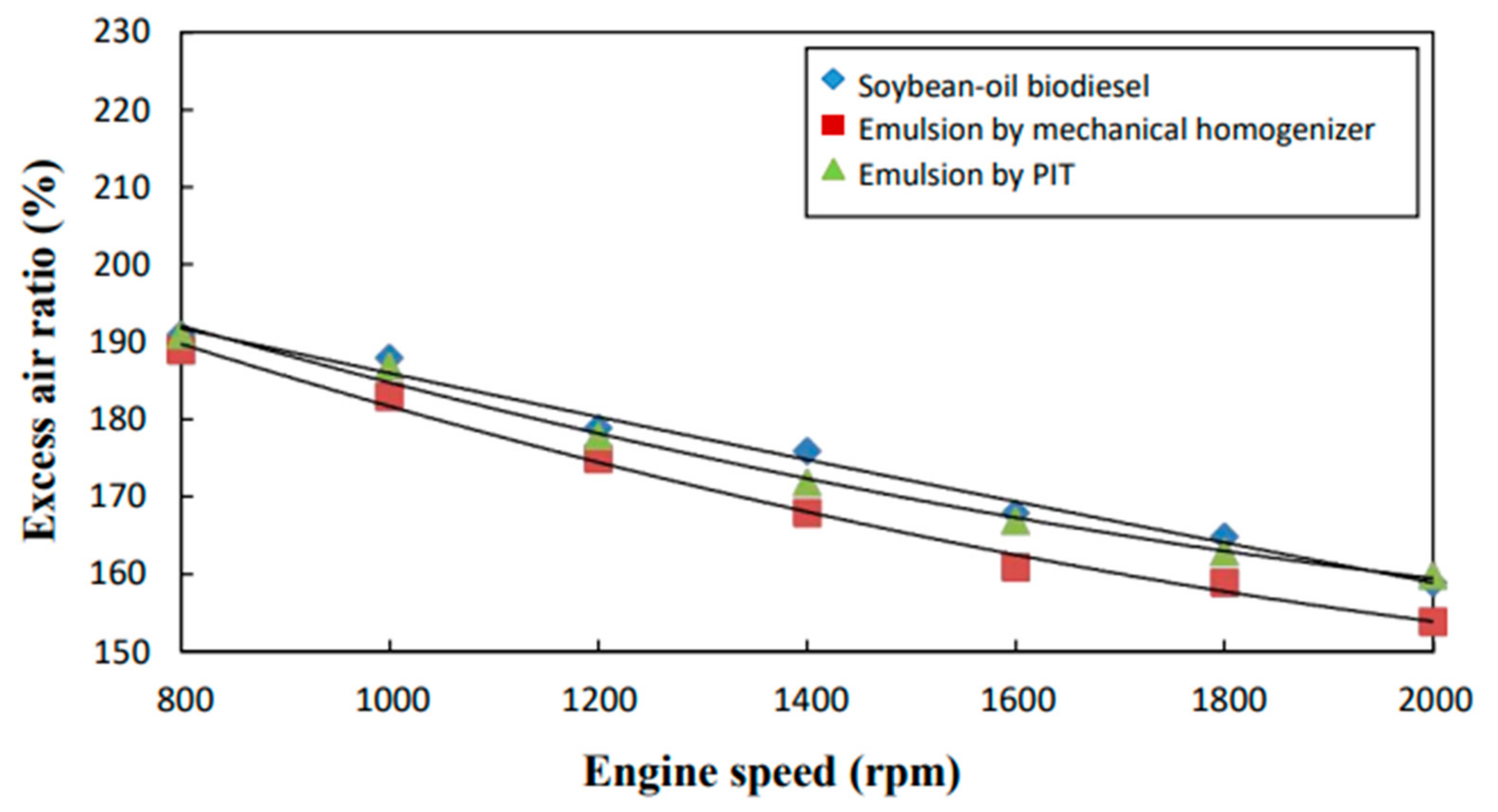
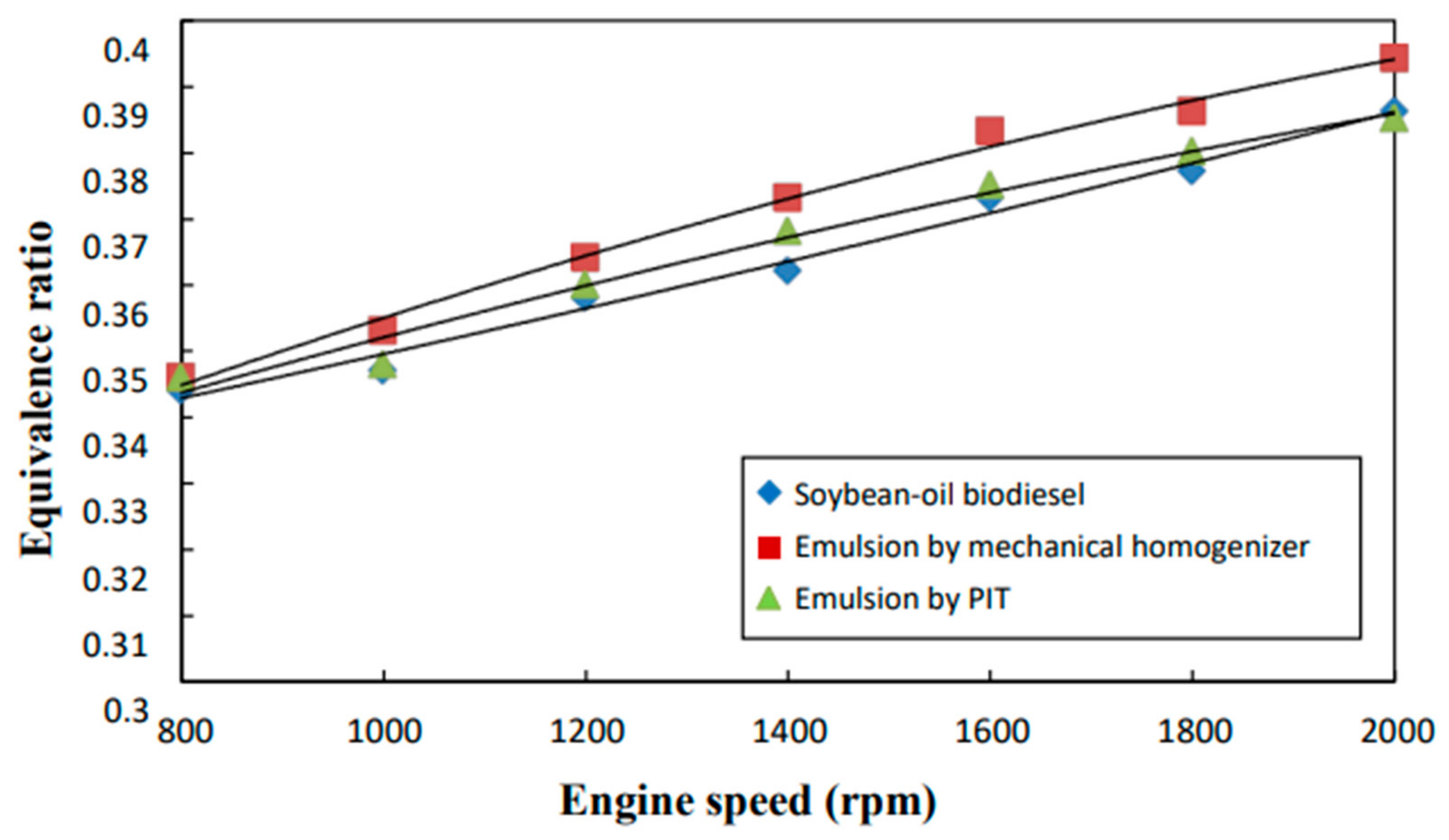
3.9. Excess Air Ratio and Equivalence Ratio
4. Conclusions
- (1)
- In comparison with those emulsions containing 20 wt. % de-ionized water, the neat biodiesel was found to have the highest amount of heat release and the lowest brake-specific fuel consumption (BSFC). The biodiesel emulsion prepared by the PIT method appeared to have a smaller mean droplet size and a lower bsfc value. On the other hand, it had a higher amount of heat release than that of the emulsion prepared by a mechanical homogenizer.
- (2)
- The neat biodiesel was observed to have a lower equivalence ratio and CO2 and CO emissions and a higher excess air ratio, higher O2 and NOx emissions, higher brake fuel conversion efficiency, and a higher exhaust gas temperature than the two biodiesel emulsions.
- (3)
- The emulsion produced by the mechanical homogenizer appeared to have lower brake fuel conversion efficiency, excess air ratio, O2 and NOx emissions, and exhaust gas temperature and higher CO and CO2 emissions and a higher equivalence ratio than the emulsion prepared by the PIT method.
- (4)
- The emulsions prepared by the phase inversion temperature (PIT) method were found to produce superior engine performance and lower emissions than those prepared by other traditional emulsification methods such as the mechanical homogenization one. The PIT method is considered a promising emulsification method for preparing emulsion fuel as an alternative environmentally friendly fuel to petro-derived diesel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamid, M.F.; Teoh, Y.H.; Idroas, M.Y.; Mohamed, M.; Sa’ad, S.; Mat, S.C.; Nguyen, H.T. A review of the emulsification method for alternative fuels used in diesel engines. Energies 2022, 15, 9429. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Mandal, A. Formation, characteristics and oil industry applications of nanoemulsions: A review. J. Pet. Sci. Eng. 2021, 206, 109042. [Google Scholar] [CrossRef]
- Ittoo, B.; Ooi, J.B.; Tran, M.V.; Jaliliantabar, F.; Najafi, G.H.; Swamy, V. Effects of dispersed multiwalled carbon nanotubes on the micro-explosion and combustion characteristics of 2-methylfuran–diesel mixture droplets. Fuel 2022, 316, 123308. [Google Scholar] [CrossRef]
- Lin, C.Y. The influences of promising feedstock variability on advanced biofuel production: A review. J. Mar. Sci. Tech-Taiw. 2021, 29, 714–730. [Google Scholar] [CrossRef]
- Sousa, A.M.; Pereira, M.J.; Matos, H.A. Oil-in-water and water-in-oil emulsions formation and demulsification. J. Pet. Sci. Eng. 2021, 210, 110041. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.; Trifkovic, M.; Bryant, S.L. A facile and economical configuration for continuous generation and separation of oil in water emulsions. Sep. Purif. Technol. 2021, 256, 117849. [Google Scholar] [CrossRef]
- Feng, J.; Esquena, J.; Rodriguez-Abreu, C.; Solans, C. Key features of nano-emulsion formation by the phase inversion temperature method. J. Dispers. Sci. Technol. 2021, 42, 1073–1081. [Google Scholar] [CrossRef]
- Choradiya, B.R.; Patil, S.B. A comprehensive review on nanoemulsion as an ophthalmic drug delivery system. J. Mol. Liq. 2021, 339, 116751. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Azmi, N.A.N.; Elgharbawy, A.A.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for food, pharmaceutical and cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef] [Green Version]
- Islam, F.; Saeed, F.; Afzaal, M.; Hussain, M.; Ikram, A.; Khalid, M.A. Food grade nanoemulsions: Promising delivery systems for functional ingredients. J. Food Sci. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Solanki, K.; Rathi, N.; Patani, P. Microemulsions: Current Trends in Novel Drug Delivery Systems. J. Pharm. Negat. Results 2022, 13, 2327–2334. [Google Scholar]
- Dado, G.P.; Knox, P.W.; Lang, R.M.; Knock, M.M. Nonlinear changes in phase inversion temperature for oil and water emulsions of nonionic surfactant mixtures. J. Surfactants Deterg. 2022, 25, 63–78. [Google Scholar] [CrossRef]
- Liu, R.; Tian, X.; Wang, Z.; Zhang, J.; Lu, P.; Huang, C. Water vapor barrier coating based on nanocellulose crystals stabilized AESO oil-in-water Pickering emulsion. Prog. Org. Coat. 2021, 159, 106479. [Google Scholar] [CrossRef]
- Ettefaghi, E.; Rashidi, A.; Ghobadian, B.; Najafi, G.; Ghasemy, E.; Khoshtaghaza, M.H.; Mazlan, M. Bio-nano emulsion fuel based on graphene quantum dot nanoparticles for reducing energy consumption and pollutants emission. Energy 2021, 218, 119551. [Google Scholar] [CrossRef]
- Masharuddin, S.M.S.; Karim, Z.A.A.; Said, M.A.M.; Amran, N.H.; Ismael, M.A. The evolution of a single droplet water-in-palm oil derived biodiesel emulsion leading to micro-explosion. Alex. Eng. J. 2022, 61, 541–547. [Google Scholar] [CrossRef]
- Lee, J.; Li, C.; Kang, S.; Park, J.; Kim, J.M.; Kim, D.H. Pt nanoparticles encapsulated in CeO2 over-layers synthesized by controlled reductive treatment to suppress CH4 formation in high-temperature water-gas shift reaction. J. Catal. 2021, 395, 246–257. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Varuvel, E.; Karthickeyan, V.; Sonthalia, A.; Kumar, G.; Saravanan, C.G.; Pugazhendhi, A. Effect of hydrogen on compression-ignition (CI) engine fueled with vegetable oil/biodiesel from various feedstocks: A review. Int. J. Hydrogen Energy 2022, 47, 37648–37667. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.W. Fuel characteristics of biodiesel produced from a high-acid oil from soybean soapstock by supercritical-methanol transesterification. Energies 2012, 5, 2370–2380. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y. Effects of the degree of unsaturation of fatty acid esters on engine performance and emission characteristics. Processes 2022, 10, 2161. [Google Scholar] [CrossRef]
- Elumalai, P.V.; Parthasarathy, M.; Hariharan, V.; Jayakar, J.; Mohammed Iqbal, S. Evaluation of water emulsion in biodiesel for engine performance and emission characteristics. J. Therm. Anal. Calorim. 2022, 147, 4285–4301. [Google Scholar] [CrossRef]
- Safaya, M.; Rotliwala, Y.C. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Mater. Today Proc. 2020, 27, 454–459. [Google Scholar] [CrossRef]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, S.; Jiang, M.; Liu, F.; Chen, K.; Zhang, Y. Effects of glycation methods on the interfacial behavior and emulsifying performance of soy protein isolate-gum Arabic conjugates. Int. J. Biol. Macromol. 2023, 233, 123554. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chiu, C.C. Effects of oxidation during long-term storage on the fuel properties of palm oil-based biodiesel. Energy Fuels 2009, 23, 3285–3289. [Google Scholar] [CrossRef]
- Formosa Oilseed Processing Co. Ltd. Test Report for Frying Vegetable Oil. Available online: https://www.fopco.com.tw/index.php (accessed on 20 November 2022).
- Prak, D.L.; Cooke, J.; Dickerson, T.; McDaniel, A.; Cowart, J. Cetane number, derived cetane number, and cetane index: When correlations fail to predict combustibility. Fuel 2021, 289, 119963. [Google Scholar] [CrossRef]
- Cho, K.; Rana, B.S.; Cho, D.W.; Beum, H.T.; Kim, C.H.; Kim, J.N. Catalytic removal of naphthenic acids over Co-Mo/γ-Al2O3 catalyst to reduce total acid number (TAN) of highly acidic crude oil. Appl. Catal. A Gen. 2020, 606, 117835. [Google Scholar] [CrossRef]
- Jamaluddin, N.A.M.; Riayatsyah, T.M.I.; Silitonga, A.S.; Mofijur, M.; Shamsuddin, A.H.; Ong, H.C.; Rahman, S.A. Techno-economic analysis and physicochemical properties of Ceiba pentandra as second-generation biodiesel based on ASTM D6751 and EN 14214. Processes 2019, 7, 636. [Google Scholar] [CrossRef] [Green Version]
- Ong, H.C.; Mofijur, M.; Silitonga, A.S.; Gumilang, D.; Kusumo, F.; Mahlia, T.M.I. Physicochemical properties of biodiesel synthesised from grape seed, philippine tung, kesambi, and palm oils. Energies 2020, 13, 1319. [Google Scholar] [CrossRef] [Green Version]
- Hoang, A.T.; Tran, Q.V.; Al-Tawaha, A.R.M.S.; Nguyen, X.P. Comparative analysis on performance and emission characteristics of an in-Vietnam popular 4-stroke motorcycle engine running on biogasoline and mineral gasoline. Renew. Energy Focus 2019, 28, 47–55. [Google Scholar] [CrossRef]
- Tran, V.D.; Le, A.T.; Hoang, A.T. An experimental study on the performance characteristics of a diesel engine fueled with ULSD-biodiesel blends. Int. J. Renew. Energy Dev. 2021, 10, 183. [Google Scholar] [CrossRef]
- Avulapati, M.M.; Megaritis, T.; Xia, J.; Ganippa, L. Experimental understanding on the dynamics of micro-explosion and puffing in ternary emulsion droplets. Fuel 2019, 239, 1284–1292. [Google Scholar] [CrossRef]
- Moussa, O.; Tarlet, D.; Massoli, P.; Bellettre, J. Parametric study of the micro-explosion occurrence of W/O emulsions. Int. J. Therm Sci. 2018, 133, 90–97. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, D.; Zhang, C. An overview of electricity powered vehicles: Lithium-ion battery energy storage density and energy conversion efficiency. Renew. Energy 2020, 162, 1629–1648. [Google Scholar] [CrossRef]
- Maawa, W.N.; Mamat, R.; Najafi, G.; De Goey, L.P.H. Performance, combustion, and emission characteristics of a CI engine fueled with emulsified diesel-biodiesel blends at different water contents. Fuel 2020, 267, 117265. [Google Scholar] [CrossRef]
- Mahabadipour, H.; Srinivasan, K.K.; Krishnan, S.R. An exergy analysis methodology for internal combustion engines using a multi-zone simulation of dual fuel low temperature combustion. Appl. Energy 2019, 256, 113952. [Google Scholar] [CrossRef]
- Fattah, I.R.; Masjuki, H.H.; Kalam, M.A.; Mofijur, M.; Abedin, M.J. Effect of antioxidant on the performance and emission characteristics of a diesel engine fueled with palm biodiesel blends. Energy Convers. Manag. 2014, 79, 265–272. [Google Scholar] [CrossRef]
- Duda, K.; Wierzbicki, S.; Mikulski, M.; Konieczny, Ł.; Łazarz, B.; Letuń-Łątka, M. Emissions from a medium-duty crdi engine fuelled with diesel: Biodiesel blends. Transp. Probl. 2021, 16, 39–49. [Google Scholar] [CrossRef]
- Xia, C.; Brindhadevi, K.; Elfasakhany, A.; Alsehli, M.; Tola, S. Performance, combustion and emission analysis of castor oil biodiesel blends enriched with nanoadditives and hydrogen fuel using CI engine. Fuel 2021, 306, 121541. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Zha, X.; Gao, G.; Li, X.; Wu, F.; Zhang, L. Internal association between combustion behavior and NOx emissions of pulverized coal MILD-oxy combustion affected by adding H2O. Energy 2023, 263, 125878. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ghobadian, B.; Najafi, G.; Hoseini, S.S.; Mofijur, M.; Mazlan, M. Impact of water–biodiesel–diesel nano-emulsion fuel on performance parameters and diesel engine emission. Fuel 2020, 280, 118576. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, F.; Duan, S.; Shao, B.; Li, T.; Tang, H.; Zhang, T. Remarkable active-site dependent H2O promoting effect in CO oxidation. Nat. Commun. 2019, 10, 3824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elumalai, P.V.; Krishna Moorthy, R.; Parthasarathy, M.; Samuel, O.D.; Owamah, H.I.; Saleel, C.A.; Afzal, A. Artificial neural networks model for predicting the behavior of different injection pressure characteristics powered by blend of biofuel-nano emulsion. Energy Sci. Eng. 2022, 10, 2367–2396. [Google Scholar] [CrossRef]
- Ghaffarzadeh, S.; Toosi, A.N.; Hosseini, V. An experimental study on low temperature combustion in a light duty engine fueled with diesel/CNG and biodiesel/CNG. Fuel 2020, 262, 116495. [Google Scholar] [CrossRef]
- Estrada, L.; Moreno, E.; Gonzalez-Quiroga, A.; Bula, A.; Duarte-Forero, J. Experimental assessment of performance and emissions for hydrogen-diesel dual fuel operation in a low displacement compression ignition engine. Heliyon 2022, 8, e09285. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Yaseen, A.A.; Atiyah, I.K. Experimental study for the effect of damaged vegetable oil biofuels on diesel engine performance and exhaust emission. J. Eng. Sci. Technol. 2021, 16, 1110–1121. [Google Scholar]
- Valera-Medina, A.; Gutesa, M.; Xiao, H.; Pugh, D.; Giles, A.; Goktepe, B.; Bowen, P. Premixed ammonia/hydrogen swirl combustion under rich fuel conditions for gas turbines operation. Int. J. Hydrogen Energy 2019, 44, 8615–8626. [Google Scholar] [CrossRef]
- Saha, D.; Sinha, A.; Roy, B.; Mishra, L. Effects of water-diesel emulsification on performance and emission characteristics of CI engine: A review. In Innovations in Energy, Power and Thermal Engineering 2022; Springer: Singapore, 2022; pp. 95–107. [Google Scholar]
| Fuel Property | Soybean Oil Biodiesel | Emulsions by PIT | Emulsions by a Mechanical Homogenizer |
|---|---|---|---|
| FAME (wt. %) | 98.7 | ─ | ─ |
| Amount of heat release (MJ/kg) | 39.62 | 31.56 | 31.28 |
| Kinematic viscosity (at 40 °C) | 4.71 | 49.68 | 52.26 |
| Specific gravity | 0.88 | 0.930 | 0.930 |
| Mean droplet size (nm) | ─ | 1378 | 1697 |
| Acid value (mg KOH/g) | 0.26 | ─ | ─ |
| Moisture content (wt. %) | 0.042 | ─ | ─ |
| Cetane index | 47.85 | ─ | ─ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Lin, K.-H. Comparison of the Engine Performance of Soybean Oil Biodiesel Emulsions Prepared by Phase Inversion Temperature and Mechanical Homogenization Methods. Processes 2023, 11, 907. https://doi.org/10.3390/pr11030907
Lin C-Y, Lin K-H. Comparison of the Engine Performance of Soybean Oil Biodiesel Emulsions Prepared by Phase Inversion Temperature and Mechanical Homogenization Methods. Processes. 2023; 11(3):907. https://doi.org/10.3390/pr11030907
Chicago/Turabian StyleLin, Cherng-Yuan, and Keng-Hung Lin. 2023. "Comparison of the Engine Performance of Soybean Oil Biodiesel Emulsions Prepared by Phase Inversion Temperature and Mechanical Homogenization Methods" Processes 11, no. 3: 907. https://doi.org/10.3390/pr11030907





