Chemical Looping Enhanced Oil Shale-to-Liquid Fuels Process: Modeling, Parameter Optimization, and Performance Analysis
Abstract
:1. Introduction
2. Process Design and Modeling of CLeSTL
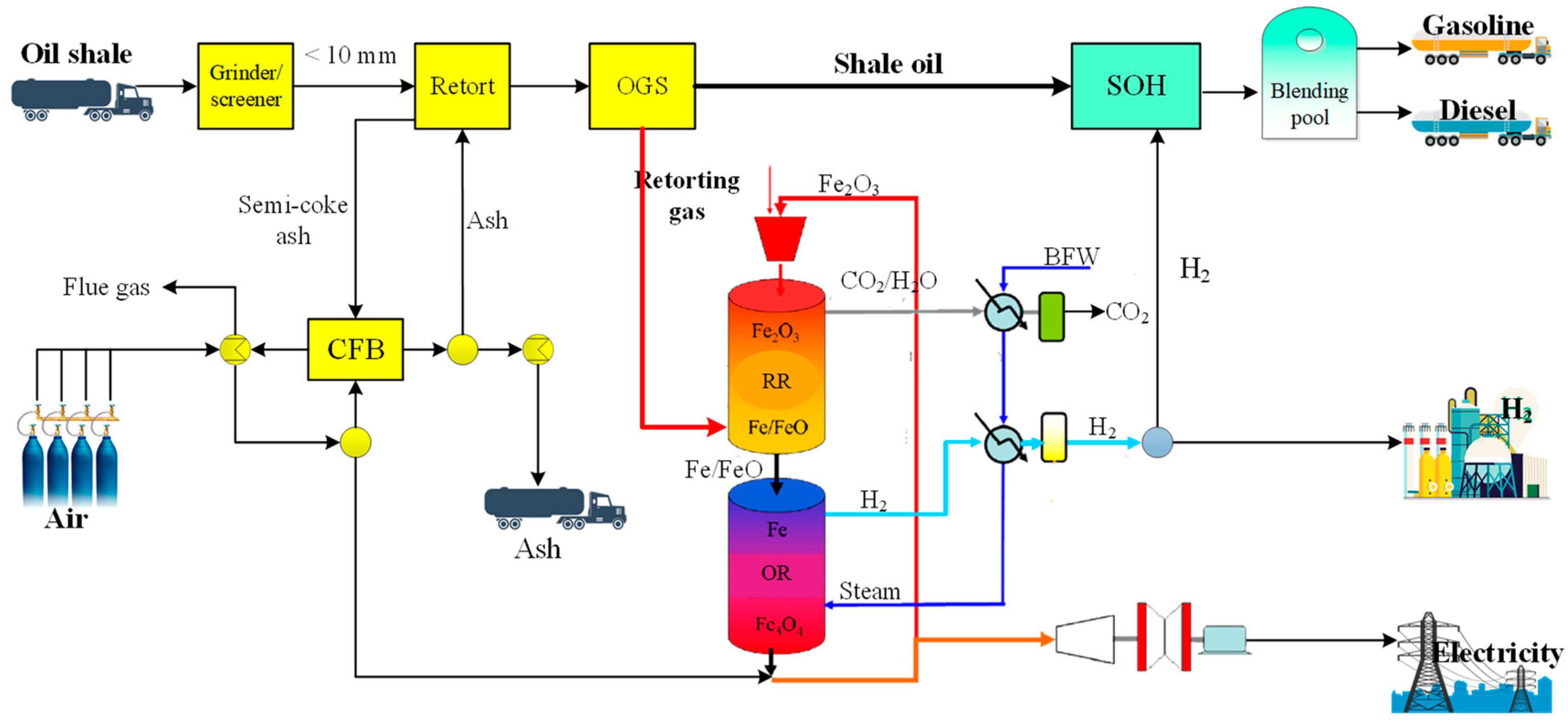
2.1. Process Design
2.2. Modeling and Simulation
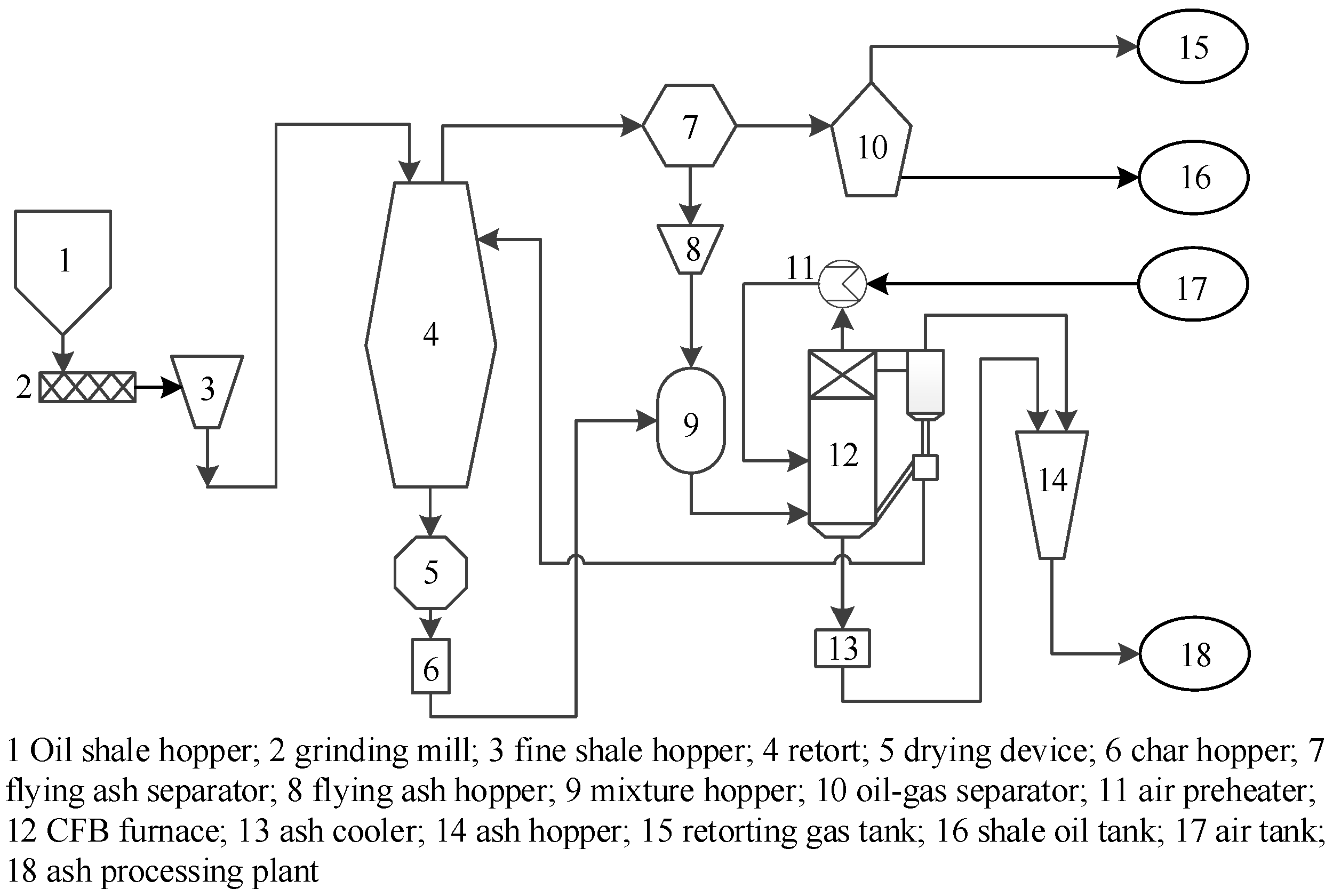
2.2.1. Oil Shale Retorting Unit
2.2.2. Oil–Gas Separation Unit
2.2.3. Thermal Balance Simulation of Retorting
2.2.4. Retorting Gas Chemical Looping for Hydrogen Production Unit
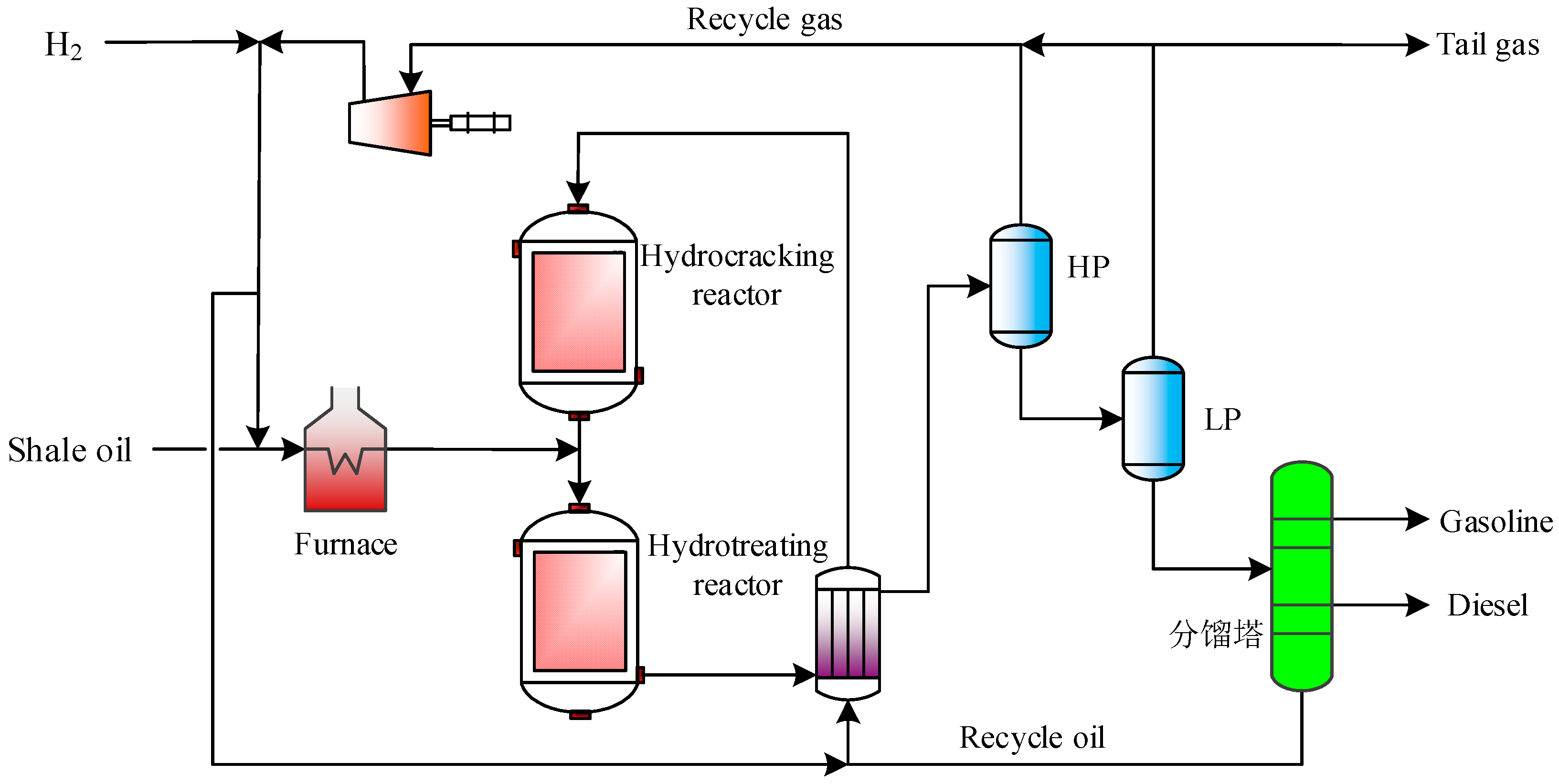
2.2.5. Shale Oil Hydrogenation Unit
3. Methodology
3.1. Technical Indicators
3.2. Economic Indicators
4. Results and Discussion
4.1. Parameter Analysis
- (1)
- Influence of blending oil shale/increasing air temperature on minimum stable burning calorific value
- (2)
- Molar flow ratio of MFe2O3/(MCO + MH2 + MCH4) and Msteam/MFe
4.2. System Simulation Analysis
4.3. Technical Performance Analysis
4.4. Economical Performance Analysis
- (1)
- Total capital investment analysis
- (2)
- Production cost analysis
- (3)
- Return on investment analysis
5. Conclusions
- (1)
- For the solid heat carrier moving bed with internals, in order to provide enough heat for 375 t/h of oil shale retorting, the combustion capacity of the semi-coke is 302.51 t/h and the addition of 9.72 t/h of oil shale in the circulating fluidized bed can ensure the stable combustion of fuel.
- (2)
- Compared with the traditional Fushun-type oil shale-to-liquid fuels process, the utilization rate of raw oil shale of the CLeSTL process is increased from 80% to 100%; the shale oil yield increased from 65% to 95.7%; and the yield of light components of shale oil increased from 20% to 64%–83%.
- (3)
- The ROIs of the CLeSTL-1, CLeSTL-2, CLeSTL-3, and CLeSTL-4 processes are 14.7%, 19.0%, 9.6%, and 15.2%, respectively. The ROI of the CLeSTL-2 process is the highest. Specifically, when the oil price is lower (50 USD/bbl), the ROI of the CLeSTL-2 process is 5%, which shows strong anti-risk ability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA (International Energy Agency). BP Word Energy Outlook. 2022. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/energy-outlook/bp-energy-outlook-2022.pdf (accessed on 1 October 2022).
- Zhou, H.; Li, H.; Duan, R.; Yang, Q. An integrated scheme of coal-assisted oil shale efficient pyrolysis and high-value conversion of pyrolysis oil. Energy 2020, 196, 117106. [Google Scholar] [CrossRef]
- Zuckerman, G. The Frackers: The Outrageous inside Story of the New Billionaire Wildcatters; Portfolio Publishing: New York, NY, USA, 2013. [Google Scholar]
- Li, X.; Zhou, H.; Wang, Y.; Qian, Y.; Yang, S. Thermoeconomic analysis of oil shale process with gas or solid heat carrier. Energy 2015, 113, 639–647. [Google Scholar]
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115121. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.; Han, X.; Tong, J. Effect of retorting temperature on product yield and characteristics of non-condensable gases and shale oil obtained by retorting Huadian oil shales. Fuel Process. Technol. 2014, 121, 9–15. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Yang, D.; Kang, Z. Characteristics of oil and gas production of oil shale pyrolysis by water vapor injection. Oil Shale 2022, 39, 153–168. [Google Scholar] [CrossRef]
- Qian, J.; Li, S. Oil Shale Retorting Technologies; China Petrochemcal Press: Beijing, China, 2014. (In Chinese) [Google Scholar]
- Qian, J.; Yin, L.; Wang, J.; Li, S.; Han, F.; He, Y. Oil Shale-Complementary Energy of Petroleum; China Petrochemcal Press: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Qian, Y.; Yang, Q.; Zhang, J.; Zhou, H.; Yang, S. Development of an integrated oil shale retorting process with coal gasification for hydrogen production. Ind. Eng. Chem. Res. 2014, 53, 19970–19978. [Google Scholar] [CrossRef]
- Lai, D.; Chen, Z.; Shi, Y.; Lin, L.; Zhan, J.; Gao, S.; Xu, G. Pyrolysis of oil shale by solid heat carrier in an innovative moving bed with internals. Fuel 2015, 159, 943–951. [Google Scholar] [CrossRef]
- Chiesa, P.; Giovanni, L.; Alberto, M.; Matteo, R.; Vincenzo, P. Three-reactors chemical looping process for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 2233–2245. [Google Scholar] [CrossRef]
- Luberti, M.; Ahn, H. Review of polybed pressure swing adsorption for hydrogen ppurification. Int. J. Hydrogen Energy 2022, 61, 6106–6124. [Google Scholar]
- Zhou, H.; Meng, W.; Wang, D.; Li, G.; Li, H.; Liu, Z.; Yang, S. A novel coal chemical looping gasification scheme for synthetic natural gas with low energy consumption for CO2 capture: Modelling, parameters optimization, and performance analysis. Energy 2021, 225, 120249. [Google Scholar] [CrossRef]
- Anaya, K.; Oni, A.O.; Kumar, A. Investigation the techno-economic and environmental performance of chemical looping technology for hydrogen production. Sustain. Energy Technol. Assess. 2023, 56, 103008. [Google Scholar]
- Xiang, W.; Chen, Y. Hydrogen and electricity from coal with carbon dioxide separation using chemical looping reactors. Energy Fuels 2007, 21, 2272–2277. [Google Scholar] [CrossRef]
- Sridhar, D.; Tong, A.; Kim, H.; Zeng, L.; Li, F.; Fan, L. Syngas chemical looping process: Design and construction of a 25 kWth sub-pilot unit. Energy Fuels 2012, 26, 2292–2302. [Google Scholar] [CrossRef]
- Tong, A.; Sridhar, D.; Sun, Z.; Kim, H.; Zeng, L.; Wang, F.; Wang, D.; Kathe, M.V.; Luo, S.; Sun, Y.; et al. Continuous high purity hydrogen generation from a syngas chemical looping 25kWth sub-pilot unit with 100% carbon capture. Fuel 2012, 103, 495–505. [Google Scholar] [CrossRef]
- Kim, H.; Wang, D.; Zeng, L.; Bayham, S.; Tong, A.; Chung, E.; Kathe, M.V.; Luo, S.; McGiveron, O.; Wang, A.; et al. Coal direct chemical looping combustion process: Design and operation of a 25 kWth sub-pilot unit. Fuel 2013, 108, 370–384. [Google Scholar] [CrossRef]
- Tong, A.; Bayham, S.; Kathe, M.; Zeng, L.; Luo, S.; Fan, L. Iron-based syngas chemical looping process and coal-direct chemical looping process development at Ohio State University. Appl. Energy 2014, 113, 1836–1845. [Google Scholar] [CrossRef]
- Bayham, S.; Kim, H.; Wang, D.; Tong, A.; Zeng, L.; McGiveron, O.; Kathe, M.V.; Chung, E.; Wang, W.; Wang, A.; et al. Iron-based coal direct chemical looping combustion process: 200-h continuous operation of a 25 kWth sub-pilot unit. Energy Fuels 2013, 27, 1347–1356. [Google Scholar] [CrossRef]
- Li, F.; Fan, L. Clean coal conversion processes-progress and challenges. Energy Environ. Sci. 2008, 1, 248–267. [Google Scholar] [CrossRef]
- Zeng, L.; He, F.; Li, F.; Fan, L. Coal-direct chemical looping gasification for hydrogen production: Reactor modeling and process simulation. Energy Fuels 2012, 26, 3680–3690. [Google Scholar] [CrossRef]
- Zhao, G.; Su, C.; Quan, H. Study on shale oil processing by single-stage reverse sequencing combination hydrocracking-hydrotreating process (FHC-FHT). Pet. Refin. Eng. 2012, 42, 36–38. (In Chinese) [Google Scholar]
- Yu, H.; Li, S.; Jin, G.; Tang, X. Hydrotreating of the diesel distillate from Huadian shale oil for production of clean fuel. J. Fuel Chem. Technol. 2010, 38, 297–301. (In Chinese) [Google Scholar]
- Yu, H.; Li, S.; Jin, G. Hydrotreating of nitrides in diesel distillate in Huadian shale oil. Petrochem. Technol. 2011, 39, 162–166. (In Chinese) [Google Scholar]
- Edrisi, A.; Mansoori, Z.; Dabir, B.; Shahnazari, A. Hydrogen, nitrogen and carbon dioxide production through chemical looping using iron-based oxygen carrier—A green plant for H2 and N2 production. Int. J. Hydrog. Energy 2014, 39, 10380–10391. [Google Scholar] [CrossRef]
- Qing, W.; Xinmin, W.; Shuo, P. Study on the structure, pyrolysis kinetics, gas release, reaction mechanism, and pathways of Fushun oil shale and kerogen in China. Fuel Process. Technol. 2022, 225, 107058. [Google Scholar] [CrossRef]
- Xie, F. Fundamental Research on Oil Shale Pyrolysis Process; Institute of Process Engineering of Chinese Academy of Sciences: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Wang, T.; Lu, S.; Zhu, Y. Study on the thermal properties of Chinese oil shale. II. The measurement of specific heat of oil shale, char, and spent shale. J. Fuel Chem. Technol. 1987, 15, 311–316. (In Chinese) [Google Scholar]
- Lin, L.; Lai, D.; Guo, E.; Zhang, C.; Xu, G. Oil shale pyrolysis in indirectly heated fixed bed with metallic plates of heating enhancement. Fuel 2016, 163, 48–55. [Google Scholar] [CrossRef]
- Cormos, C. Evaluation of iron based chemical looping for hydrogen and electricity co-production by gasification process with carbon capture and storage. Int. J. Hydrog. Energy 2010, 35, 2278–2289. [Google Scholar] [CrossRef]
- Rydén, M.; Arjmand, M. Continuous hydrogen production via the steam-iron reaction by chemical looping in a circulating fluidized-bed reactor. Int. J. Hydrog. Energy 2012, 37, 4843–4854. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, Y.; Yang, Q.; Wang, D.; Li, H.; Li, G.; Yang, Y.; Fan, Z.; Ji, D.; Li, N.; et al. A new scheme for ammonia and fertilizer generation by coal direct chemical looping hydrogen process: Concept design, parameter optimization, and performance analysis. J. Clean. Prod. 2022, 363, 132445. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, S.; Yang, Q.; Huang, W.; Yu, P.; Zhang, D. Comparative techno-economic analysis of oil-based and coal-based ethylene glycol processes. Energy Convers. Manag. 2019, 198, 111814. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Zhang, J.; Xing, J.; Jia, J.; Cui, P. Development and techno-economic evaluation of coal to ethylene glycol process and allam power cycle and carbon capture and storage and integration process. Fuel 2023, 332, 126121. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Q.; Fan, Y.; Zhang, D.; Yu, J. Conceptual design and techno-economic analysis of a coproduction system for ethylene glycol and LNG from steel mill off-gases. Fuel 2022, 318, 123693. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Meng, W.; Wang, K.; Li, G.; Yang, Y.; Fan, Z.; Wang, D.; Ji, D. Comparative investigation of CO2-to-methanol process using different CO2 capture technologies. Fuel 2023, 338, 127359. [Google Scholar] [CrossRef]














| Proximate Analysis (wt.%, ar) | Elemental Analysis (wt.%, ar) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| M | FC | V | A | C | H | O | N | S | |
| Oil shale | 3.43 | 1.77 | 22.83 | 71.97 | 50.16 | 6.37 | 38.12 | 1.99 | 3.35 |
| Key Parameters | Value | Key Parameters | Value |
|---|---|---|---|
| OSR unit a | OGS unit c | ||
| Retorting temperature (°C) | 500 | Washing tower temperature (°C) | 118 |
| Retorting pressure (MPa) | 0.1 | Cooling tower temperature (°C) | 51 |
| Oil shale flow rate (t/h) | 375 | Electrostatic tower temperature (°C) | 58 |
| Ash/Oil shale (t/t) | SOH unit d | ||
| CLH unit b | Temperature (°C) | 360 e/390 f | |
| Fuel reactor’s temperature (°C) | 900 | Pressure (MPa) | 4.0 e/15.7 f |
| Fuel reactor’s pressure (MPa) | 3.0 | LHSV (h−1) | 1.0 e/0.8 f |
| Steam reactor’s temperature (°C) | 750 | V (H2)/V (Feed oil) | 430 e/800 f |
| Steam reactor’s pressure (MPa) | 3.0 | Catalyst | WNi e/MoNi f |
| Air reactor’s temperature (°C) | 1170 | CFB unit g | |
| Air reactor’s pressure (MPa) | 0.1 | Combustion temperature (°C) | 800 |
| n (Fe2O3)/m (oil shale) | 1.5 | Adiabatic efficiency (%) | 95 |
| n (H2O)/m (oil shale) | 5 | Isentropic efficiency (%) | 85 |
| Elemental Analyses (wt.%) | C | H | O | N | S |
|---|---|---|---|---|---|
| Shale oil | 84.60 | 11.16 | 2.73 | 1.14 | 0.37 |
| Semi-coke | 49.42 | 5.42 | 38.79 | 2.32 | 4.05 |
| Component | Mass Fraction (%) | |
|---|---|---|
| Simulation Data | Industrial Data | |
| Semi-coke | 80.67 | 81.01 |
| CO | 0.40 | 0.38 |
| CO2 | 3.93 | 3.91 |
| CH4 | 0.57 | 0.56 |
| C2H4 | 0.21 | - |
| C2H6 | 0.21 | - |
| C3H6 | 0.31 | - |
| C3H8 | 0.02 | - |
| H2 | 0.24 | 0.25 |
| NH3 | 0.01 | - |
| H2S | 0.05 | - |
| H2O | 3.92 | 3.90 |
| C15H12 | 9.45 | 9.28 |
| Stream | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Water (t/h) | 20.8 | 176.84 | 289.38 | 2.03 | 174.81 | 445.42 | 873.26 | 2.03 | 0.00 |
| Retorting gas (t/h) | 22.35 | 21.95 | 0.40 | 21.95 | 0.00 | 0.00 | 0.00 | 21.95 | 0.00 |
| Shale oil (t/h) | 35.44 | 14.98 | 20.46 | 6.42 | 8.56 | 0.00 | 0.00 | 3.19 | 32.25 |
| Total flow (t/h) | 78.59 | 213.77 | 310.24 | 30.4 | 183.37 | 445.42 | 873.26 | 27.17 | 32.25 |
| Temperature (°C) | 500 | 120 | 116 | 52 | 49 | 66 | 30 | 45 | 70 |
| Pressure (MPa) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Item | Price (CNY) | OLFH | OFFH | ||
|---|---|---|---|---|---|
| Output | Income a | Output | Income a | ||
| Gasoline (t) | 8150 | 4.5 × 104 | 369 | 5.7 × 104 | 466 |
| Diesel (t) | 6553 | 12.1 × 104 | 789 | 17.5 × 104 | 1142 |
| Residual (t) | 2080 | 7.9 × 104 | 165 | - | - |
| Hydrogen (t) | 23,000 | 3.0 × 103 | 68 | - | - |
| Electricity (kWh) | 0.5 | 8.7 × 107 | 44 | 8.7 × 107 | 44 |
| Total income | 1391 | 1608 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yang, Y.; Zhou, H. Chemical Looping Enhanced Oil Shale-to-Liquid Fuels Process: Modeling, Parameter Optimization, and Performance Analysis. Processes 2023, 11, 929. https://doi.org/10.3390/pr11030929
Wang Q, Yang Y, Zhou H. Chemical Looping Enhanced Oil Shale-to-Liquid Fuels Process: Modeling, Parameter Optimization, and Performance Analysis. Processes. 2023; 11(3):929. https://doi.org/10.3390/pr11030929
Chicago/Turabian StyleWang, Qiang, Yong Yang, and Huairong Zhou. 2023. "Chemical Looping Enhanced Oil Shale-to-Liquid Fuels Process: Modeling, Parameter Optimization, and Performance Analysis" Processes 11, no. 3: 929. https://doi.org/10.3390/pr11030929
APA StyleWang, Q., Yang, Y., & Zhou, H. (2023). Chemical Looping Enhanced Oil Shale-to-Liquid Fuels Process: Modeling, Parameter Optimization, and Performance Analysis. Processes, 11(3), 929. https://doi.org/10.3390/pr11030929









