Processing Strategies for Extraction and Concentration of Bitter Acids and Polyphenols from Brewing By-Products: A Comprehensive Review
Abstract
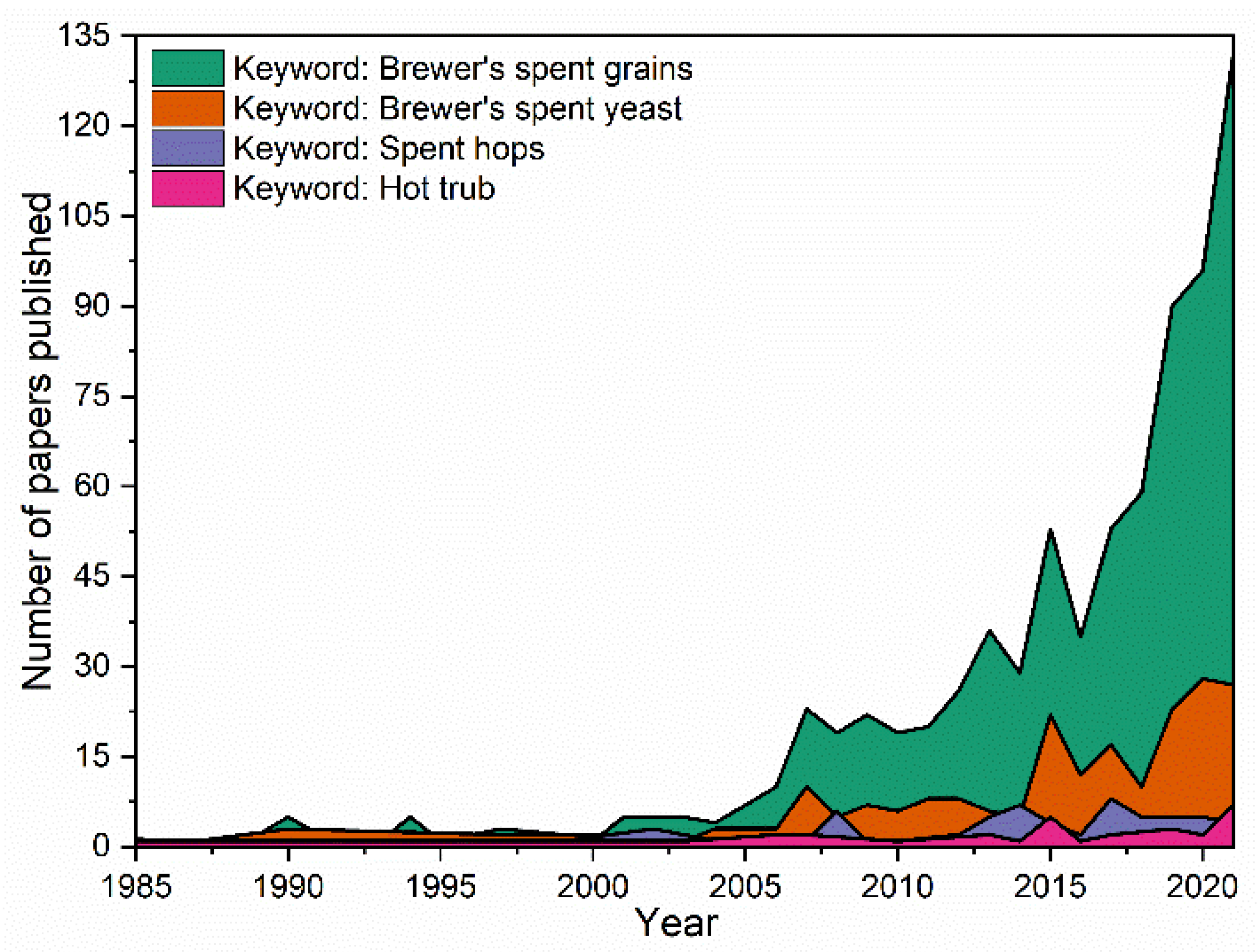
:1. Introduction
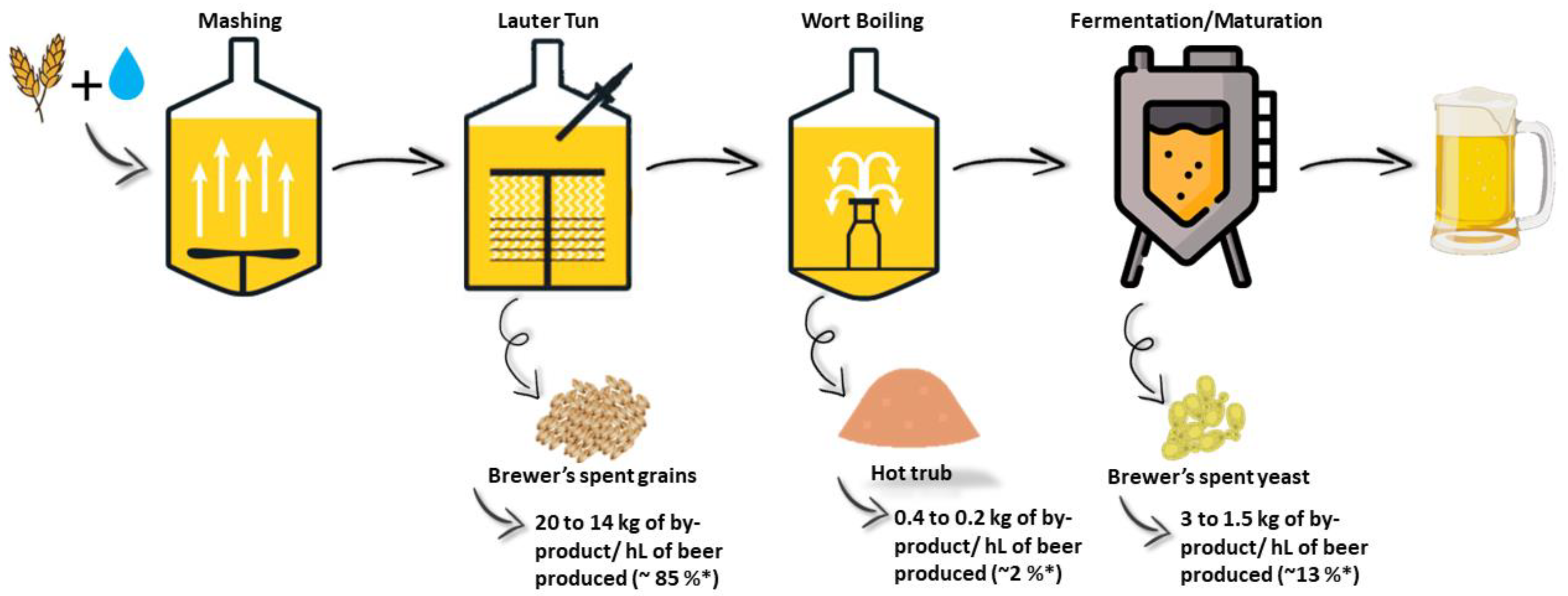
2. General Aspects of Brewing By-Products Generation
2.1. Brewer’s Spent Grains as a Source of New Products
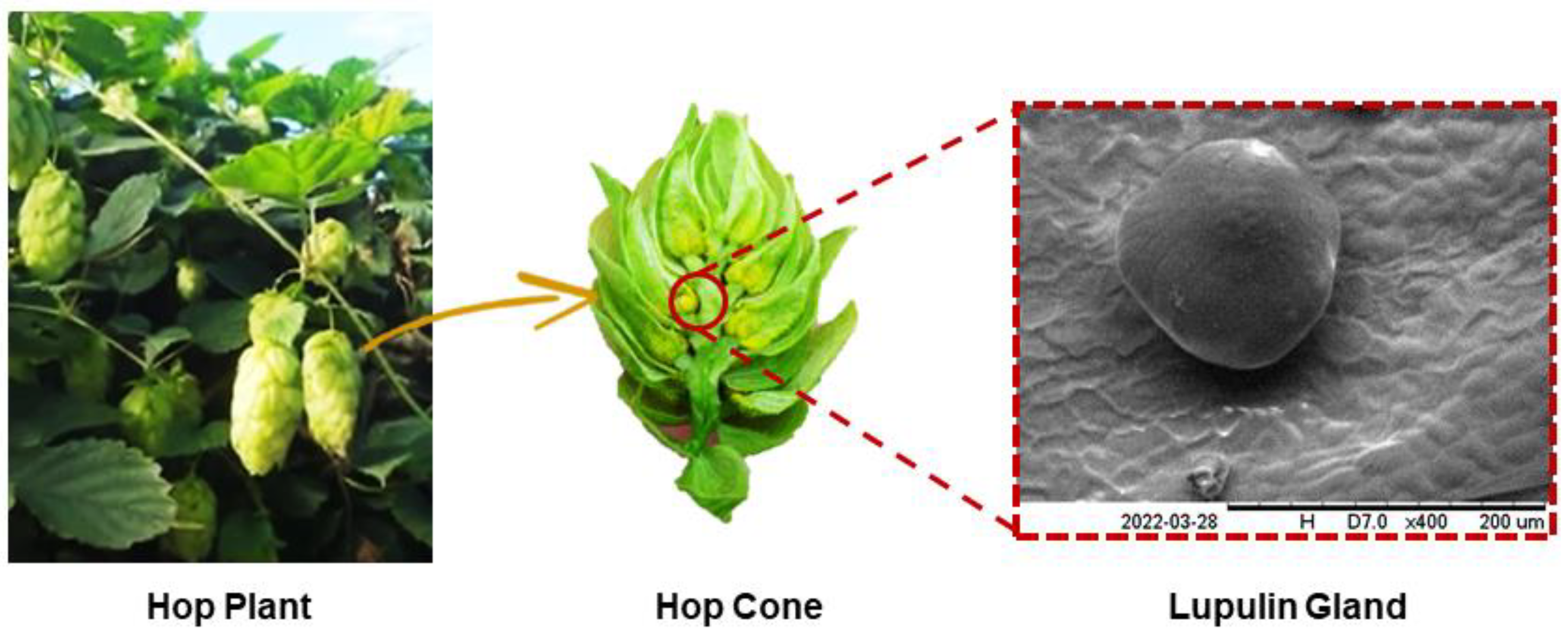
2.2. Hot Trub and Spent Hops as a Source of New Products
2.3. Brewer’s Spent Yeast as a Source of New Products
3. Characteristics of Bitter Acids and Xanthohumol
3.1. Bitter Acids
3.2. Polyphenols
4. Trends for the Extracting Polyphenols and Bitter Acids from Brewing By-Products
4.1. High-Intensity Ultrasound Technology
4.2. Microwave Technology
4.3. High-Pressure Fluid Technologies
4.4. Ohmic Heating Technology
4.5. Pulsed Electric Field Technology
4.6. Deep Eutectic Solvents
4.7. Comparison between Emerging Technologies and Alternative Solvents
5. Methods of Isolation and Concentration of Bitter Acids and Polyphenols Obtained by Beer By-Products
5.1. Adsorption Process
5.2. Membrane Technologies
6. Industrial Aspects: Value of the Extracted Compounds, Process Scale-Up and Economic Evaluation and Patents
7. Perspectives for Further Studies and Final Considerations
Author Contributions
Funding
Conflicts of Interest
References
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-Industrial by-Products: Valuable Sources of Bioactive Compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- FAO The State of Food and Agriculture 2021. Making Agrifood Systems More Resilient to Shocks and Stresses; FAO: Rome, Italy, 2021; ISBN 978-92-5-134329-6. [Google Scholar]
- Conway, J. Worldwide Beer Production. 2021. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 31 January 2023).
- Mathias, T.R.D.S.; Mello, P.P.M.d.M.; Sérvulo, E.F.C. Solid Wastes in Brewing Process: A Review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-Products in the Malting and Brewing Industries—Re-Usage Possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Mathias, T.R.d.S.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and Determination of Brewer’s Solid Wastes Composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Wolny-Koładka, K.; Zdaniewicz, M.; Jarosz, R. Biological Activity of Composts Obtained from Hop Waste Generated during the Brewing. Biomass Convers. Biorefinery 2022, 12, 1271–1279. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent Brewer’s Yeast as a Source of High Added Value Molecules: A Systematic Review on Its Characteristics, Processing and Potential Applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Vicente de Andrade Silva, G.; Demaman Arend, G.; Antonio Ferreira Zielinski, A.; Di Luccio, M.; Ambrosi, A. Xanthohumol Properties and Strategies for Extraction from Hops and Brewery Residues: A Review. Food Chem. 2023, 404, 134629. [Google Scholar] [CrossRef]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of Brewery Wastes in Food Industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef]
- Rodriguez, L.M.; Camina, J.L.; Borroni, V.; Pérez, E.E. Protein Recovery from Brewery Solid Wastes. Food Chem. 2023, 407, 134810. [Google Scholar] [CrossRef]
- Türker, M.; Selimoğlu, S.M.; Taşpınar-Demir, H. Waste (Water) to Feed Protein: Effluent Characteristics, Protein Recovery, and Single-Cell Protein Production from Food Industry Waste Streams. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–244. [Google Scholar]
- Olivares-Galván, S.; Marina, M.L.; García, M.C. Extraction of Valuable Compounds from Brewing Residues: Malt Rootlets, Spent Hops, and Spent Yeast. Trends Food Sci. Technol. 2022, 127, 181–197. [Google Scholar] [CrossRef]
- Lewis, M.J.; Bamforth, C.W. Essays in Brewing Science; Springer Science+Business: New York, NY, USA, 2006; ISBN 0125610017 (v. 1)r0125610033 (v. 3). [Google Scholar]
- Jaskula-Goiris, B.; de Cooman, L.; Goiris, K. Humulus Lupulus: Hop Alpha-Acids Isomerization—A Review. BrewingScience 2018, 71, 85–95. [Google Scholar] [CrossRef]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing Science and Practice; Woodhead Publishing: Sawston, UK, 2004; ISBN 0849325471. [Google Scholar]
- Kunze, W. Technology Brewing and Malting, 6th ed.; Hendel, O., Ed.; VLB: Berlin, Germany, 2019. [Google Scholar]
- Ervin, V.; Alli, I.; Smith, J.P.; Li, Z. Extraction and Precipitation of Proteins From Brewer’s Spent Grain. Can. Inst. Food Sci. Technol. J. 1989, 22, 216–221. [Google Scholar] [CrossRef]
- Kabel, M.A.; Schols, H.A.; Voragen, A.G.J. Complex Xylo-Oligosaccharides Identified from Hydrothermally Treated Eucalyptus Wood and Brewery’s Spent Grain. Carbohydr. Polym. 2002, 50, 191–200. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Roberto, I.C. Lignin Recovery from Brewer’s Spent Grain Black Liquor. Carbohydr. Polym. 2007, 70, 218–223. [Google Scholar] [CrossRef]
- Torres-Mayanga, P.C.; Azambuja, S.P.H.; Tyufekchiev, M.; Tompsett, G.A.; Timko, M.T.; Goldbeck, R.; Rostagno, M.A.; Forster-Carneiro, T. Subcritical Water Hydrolysis of Brewer’s Spent Grains: Selective Production of Hemicellulosic Sugars (C-5 Sugars). J. Supercrit. Fluids 2019, 145, 19–30. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Viganó, J.; Castro, L.E.N.; Maciel-Silva, F.W.; Rostagno, M.A.; Mussatto, S.I.; Forster-Carneiro, T. Recovery of Sugars and Amino Acids from Brewers’ Spent Grains Using Subcritical Water Hydrolysis in a Single and Two Sequential Semi-Continuous Flow-through Reactors. Food Res. Int. 2022, 157, 111470. [Google Scholar] [CrossRef]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.J.B.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.D.; Freire, C.S.R. Antioxidant and Antimicrobial Films Based on Brewers Spent Grain Arabinoxylans, Nanocellulose and Feruloylated Compounds for Active Packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and P-Coumaric Acids Extraction by Alkaline Hydrolysis of Brewer’s Spent Grain. Ind. Crops Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Ainsworth, P.; Ibanoǧlu, Ş.; Plunkett, A.; Ibanoǧlu, E.; Stojceska, V. Effect of Brewers Spent Grain Addition and Screw Speed on the Selected Physical and Nutritional Properties of an Extruded Snack. J. Food Eng. 2007, 81, 702–709. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Crofton, E.; Scannell, A.G.M.; Hannon, J.A.; Kilcawley, K.N.; Gallagher, E. Sensory Properties and Aromatic Composition of Baked Snacks Containing Brewer’s Spent Grain. J. Cereal Sci. 2013, 57, 384–390. [Google Scholar] [CrossRef]
- Vieira, E.; Rocha, M.A.M.; Coelho, E.; Pinho, O.; Saraiva, J.A.; Ferreira, I.M.P.L.V.O.; Coimbra, M.A. Valuation of Brewer’s Spent Grain Using a Fully Recyclable Integrated Process for Extraction of Proteins and Arabinoxylans. Ind. Crops Prod. 2014, 52, 136–143. [Google Scholar] [CrossRef]
- Laine, C.; Kemppainen, K.; Kuutti, L.; Varhimo, A.; Asikainen, S.; Grönroos, A.; Määttänen, M.; Buchert, J.; Harlin, A. Extraction of Xylan from Wood Pulp and Brewer’s Spent Grain. Ind. Crops Prod. 2015, 70, 231–237. [Google Scholar] [CrossRef]
- He, Y.; Kuhn, D.D.; Ogejo, J.A.; O’Keefe, S.F.; Fraguas, C.F.; Wiersema, B.D.; Jin, Q.; Yu, D.; Huang, H. Wet Fractionation Process to Produce High Protein and High Fiber Products from Brewer’s Spent Grain. Food Bioprod. Process. 2019, 117, 266–274. [Google Scholar] [CrossRef]
- Sibhatu, H.K.; Anuradha Jabasingh, S.; Yimam, A.; Ahmed, S. Ferulic Acid Production from Brewery Spent Grains, an Agro-Industrial Waste. LWT 2021, 135, 110009. [Google Scholar] [CrossRef]
- He, Y.; Kuhn, D.D.; O’Keefe, S.F.; Ogejo, J.A.; Fraguas, C.F.; Wang, H.; Huang, H. Protein Production from Brewer’s Spent Grain via Wet Fractionation: Process Optimization and Techno-Economic Analysis. Food Bioprod. Process. 2021, 126, 234–244. [Google Scholar] [CrossRef]
- Estévez, A.; Padrell, L.; Iñarra, B.; Orive, M.; Martin, D.S. Brewery By-Products (Yeast and Spent Grain) as Protein Sources in Gilthead Seabream (Sparus Aurata) Feeds. Aquaculture 2021, 543, 736921. [Google Scholar] [CrossRef]
- de Campos, K.C.G.; de Farias, A.K.N.; Becker, G.; de Britto, G.C.S.; Soares, W.P.; Nascimento, E.; Scabora, M.H.; Rodrigues, E.C.; Picanço, N.F.M.; de Faria, R.A.P.G. Quality Measurements of Cuiabana-Type Pork Sausages Added with Brewing by-Product Flours. Meat Sci. 2021, 179, 108441. [Google Scholar] [CrossRef]
- Talens, C.; Llorente, R.; Simó-Boyle, L.; Odriozola-Serrano, I.; Tueros, I.; Ibargüen, M. Hybrid Sausages: Modelling the Effect of Partial Meat Replacement with Broccoli, Upcycled Brewer’s Spent Grain and Insect Flours. Foods 2022, 11, 3396. [Google Scholar] [CrossRef]
- Cuomo, F.; Trivisonno, M.C.; Iacovino, S.; Messia, M.C.; Marconi, E. Sustainable Re-Use of Brewer’s Spent Grain for the Production of High Protein and Fibre Pasta. Foods 2022, 11, 642. [Google Scholar] [CrossRef]
- Öztürk, S.; Özboy, Ö.; Cavidoǧlu, I.; Köksel, H. Effects of Brewer’s Spent Grain on the Quality and Dietary Fibre Content of Cookies. J. Inst. Brew. 2002, 108, 23–27. [Google Scholar] [CrossRef]
- Wallen, S.E.; Marshall, H.F. Protein Quality Evaluation of Spent Hops. J. Agric. Food Chem. 1979, 27, 635–636. [Google Scholar] [CrossRef]
- Rayment, I. Encyclopedia of Physical Science and Technology, 3rd ed.; Meyes, R.A., Ed.; Elsevier Science Ltd.: Tarzana, CA, USA, 2001; ISBN 978-0-12-227410-7. [Google Scholar]
- Iimure, T.; Nankaku, N.; Kihara, M.; Yamada, S.; Sato, K. Proteome Analysis of the Wort Boiling Process. Food Res. Int. 2012, 45, 262–271. [Google Scholar] [CrossRef]
- Chadwick, L.R.; Nikolic, D.; Burdette, J.E.; Overk, C.R.; Bolton, J.L.; van Breemen, R.B.; Fröhlich, R.; Fong, H.H.S.; Farnsworth, N.R.; Pauli, G.F. Estrogens and Congeners from Spent Hops (Humulus lupulus). J. Nat. Prod. 2004, 67, 2024–2032. [Google Scholar] [CrossRef]
- Anioł, M.; Huszcza, E.; Bartmańska, A.; Zołnierczyk, A.; Ma̧czka, W.; Wawrzeńczyk, C. Trace Analysis of Hop Essential Oils in Spent Hop. J. Am. Soc. Brew. Chem. 2007, 65, 214–218. [Google Scholar] [CrossRef]
- Anioł, M.; Żołnierczyk, A. Extraction of Spent Hops Using Organic Solvents. J. Am. Soc. Brew. Chem. 2008, 66, 208–214. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.R.; Chen, S.-N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F.; et al. Pharmacokinetics of Prenylated Hop Phenols in Women Following Oral Administration of a Standardized Extract of Hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef] [Green Version]
- Jackowski, J.; Hurej, M.; Rój, E.; Popłoński, J.; Kośny, L.; Huszcza, E. Antifeedant Activity of Xanthohumol and Supercritical Carbon Dioxide Extract of Spent Hops against Stored Product Pests. Bull. Entomol. Res. 2015, 105, 456–461. [Google Scholar] [CrossRef]
- Grudniewska, A.; Pastyrczyk, N. New Insight for Spent Hops Utilization: Simultaneous Extraction of Protein and Xanthohumol Using Deep Eutectic Solvents. Biomass Convers. Biorefinery 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Grudniewska, A.; Popłoński, J. Simple and Green Method for the Extraction of Xanthohumol from Spent Hops Using Deep Eutectic Solvents. Sep. Purif. Technol. 2020, 250, 117196. [Google Scholar] [CrossRef]
- Moens, E.; Bolca, S.; van de Wiele, T.; van Landschoot, A.; Goeman, J.L.; Possemiers, S.; Verstraete, W. Exploration of Isoxanthohumol Bioconversion from Spent Hops into 8-Prenylnaringenin Using Resting Cells of Eubacterium Limosum. AMB Express 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, I.R.; Lederer, C.; Shellhammer, T.H. Bitterness-Modifying Properties of Hop Polyphenols Extracted from Spent Hop Material. J. Am. Soc. Brew. Chem. 2008, 66, 174–183. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Girardi, J.; Cosci, F.; Conti, B. Not Just for Beer: Evaluation of Spent Hops (Humulus Lupulus L.) as a Source of Eco-Friendly Repellents for Insect Pests of Stored Foods. J. Pest Sci. 2015, 88, 583–592. [Google Scholar] [CrossRef]
- Saraiva, B.R.; da Silva, L.H.M.; Anjo, F.A.; Vital, A.C.P.; da Silva, J.B.; Bruschi, M.L.; Matumoto Pintro, P.T. Technological and Sensorial Properties of Liquid Nitrogen Ice Cream Enriched with Protein from Brewing Waste (Trub). Int. J. Food Sci. Technol. 2020, 55, 1962–1970. [Google Scholar] [CrossRef]
- Codina-Torrella, I.; Rodero, L.; Almajano, M.P. Brewing By-Products as a Source of Natural Antioxidants for Food Preservation. Antioxidants 2021, 10, 1512. [Google Scholar] [CrossRef]
- Censi, R.; Vargas Peregrina, D.; Gigliobianco, M.R.; Lupidi, G.; Angeloni, C.; Pruccoli, L.; Tarozzi, A.; di Martino, P. New Antioxidant Ingredients from Brewery By-Products for Cosmetic Formulations. Cosmetics 2021, 8, 96. [Google Scholar] [CrossRef]
- Saraiva, B.R.; Anjo, F.A.; Vital, A.C.P.; Matumoto-Pintro, P.T. Soluble Protein Isolate from Brewing By-product (Trub) Using the Box-Behnken Design. J. Food Process. Preserv. 2021, 45, e15871. [Google Scholar] [CrossRef]
- Saraiva, B.R.; Zancheta, J.C.; Sversut Gibin, M.; Anjo, F.A.; Lazzari, A.; Machado Filho, E.R.; Sato, F.; Matumoto-Pintro, P. Brewing By-Product Valorisation: Trub Debittered for Nutritional and Quality Improvement of Pasta. Int. J. Food Sci. Nutr. 2022, 73, 915–926. [Google Scholar] [CrossRef]
- Cerqueira e Silva, K.F.; Rabelo, R.S.; Feltre, G.; Hubinger, M. Bitter Substances Recovery from Hot Trub: A Study of Polymeric Membranes Performance in a Sequential Mode with Fouling Investigation. Sep. Purif. Technol. 2022, 303, 122241. [Google Scholar] [CrossRef]
- Gandolpho, B.C.G.; Almeida, A.R.; Gandolpho, G.M.; Freitas, D.Z.; Gasparini, O.C.; MacHado, M.H.; Barreto, P.L.M. Optimization of Brewing Waste’s (TRUB) Phenolic Compounds Extraction by Ultrasound Assisted Using Response Surface Methodology. Quim. Nova 2021, 44, 478–483. [Google Scholar] [CrossRef]
- Oosterveld, A.; Voragen, A.G.J.; Schols, H.A. Characterization of Hop Pectins Shows the Presence of an Arabinogalactan-Protein. Carbohydr. Polym. 2002, 49, 407–413. [Google Scholar] [CrossRef]
- Jerkovic, V.; Collin, S. Fate of Resveratrol and Piceid through Different Hop Processings and Storage Times. J. Agric. Food Chem. 2008, 56, 584–590. [Google Scholar] [CrossRef]
- Gibson, D.L.; Dwivedi, B.K. Production of Meat Substitutes from Spent Brewers’ Yeast and Soy Protein. Can. Inst. Food Technol. J. 1970, 3, 113–115. [Google Scholar] [CrossRef]
- Vieira, E.; Brandão, T.; Ferreira, I.M.P.L.V.O. Evaluation of Brewer’s Spent Yeast To Produce Flavor Enhancer Nucleotides: Influence of Serial Repitching. J. Agric. Food Chem. 2013, 61, 8724–8729. [Google Scholar] [CrossRef]
- da Silva Araújo, V.B.; de Melo, A.N.F.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; de Souza, E.L.; Magnani, M. Followed Extraction of β-Glucan and Mannoprotein from Spent Brewer’s Yeast (Saccharomyces Uvarum) and Application of the Obtained Mannoprotein as a Stabilizer in Mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- dos Santos, M.T.R.; de Aguiar, P.F.; de Almeida e Silva, J.B.; de Mello, P.P.M.; Sérvulo, E.F.C. Brewery Wastes Reuse for Protease Production by Lactic Acid Bacteria Fermentation. Food Technol. Biotechnol. 2017, 55, 218–224. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Impact of New Ingredients Obtained from Brewer’s Spent Yeast on Bread Characteristics. J. Food Sci. Technol. 2018, 55, 1966–1971. [Google Scholar] [CrossRef]
- León-González, M.E.; Gómez-Mejía, E.; Rosales-Conrado, N.; Madrid-Albarrán, Y. Residual Brewing Yeast as a Source of Polyphenols: Extraction, Identification and Quantification by Chromatographic and Chemometric Tools. Food Chem. 2018, 267, 246–254. [Google Scholar] [CrossRef]
- Marson, G.V.; Saturno, R.P.; Comunian, T.A.; Consoli, L.; Machado, M.T.D.C.; Hubinger, M.D. Maillard Conjugates from Spent Brewer’s Yeast by-Product as an Innovative Encapsulating Material. Food Res. Int. 2020, 136, 109365. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Saturno, R.P.; Marson, G.V.; Hubinger, M.D. Spent Brewer’s Yeast Proteins and Cell Debris as Innovative Emulsifiers and Carrier Materials for Edible Oil Microencapsulation. Food Res. Int. 2021, 140, 109853. [Google Scholar] [CrossRef] [PubMed]
- Marson, G.V.; Lacour, S.; Hubinger, M.D.; Belleville, M.-P. Serial Fractionation of Spent Brewer’s Yeast Protein Hydrolysate by Ultrafiltration: A Peptide-Rich Product with Low RNA Content. J. Food Eng. 2022, 312, 110737. [Google Scholar] [CrossRef]
- Rubio, F.T.V.; Haminiuk, C.W.I.; Santos, P.D.D.F.; Martelli-Tosi, M.; Thomazini, M.; Balieiro, J.C.D.C.; Makimori, G.Y.F.; Fávaro-Trindade, C.S. Investigation on Brewer’s Spent Yeast as a Bio-Vehicle for Encapsulation of Natural Colorants from Pumpkin (Cucurbita moschata) Peels. Food Funct. 2022, 13, 10096–10109. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-W.; Fu, J.-J.; Li, J.-J.; Tang, Y.; Shao, Z.-W.; Zhou, D.-Y.; Song, L. Efficient Encapsulation of Curcumin into Spent Brewer’s Yeast Using a PH-Driven Method. Food Chem. 2022, 394, 133537. [Google Scholar] [CrossRef] [PubMed]
- Life Yeast Background. Available online: https://lifeyeast.com/background/ (accessed on 25 June 2021).
- Stevens, R. The Chemistry of Hop Constituents. Chem. Rev. 1967, 67, 19–71. [Google Scholar] [CrossRef]
- Jaskula, B.; Kafarski, P.; Aerts, G.; de Cooman, L. A Kinetic Study on the Isomerization of Hop α-Acids. J. Agric. Food Chem. 2008, 56, 6408–6415. [Google Scholar] [CrossRef]
- Spetsig, L.-O.; Bjerrum, J.; Lundén, R.; Prydz, H. Electrolytic Constants and Solubilities of Humulinic Acid, Humulone, and Lupulone. Acta Chem. Scand. 1955, 9, 1421–1424. [Google Scholar] [CrossRef]
- Wietstock, P.C.; Shellhammer, T.H. Chelating Properties and Hydroxyl-Scavenging Activities of Hop α- and Iso-α-Acids. J. Am. Soc. Brew. Chem. 2011, 69, 133–138. [Google Scholar] [CrossRef]
- Karabín, M.; Rýparová, A.; Jelínek, L.; Kunz, T.; Wietstock, P.; Methner, F.-J.; Dostálek, P. Relationship of Iso- α -Acid Content and Endogenous Antioxidative Potential during Storage of Lager Beer. J. Inst. Brew. 2014, 120, 212–219. [Google Scholar] [CrossRef]
- Mertens, T.; Kunz, T.; Gibson, B.R. Transition Metals in Brewing and Their Role in Wort and Beer Oxidative Stability: A Review. J. Inst. Brew. 2022, 128, 77–95. [Google Scholar] [CrossRef]
- Schönberger, C. The Role of Hops in Flavour stability – Some Aspects. BrewingScience 2012, 65, 130–133. [Google Scholar]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Zawadzki, A.; Alloo, C.; Grossi, A.B.; do Nascimento, E.S.P.; Almeida, L.C.; Bogusz Junior, S.; Skibsted, L.H.; Cardoso, D.R. Effect of Hop β-Acids as Dietary Supplement for Broiler Chickens on Meat Composition and Redox Stability. Food Res. Int. 2018, 105, 210–220. [Google Scholar] [CrossRef]
- Kramer, B.; Thielmann, J.; Hickisch, A.; Muranyi, P.; Wunderlich, J.; Hauser, C. Antimicrobial Activity of Hop Extracts against Foodborne Pathogens for Meat Applications. J. Appl. Microbiol. 2015, 118, 648–657. [Google Scholar] [CrossRef]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of Hop Extract as Antifungal Ingredient for Bread Making and Selection of Autochthonous Resistant Starters for Sourdough Fermentation. Int. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef]
- Xu, D.; Chen, T.; Liu, Y. The Physical Properties, Antioxidant and Antimicrobial Activity of Chitosan–Gelatin Edible Films Incorporated with the Extract from Hop Plant. Polym. Bull. 2021, 78, 3607–3624. [Google Scholar] [CrossRef]
- Arruda, T.R.; Bernardes, P.C.; e Moraes, A.R.F.; Marques, C.S.; Pinheiro, P.F.; de Oliveira, T.V.; Ferreira, S.O.; Naves, E.A.A.; Soares, N.D.F.F. Beyond Brewing: β-Acid Rich Hop Extract in the Development of a Multifunctional Polylactic Acid-Based Food Packaging. Int. J. Biol. Macromol. 2023, 228, 23–39. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Kondo, K.; Nakayama, H. Iso-α-Acids, Hop-Derived Bitter Components of Beer, Attenuate Age-Related Inflammation and Cognitive Decline. Front. Aging Neurosci. 2019, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Yoshikawa, M.; Takaichi, Y.; Michikawa, M.; Uchida, K.; Nakayama, H.; Takashima, A. Iso-α-Acids, Bitter Components in Beer, Suppress Inflammatory Responses and Attenuate Neural Hyperactivation in the Hippocampus. Front. Pharmacol. 2019, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Hoshi, A.; Ayabe, T.; Ohya, R.; Uchida, S.; Yamada, K.; Kondo, K.; Kitaoka, S.; Furuyashiki, T. Iso-α-Acids, the Bitter Components of Beer, Improve Hippocampus-Dependent Memory through Vagus Nerve Activation. FASEB J. 2019, 33, 4987–4995. [Google Scholar] [CrossRef] [Green Version]
- Ano, Y.; Takaichi, Y.; Uchida, K.; Kondo, K.; Nakayama, H.; Takashima, A. Iso-α-Acids, the Bitter Components of Beer, Suppress Microglial Inflammation in RTG4510 Tauopathy. Molecules 2018, 23, 3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Polyphenols in Foods Are More Complex than Often Thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef] [Green Version]
- Guido, L.F.; Moreira, M.M. Techniques for Extraction of Brewer’s Spent Grain Polyphenols: A Review. Food Bioprocess. Technol. 2017, 10, 1192–1209. [Google Scholar] [CrossRef] [Green Version]
- Proestos, C.; Komaitis, M. Antioxidant Capacity of Hops. Beer Health Dis. Prev. 2009, 467–474. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop Compounds: Extraction Techniques, Chemical Analyses, Antioxidative, Antimicrobial, and Anticarcinogenic Effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.F.; Page, J.E. Xanthohumol and Related Prenylflavonoids from Hops and Beer: To Your Good Health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.F. Xanthohumol and Structurally Related Prenylflavonoids for Cancer Chemoprevention and Control. In Natural Products for Cancer Chemoprevention: Single Compounds and Combinations; Springer International Publishing: Cham, Switzerland, 2020; pp. 320–350. ISBN 9783030398552. [Google Scholar]
- Wunderlich, S.; Zürcher, A.; Back, W. Enrichment of Xanthohumol in the Brewing Process. Mol. Nutr. Food Res. 2005, 49, 874–881. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Depigmenting Effect of Xanthohumol from Hop Extract in MNT-1 Human Melanoma Cells and Normal Human Melanocytes. Biochem. Biophys. Rep. 2021, 26, 100955. [Google Scholar] [CrossRef]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.J.; Conte, J.; Natrajan, P.; Haas, G.; Gonzalez, S. Direct Inhibition of Elastase and Matrixmetalloproteinases and Stimulation of Biosynthesis of Fibrillar Collagens, Elastin, and Fibrillins by Xanthohumol. Int. J. Cosmet. Sci. 2010, 32, 395–396. [Google Scholar] [CrossRef]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Lund, M.N.; Tiwari, B.K. Ultrasound Processing of Coffee Silver Skin, Brewer’s Spent Grain and Potato Peel Wastes for Phenolic Compounds and Amino Acids: A Comparative Study. J. Food Sci. Technol. 2021, 58, 2273–2282. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Diez, M.T.S.; Blanco, B.; Beltrán, S.; Trigueros, E.; Benito-Román, O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, R.; Ciccoritti, R.; Ceccantoni, B.; Bellincontro, A.; Amoriello, T. Optimization of Phenolic Compound Extraction from Brewers’ Spent Grain Using Ultrasound Technologies Coupled with Response Surface Methodology. Sustainability 2022, 14, 3309. [Google Scholar] [CrossRef]
- Senna Ferreira Costa, F.; Roquete Amparo, T.; Brandão Seibert, J.; Silveira, B.M.; Gomes da Silva, R.; Inocêncio Pereira, D.; Gontijo Garcia Barbosa, R.; dos Santos, O.D.H.; Brandão, G.C.; de Medeiros Teixeira, L.F.; et al. Reuse of Hot Trub as an Active Ingredient with Antioxidant and Antimicrobial Potential. Waste Biomass Valorization 2021, 12, 2037–2047. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Sologubik, C.A.; Fernández, M.B.; Manrique, G.D.; D’Alessandro, L.G. Extraction of Antioxidant Phenolic Compounds from Brewer’s Spent Grain: Optimization and Kinetics Modeling. Antioxidants 2018, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Zago, E.; Tillier, C.; de Leener, G.; Nandasiri, R.; Delporte, C.; Bernaerts, K.V.; Shavandi, A. Sustainable Production of Low Molecular Weight Phenolic Compounds from Belgian Brewers’ Spent Grain. Bioresour. Technol. Rep. 2022, 17, 100964. [Google Scholar] [CrossRef]
- Stefanello, F.S.; dos Santos, C.O.; Bochi, V.C.; Fruet, A.P.B.; Soquetta, M.B.; Dörr, A.C.; Nörnberg, J.L. Analysis of Polyphenols in Brewer’s Spent Grain and Its Comparison with Corn Silage and Cereal Brans Commonly Used for Animal Nutrition. Food Chem. 2018, 239, 385–401. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Campillo, V.; Coca, M.; Lucas, S.; García-Cubero, M.T. Microwave-assisted Deep Eutectic Solvent Extraction of Phenolic Compounds from Brewer’s Spent Grain. J. Chem. Technol. Biotechnol. 2021, 96, 481–490. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Impact of the Use of Pressurized Liquids on the Extraction and Functionality of Proteins and Bioactives from Brewer’s Spent Grain. Food Chem. 2021, 359, 129874. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Riaño, P.; Sanz, M.T.; Benito-Román, O.; Beltrán, S.; Trigueros, E. Subcritical Water as Hydrolytic Medium to Recover and Fractionate the Protein Fraction and Phenolic Compounds from Craft Brewer’s Spent Grain. Food Chem. 2021, 351, 129264. [Google Scholar] [CrossRef] [PubMed]
- Galván, S.O.; González-García, E.; Marina, M.L.; García, M.C. Comparative Study of Factors Affecting the Recovery of Proteins from Malt Rootlets Using Pressurized Liquids and Ultrasounds. Curr. Res. Food Sci. 2022, 5, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Riaño, P.; Melgosa, R.; Trigueros, E.; Illera, A.E.; Beltrán, S.; Sanz, M.T. Valorization of Brewer’s Spent Grain by Consecutive Supercritical Carbon Dioxide Extraction and Enzymatic Hydrolysis. Food Chem. 2022, 396, 133493. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Vilas-Boas, A.; Machado, M.; Costa, E.M.; Silva, S.; Pereira, R.N.; Campos, D.; Teixeira, J.A.; Pintado, M. Exploring the Bioactive Potential of Brewers Spent Grain Ohmic Extracts. Innov. Food Sci. Emerg. Technol. 2022, 76, 102943. [Google Scholar] [CrossRef]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed Electric Field (PEF) as Pre-Treatment to Improve the Phenolic Compounds Recovery from Brewers’ Spent Grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Sanchez-Camargo, A.D.P.; Benvenutti, L.; Ferro, D.M.; Dias, J.L.; Ferreira, S.R.S. High-Pressure Fluid Technologies: Recent Approaches to the Production of Natural Pigments for Food and Pharmaceutical Applications. Trends Food Sci. Technol. 2021, 118, 850–869. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–398. [Google Scholar] [CrossRef]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical Carbon Dioxide Extraction of Plant Phytochemicals for Biological and Environmental Applications—A Review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Roobab, U.; Khan, A.W.; Irfan, M.; Madni, G.M.; Zeng, X.-A.; Nawaz, A.; Walayat, N.; Manzoor, M.F.; Aadil, R.M. Recent Developments in Ohmic Technology for Clean Label Fruit and Vegetable Processing: An Overview. J. Food Process Eng. 2022, 45, e14045. [Google Scholar] [CrossRef]
- Cristianini, M.; Guillén Sánchez, J.S. Extraction of Bioactive Compounds from Purple Corn Using Emerging Technologies: A Review. J. Food Sci. 2020, 85, 862–869. [Google Scholar] [CrossRef]
- Schendel, R.R. Phenol Content in Sprouted Grains. In Sprouted Grains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–315. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-Products. Food Bioprocess Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Liu, Z.; Esveld, E.; Vincken, J.-P.; Bruins, M.E. Pulsed Electric Field as an Alternative Pre-Treatment for Drying to Enhance Polyphenol Extraction from Fresh Tea Leaves. Food Bioprocess Technol. 2019, 12, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The Effect of Pulsed Electric Field as a Pre-Treatment Step in Ultrasound Assisted Extraction of Phenolic Compounds from Fresh Rosemary and Thyme by-Products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644. [Google Scholar] [CrossRef]
- el Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption Isotherm Models: A Comprehensive and Systematic Review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and Isolation of Phenolic Compounds. Methods Mol. Biol. 2012, 864, 427–464. [Google Scholar] [CrossRef]
- Gkika, D.A.; Mitropoulos, A.C.; Kyzas, G.Z. Why Reuse Spent Adsorbents? The Latest Challenges and Limitations. Sci. Total Environ. 2022, 822, 153612. [Google Scholar] [CrossRef] [PubMed]
- Biendl, M. Isolation of Prenylflavonoids from Hops. Acta Hortic. 2013, 1010, 131–140. [Google Scholar] [CrossRef]
- Ideia, P.; Sousa-Ferreira, I.; Castilho, P.C. A Novel and Simpler Alkaline Hydrolysis Methodology for Extraction of Ferulic Acid from Brewer’s Spent Grain and Its (Partial) Purification through Adsorption in a Synthetic Resin. Foods 2020, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.J.; Vieira, J.S.; Gonçalves, L.M.; Pacheco, J.G.; Guido, L.F.; Barros, A.A. Isolation of Phenolic Compounds from Hop Extracts Using Polyvinylpolypyrrolidone: Characterization by High-Performance Liquid Chromatography–Diode Array Detection–Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2010, 1217, 3258–3268. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Gigliobianco, M.R.; Peregrina, D.V.; Sagratini, G.; Censi, R.; di Martino, P. Quantification of Phenolic Compounds in Different Types of Crafts Beers, Worts, Starting and Spent Ingredients by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1612, 460622. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane Technologies Assisting Plant-Based and Agro-Food by-Products Processing: A Comprehensive Review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Moncada, J.; Roberto, I.C.; Cardona, C.A. Techno-Economic Analysis for Brewer’s Spent Grains Use on a Biorefinery Concept: The Brazilian Case. Bioresour. Technol. 2013, 148, 302–310. [Google Scholar] [CrossRef]
- Varga, Á.; Márki, E. Microfiltration: A Novel Technology for Removal of Trub from Hopped Wort. J. Food Process. Eng. 2019, 42, e13200. [Google Scholar] [CrossRef] [Green Version]
- Samriddhi, C.; Roshan, D. Polyphenol Market by Product Type (Apple, Green Tea, Grape Seeds and Others), Type (Flavonoid, Resveratrol, Phenolic Acid and Lignin), and Application (Functional Foods, Functional Beverages, Dietary Supplements, and Others): Global Opportunity Analysis and Industry Forecast 2021–2030; Allied Market Research: Maharashtra, India, 2022. [Google Scholar]
- Bloomberg Polyphenols Market Size Worth $2.9 Billion by 2030: Grand View Research, Inc. Available online: https://www.bloomberg.com/press-releases/2022-03-30/polyphenols-market-size-worth-2-9-billion-by-2030-grand-view-research-inc (accessed on 9 March 2023).
- Market Research Firm. Cosmetic Antioxidants Market by Source (Natural, Chemically Derived), Type (Vitamins, Enzymes, Polyphenols), Function (Anti-Aging, Hair Conditioning, UV Protection), and Application (Skincare, Hair Care, Make up)—Global Forescasts to 2025; MarketsandMarkets Research: Maharashtra, India, 2023. [Google Scholar]
- DermoTech Beauty. Where the American Scientists Cannot Tread, There the Polish Ones Will Find the Way. Available online: https://proxn.eu/ (accessed on 8 March 2023).
- Steiner, S.H. Guidelines for Hop Buying; Hopsteiner GmbH & Co: Mainburg, Germany, 2022; Available online: https://www.hopsteiner.com/wp-content/uploads/2022/11/Guidelines_2022_online.pdf (accessed on 9 March 2023).
- Barth, S.J. BarthHaas Report: Hops 2019/2020; BarthHaas GmbH & Co. KG: Nuremberg, Germany, 2020; Available online: https://www.barthhaas.com/fileadmin/user_upload/01-barthhaas-2022/Downloads/Reports_Broschures/BarthHaas_Reports/English/2010-2020/barthhaas_report_2020_en.pdf (accessed on 9 March 2023).
- Barth, S.J. BarthHaas Report: Hops 2020/2021; BarthHaas GmbH & Co. KG: Nuremberg, Germany, 2021; Available online: https://www.barthhaas.com/fileadmin/user_upload/01-barthhaas-2022/Downloads/Reports_Broschures/BarthHaas_Reports/BarthHaas_Report_Hops_2020_21.pdf (accessed on 9 March 2023).
- BarthHaas. Whole Hops & Pellets For The UK & Europe. Available online: https://www.barthhaasx.com/ (accessed on 27 December 2022).
- Alonso-Riaño, P.; Ramos, C.; Trigueros, E.; Beltrán, S.; Sanz, M.T. Study of Subcritical Water Scale-up from Laboratory to Pilot System for Brewer’s Spent Grain Valorization. Ind. Crops. Prod. 2023, 191, 115927. [Google Scholar] [CrossRef]
- Colpo, I.; Rabenschlag, D.R.; de Lima, M.S.; Martins, M.E.S.; Sellitto, M.A. Economic and Financial Feasibility of a Biorefinery for Conversion of Brewers’ Spent Grain into a Special Flour. J. Open Innov. Technol. Mark. Complex. 2022, 8, 79. [Google Scholar] [CrossRef]
| Recovered Molecule | Product | Technique | Ref |
|---|---|---|---|
| Proteins | Proteins concentrate | Alkaline extraction associated with temperature variation (from 27 to 100 °C) and precipitation at the isoelectric point | [20] |
| Xylo-oligosaccharides | - | Hydrolysis via hydrothermal treatment followed by fractionation using anion-exchange chromatography and size exclusion chromatography | [21] |
| Lignin | - | pH adjustment to lignin precipitation | [22] |
| Ferulic acid and p-coumaric acid | - | Alkaline hydrolysis | [26] |
| Fiber | Snack product | Extruded chickpea-based using brewer’s spent grains as replacement maize flour | [27] |
| - | Baked snacks | Snacks formulation using brewer’s spent grains as replacement wheat flour | [28] |
| Proteins/hemicellulose | Proteins and arabinoxylans | Sequential extraction of proteins and arabinoxylans using an alkaline solution and ethanol | [29] |
| Xylan | - | Alkaline extraction followed by ultrafiltration and diafiltration | [30] |
| Hemicellulose | - | hydrolysis via flow-through subcritical water reactor | [23] |
| Proteins/fibers | Proteins and fiber concentrate | Fractionation process via the combination of wet milling with chemical and enzymatic incubation | [31] |
| Arabinoxylans | Films | Arabinoxylans extraction via precipitation in pH adjustment followed by microwave treatment. The film was produced by the casting method. | [25] |
| Ferulic acid | - | Extraction via dilute acid hydrolysis followed by alkaline hydrolysis | [32] |
| Proteins/fibers | Proteins and fiber concentrate | Fraction process via wet milling with enzymatic incubation | [33] |
| Proteins | Fishmeal | Extruded | [34] |
| Fiber/proteins | Pork sausages | Brewer’s spent grains flour as the replacement of a small part of pig meat | [35] |
| Fiber/proteins | Hybrid sausages | Brewer’s spent grains flour as the replacement for broccoli and insect flour | [36] |
| Fiber/proteins | Dry Pasta | Extrusion | [37] |
| Xylose/arabinose/Amino acids | - | Hydrolysis via flow-through subcritical water reactor | [24] |
| By-Products | Molecule Recovered | Products | Techniques | Phytochemical Yields | Ref |
|---|---|---|---|---|---|
| spent hops | Desmethylxanthohumol/xanthohumol | - | Extraction by polarity using apolar solvents | Xanthohumol (0.04%) Desmethylxanthohumol (0.00056%) | [42] |
| spent hops | Essential oil | - | Extraction via hydro-distillation | β-Myrcene (241 μg/g) Limonene (7.83 μg/g) Linalool (8.08 μg/g) Geraniol (3.08 μg/g) 2-undecanone (1.36 μg/g) β-caryophyllene (11.2 μg/g) α-humulene (19.1 μg/g) | [43] |
| spent hops | Bitter acids | - | Extraction by polarity using apolar solvents | Spent hops before isomerization: Isocohumulone (65.59 μg/g) Isohumulone (134.97 μg/g) Isoadhumulone (21.09 μg/g) Cohumulone (175.91 μg/g) N+adhumulone (580.70 μg/g) Colupulone (57.42 μg/g) Adlupulone (15.65 μg/g) Spent hops after isomerization: Isocohumulone (129.76 μg/g) Isohumulone (399.78 μg/g) Isoadhumulone (51.9 μg/g) Cohumulone (44.39 μg/g) N+adhumulone (58.73 μg/g) Colupulone (21.41 μg/g) Adlupulone (10.68 μg/g) | [44] |
| spent hops | Xanthohumol/isoxanthohumol/6-prenylnaringenin/8-prenylnaringenin | Encapsulated in green gelatin capsules | Extract was produced via the enzymatic approach | 8-Prenylnaringenin (0.42 g/100 g dw) 6-Prenylnaringenin (2.18 g/100 g dw) Isoxanthohumol (1.35 g/100 g dw) Xanthohumol (35.78 g/100 g dw) | [45] |
| spent hops | Xanthohumol/Isoxanthohumol | - | Extraction via Supercritical carbon dioxide | - | [46] |
| spent hops | Proteins/Xanthohumol | - | Extraction using deep eutectic solvents | Xanthohumol (from 0.52 to 1.92 mg/g SH) Protein content (40 and 64%) | [47] |
| spent hops | Xanthohumol | - | Extraction via deep eutectic solvents | Xanthohumol (2.30 mg/g SH) | [48] |
| spent hops | Xanthohumol | - | Extraction by polarity using apolar solvents was followed by the bioconversion method (via enzymes) to 8-prenylnaringenin | Xanthohumol (from 0.36 to 2.14 %m) | [49] |
| spent hops | Polyphenols | - | Extraction by polarity using water and ethanol | α-Acids (0.011%m) Total polyphenols (10.45 g/L) Humulinones (0.031%m) | [50] |
| hot trub | Essential oils | - | Extraction via hydro-distillation | Myrcene (24.2 %) a-Humulene (16.2%) b-Caryophyllene (6.6%) 2-Undecanone (4.7%) Humulene oxide II (4.0%) 2-Methylbutyl isobutyrate (3.6%) d-Cadinene (3.3%) Linalool (1.9%) Limonene (1.2%) | [51] |
| hot trub | Proteins | Ice cream | Proteins flour of hot trub was added in the ice cream formulation as proteins enrichment | - | [52] |
| hot trub | Phenolic compounds | - | Extraction by polarity using water, methanol, and acetone | TPC (24.84 μmol GAE/g w.s.) | [53] |
| hot trub | Phenolic compounds | - | Extraction by polarity using water and hydroethanolic solution (70% v/v) | TPC (from 7.40 to 15.98 μmol GAE/g) | [54] |
| hot trub | Proteins | Proteins isolated | Alkaline extraction and precipitation at the isoelectric point | Protein content (94.56 %dw) TPC (35.36 mg GAE/g) Flavonoids (4.06 mg QE/g) | [55] |
| hot trub | Proteins | Pasta | Proteins flour of debittered hot trub for pasta production | Protein content (45.72%) | [56] |
| hot trub | Bitter acids | - | Extraction by polarity using hydroethanolic solution (30% v/v) and pH 7 that was followed by membrane filtration | N+-adlupulone (6.4 mg/g) Colupulone (6.3 mg/g) Iso-α-acids (28.23 IBU) N+-adhumulone (2.3 mg/g) Cohumulone (2.1 mg/g) | [57] |
| Recovered Molecule | Product | Technique | Ref |
|---|---|---|---|
| - | Meat substitute | Brewer’s spent yeast hydrolysate was used as an ingredient in the formulation of meat products | [61] |
| Nucleotide | Flavor enhancer | Mechanic disruption treatment and chemical hydrolysis of RNA | [62] |
| β-glucan/mannoproteins | Mayonnaise stabilizing | Proteolysis treatment | [63] |
| - | Substrate for production of proteolytic enzymes | Brewer’s spent yeast was used to support bacterial growth | [64] |
| β-glucan/proteins/proteolytic enzymes | Fortification of bread formulation | Chemical autolysis and mechanic disruption treatment | [65] |
| Phenolic compounds | - | Extraction by polarity using the hydroethanolic solution | [66] |
| Maillard conjugates | Wall material for encapsulated products | Enzymatic hydrolysis and heat treatment | [67] |
| Proteins/peptides | Emulsifying material and carrier agent for encapsulated products | Enzymatic hydrolysis was followed by ultrafiltration in concentration mode | [68] |
| Peptides | - | Enzymatic hydrolysis was followed by sequential membranes filtration | [69] |
| - | Emulsifying material and carrier agent for encapsulated products | The incorporation of carotenoids in brewer’s spent yeast via ultra-turrax and ultrasonic methods was followed by atomization in spray dried | [70] |
| - | Wall material for encapsulated products | Encapsulation of curcumin into brewer’s spent yeast using a pH-driven method and then lyophilized | [71] |
| Technology/Equipment | By-Product | Conditions Optimized | Results ¹ | Ref |
|---|---|---|---|---|
| Ultrasound/1.2-cm probe system | brewer’s spent grains | F (n-i), NP (49.5 W), T (25 °C), S/F (20), t (30 min), solvent (water) | TPC (0.66 mg GAE/g) | [103] |
| Ultrasound/13-mm probe system | brewer’s spent grains | F (20 kHz), NP (750 W), pulse mode (5 sec on and 5 sec off), T (47 °C), S/F (21.7), t (30 min), solvent (20% ethanol aqueous solution) | TPC (3.55 mg GAE/g) p-hydroxybenzoic acid (10 µg/g) Ferulic acid (9.5 µg/g) Sinapic acid (13.5 µg/g) | [104] |
| Ultrasound/bath system | brewer’s spent grains | F (37 kHz), NP (n-i), T (80 °C), S/F (20), t (50 min), solvent (65 ethanol: 35 water) | TPC (0.1 mg GAE/g) Ferulic acid (about 1.5 ± 0.2 mg/L), Vanillic acid (0.8 ± 0.2 mg/L) p-coumaric acid (0.12 ± 0.03 mg/L) | [105] |
| Ultrasound/bath system | hot trub | F (n-i), NP (n-i), T (n-i), S/F (4.46), t (60 min), solvent (ethanol) | TPC (8.93 mg GAE/g) TFC (1.31 mg QE/g) | [106] |
| Ultrasound and microwave/probe system and open-system microwave oven | brewer’s spent grains | Ultrasound F (20 kHz), NP (45 W) Microwave F (2.45 GHz), NP (800 W) General information T (80 °C), S/F (30), solvent (72% ethanol aqueous solution), t (2 h) | Ultrasound TPC (4.11 mg GAE/g) Microwave TPC (3.91 mg GAE/g) | [107] |
| Microwave/domestic device | brewer’s spent grains | Microwave pre-treatment F (2.45 GHz), NP (600 W and 800 W), t (30 min), sample mass (0.5 g) Magnetic stirring in an oil bath extraction 2 × [S/F (30), solvent (70 ethanol: 30 water, v/v), T (80 °C), t (60 min)] | Light barley: TPC (12.5 mg GAE/g) 4-Vinylguaiacol (2 µg/mL) Ferulic acid (34 µg/mL) p-Coumaric acid (7 µg/mL) Red barley: TPC (13.23 mg GAE/g) 4-Vinylguaiacol (0.7 µg/mL) Ferulic acid (14.83 µg/mL) p-Coumaric acid (9.1 µg/mL) | [108] |
| Microwave/system equipped 16-carrousel containers under magnetic stirring at 200 rpm | brewer’s spent grains | F (n-i), NP (n-i), S/F (20), T (100 °C), t (15 min), solvent (0.75% NaOH aqueous solution) | TPC (13.7 mg GAE/g) TFC (2.6 mg QE/g) | [109] |
| Microwave/multiwave reactor with rotor-type equipment | brewer’s spent grains | F (50 Hz), NP (n-i), S/F (10), t (13.3 min), T (100 °C), solvent [37.46% (v/v) water in the DES (1 choline chloride: 2 glycerol)] | TPC (2.89 mg GAE/g) 4-hydroxybenzoic acid (14.4%) Vanillic acid (7%) Vanillin (9.4%) Syringic acid (8.2%) Syringaldehyde (14%) Coumaric acid (20.6%) Ferulic acid (26.4%) | [110] |
| High-pressure and ultrasound/pressurized liquid extractor and probe system | brewer’s spent grains | Preheated conditions (1500 psi for 6 min), T (155 °C), t (17 min), P (n-i), solvent (35 ethanol: 65 water), S/F (11.33), Cycles (5) | TPC (17.2 mg GAE/g) | [111] |
| High-pressure/pressurized liquid extractor | rewer’s spent grains | Preheated conditions (30 min at increasing pressure), P (5 MPa), T (185 °C), t (240 min), solvent (subcritical water), S/F (80) | TPC (33 mg GAE/g) | [112] |
| High-pressure and ultrasound/pressurized liquid extractor and probe system | malt rootlets | High-pressure Preheated conditions (1500 psi for 6 min), P (5 MPa), T (164 °C), t (15 min), solvent (ethanol:water mixture 33:67), S/F (n-i) Ultrasound F (n-i), NP (130 W), Amplitude (30%), S/F (50), T (room temperature), t (5 min) | High-pressure TPC (14.8 mg GAE/g) Ultrasound TPC (3.1 mg GAE/g) | [113] |
| High-pressure/supercritical CO2 | brewer’s spent grains | P (40 MPa), T (80 °C), solvent (sCO2) | TPC (0.94 mg GAE/g) TF (0.219 mg QE/g) | [114] |
| Ohmic heating | brewer’s spent grains | V (45 V to 75 V), distance between electrodes (8 cm), T (35 °C), t (30 min) solvents (ethanol: water mixture 60:40 v/v) | TPC (11.57 mg GAE/g) 4-hydroxybenzoic acid (125 μg/g) Vanillin (27.8 μg/g) Catechin (116 μg/g) Vanillic acid (23 μg/g) Ferulic acid (35.8 μg/g) | [115] |
| Pulsed electric field (pretreatment) | brewer’s spent grains | Pretreatment conditions: E (2.5 kV/cm), F (50 kHz), t (14.5 s), S/F (1), solvent (water) | Free polyphenols (101 µg/g) Flavan-3-ols (10.1 µg/g) Flavonoids (60 µg/g) Phenolic acid derivates (55 µg/g) | [116] |
| Technology | Energy | Temperature | Extraction Time | S/F | Solvent |
|---|---|---|---|---|---|
| Ultrasound | F (20–37 kHz), Po (49.5–800 W) | 25–80 °C | 30–120 min | 4.46–20 | Water, ethanol, ethanol-water mixtures [103] |
| Microwave | F (50 Hz–2.45–GHz), Po (600–800 W) | 80–100 °C | 13.3–120 min | 10–30 | Ethanol–water mixtures, NaOH aqueous solution, solutions with DES [108] |
| High-pressure | P (5 MPa) | 155–185 °C | 15–240 min | 11.3–80 | Water, ethanol–water mixtures [111] |
| Ohmic heating | V (45–75 V) | 80 °C | 30 min | n-i | Ethanol–water mixture [115] |
| Pulsed electric field | E (2.5 kV/cm), F (50 kHz) | n-i | 14.5 s (pre-treatment) | 1 | Water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, K.F.C.e.; Strieder, M.M.; Pinto, M.B.C.; Rostagno, M.A.; Hubinger, M.D. Processing Strategies for Extraction and Concentration of Bitter Acids and Polyphenols from Brewing By-Products: A Comprehensive Review. Processes 2023, 11, 921. https://doi.org/10.3390/pr11030921
Silva KFCe, Strieder MM, Pinto MBC, Rostagno MA, Hubinger MD. Processing Strategies for Extraction and Concentration of Bitter Acids and Polyphenols from Brewing By-Products: A Comprehensive Review. Processes. 2023; 11(3):921. https://doi.org/10.3390/pr11030921
Chicago/Turabian StyleSilva, Klycia Fidélis Cerqueira e, Monique Martins Strieder, Mariana Barreto Carvalhal Pinto, Maurício Ariel Rostagno, and Miriam Dupas Hubinger. 2023. "Processing Strategies for Extraction and Concentration of Bitter Acids and Polyphenols from Brewing By-Products: A Comprehensive Review" Processes 11, no. 3: 921. https://doi.org/10.3390/pr11030921
APA StyleSilva, K. F. C. e., Strieder, M. M., Pinto, M. B. C., Rostagno, M. A., & Hubinger, M. D. (2023). Processing Strategies for Extraction and Concentration of Bitter Acids and Polyphenols from Brewing By-Products: A Comprehensive Review. Processes, 11(3), 921. https://doi.org/10.3390/pr11030921






