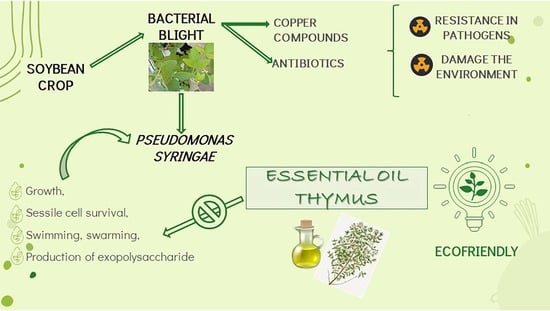
Inhibitory Potential of Thymus vulgaris Essential Oil against Growth, Biofilm Formation, Swarming, and Swimming in Pseudomonas syringae Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Media, and Culture Conditions
2.2. Essential Oil
2.3. Effect of TEO on the Growth of P. syringae
2.4. Effect of TEO on Sessile Cell Survival
2.5. Effect of TEO on the Production of Extracellular Polysaccharides
2.6. Effect of TEO on Swimming and Swarming Motility
2.7. Statistical Analysis
3. Results
3.1. Effect of TEO on the Growth of P. Syringae
3.2. Effects of TEO on Sessile Cell Survival
3.3. Effect of TEO on the Production of Extracellular Polysaccharides
3.4. Effect of TEO on Swimming and Swarming Motility
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buttimer, C.; McAuliffe, O.; Ross, R.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Gnanaprakasam, P.D.; Vanisree, A.J. Recurring detrimental impact of agrochemicals on the ecosystem and a look at organic agriculture as a possible rescue. Environ. Sci. Pollut. Res. 2022, 29, 75103–75112. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture-Trends and Challenges. Rome. 2017. Available online: https://efaidnbmnnnibpcajpcglclefindmkaj/https://www.fao.org/3/i6583e/i6583e.pdf (accessed on 7 December 2022).
- Martins, P.; Merfa, M.; Takita, M.; De Souza, A. Persistence in Phytopathogenic Bacteria: Do We Know Enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Baltrus, D.A.; Nishimura, M.T.; Romanchuk, A.; Chang, J.H.; Mukhtar, M.S.; Cherkis, K.; Roach, J.; Grant, S.R.; Jones, C.J.; Dangl, J.L. Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Pseudomonas syringae Isolates. PLoS Pathog. 2011, 7, 7. [Google Scholar] [CrossRef]
- Beoletto, V.G.; De Las Mercedes Oliva, M.; Marioli, J.M.; Carezzano, M.E.; Demo, M.S. Antimicrobial natural products against bacterial biofilms. In Antibiotic Resistance: Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Elsevier: London, UK, 2016; pp. 290–307. [Google Scholar]
- Carezzano, E.; Sotelo, J.; Primo, E.; Reinoso, E.; Paletti Rovey, M.F.; Demo, M.; Giordano, W.; Oliva, M.D.L.M. Inhibitory effect of Thymus vulgaris and Origanum vulgare EO on virulence factors of phytopathogenic Pseudomonas syringae strains. Plant Biol. 2017, 19, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Ruinelli, M.; Blom, J.; Smits, T.H.M.; Pothier, J.F. Comparative genomics and pathogenicity potential of members of the Pseudomonas syringae species complex on Prunus spp. BMC Genom. 2019, 20, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hockett, K.L.; Burch, A.Y.; Lindow, S.E. Thermo-Regulation of Genes Mediating Motility and Plant Interactions in Pseudomonas syringae. PLoS ONE 2013, 8, e59850. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Karig, D.; Kumar, A.; Ardekani, A.M. Interplay of Physical Mechanisms and Biofilm Processes: Review of Microfluidic Methods. Lab Chip. 2015, 15, 23–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Bogino, P.; Abod, A.; Nievas, F.; Giordano, W. Water-limiting conditions alter the structure and biofilm-forming ability of bacterial multispecies communities in the alfalfa rhizosphere. PLoS ONE 2013, 8, e79614. [Google Scholar] [CrossRef] [Green Version]
- Primo, E.; Bogino, P.; Cossovich, S.; Foresto, E.; Nievas, F.; Giordano, W. Exopolysaccharide II is relevant for the survival of Sinorhizobium meliloti under water deficiency and salinity stress. Molecules 2020, 25, 4876. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouteiller, M.; Dupont, C.; Bourigault, Y.; Latour, X.; Barbey, C.; Konto-Ghiorghi, Y.; Merieau, A. Pseudomonas Flagella: Generalities and Specificities. Int. J. Mol. Sci. 2021, 22, 3337. [Google Scholar] [CrossRef]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Schaad, N.W.; Jones, J.B.; Chun, W. Laboratory Guide for Identification of Plant Pathogenic Bacteria, 2nd ed.; The American Phytopathological Society Press: St. Paul, MN, USA, 2001. [Google Scholar]
- Ni, P.; Wang, L.; Deng, B.; Jiu, S.; Ma, C.; Zhang, C.; Almeida, A.; Wang, D.; Xu, W.; Wang, S. Combined application of bacteriophages and carvacrol in the control of Pseudomonas syringae pv actinidiae planktonic and biofilm forms. Microorganisms 2020, 8, 837. [Google Scholar] [CrossRef]
- Masak, J.; Cejková, A.; Schreiverová, O.; Rezanka, T. Pseudomonas biofilms: Possibilities of their control. FEMS Microbiol. Ecol. 2014, 89, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Topa, S.; Subramoni, S.; Palombo, E.; Kingshott, P.; Rice, S.; Blackall, L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097. [Google Scholar] [CrossRef]
- Soylu, S.; Soylu, E.M.; Bozkurt, A.; Kaya, A.D. Antibacterial activities of EO from oregano, thyme, rosemary and lavender plants against Pseudomonas savastanoi pv phaseolicola, the causal agent of halo blight of bean. Ovidius Univ. Ann. Med. Sci. -Pharm. 2003, 1, 40–44. [Google Scholar]
- Vasinauskiene, M.; Radusiene, J.; Zitikaite, I.; Surviliene, E. Antibacterial activities of EO from aromatic and medicinal plants against growth of phytopathogenic bacteria. Agron. Res. 2006, 4, 437–440. [Google Scholar]
- Oliva, M.M.; Carezzano, M.E.; Giuliano, M.; Daghero, J.; Zygadlo, J.; Bogino, P.; Giordano, W.; Demo, M. Antimicrobial activity of EO of Thymus vulgaris and Origanum vulgare on phytopathogenic strains isolated from soybean. Plant Biol. 2014, 17, 758–765. [Google Scholar] [CrossRef]
- Sotelo, J.P.; Oddino, C.; Giordano, D.F.; Carezzano, M.E.; Oliva, M.D.L.M. Effect of Thymus vulgaris essential oil on soybeans seeds infected with Pseudomonas syringae. Physiol. Mol. Plant Pathol. 2021, 116, 101735. [Google Scholar] [CrossRef]
- Wu, J.E.; Lin, J.; Zhong, Q. Physical and antimicrobial characteristics of thyme oil emulsified with soluble soybean polysaccharide. Food Hydrocoll. 2019, 39, 144–150. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.M.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Lizardi-Mendoza, J.; Madera-Santana, T.J.; Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Ayala-Zavala, J.F. Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control. 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. South Afr. J. Bot. 2016, 103, 89–94. [Google Scholar] [CrossRef]
- Cady, N.C.; McKean, K.A.; Behnke, J.; Kubec, R.; Mosier, A.P.; Kasper, S.H.; Burz, D.S.; Musah, R.A. Inhibition of Biofilm Formation, Quorum Sensing and Infection in Pseudomonas aeruginosa by Natural Products-Inspired Organosulfur Compounds. PLoS ONE 2012, 7, e38492. [Google Scholar] [CrossRef] [Green Version]
- Ude, S.; Arnold, D.L.; Moon, C.D.; Timms-Wilson, T.; Spiers, A.J. Biofilm formation and cellulose expression among diverse environmental Pseudomonas isolates. Environ. Microbiol. 2006, 8, 1997–2011. [Google Scholar] [CrossRef]
- Grace, A.; Sahu, R.; Owen, D.R.; Dennis, V.A. Pseudomonas aeruginosa reference strains PAO1 and PA14: A genomic, phenotypic, and therapeutic review. Front. Microbiol. 2022, 13, 1023523. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mendoza, D.; Aragon, I.; Ramirez, H.; Jimenez, L.; Ramos, C.; Gallegos, M.; Sanjuan, J. Responses to Elevated c-di-GMP Levels in Mutualistic and Pathogenic Plant-Interacting Bacteria. PLoS ONE 2014, 9, e91645. [Google Scholar] [CrossRef] [Green Version]
- Quiñones, B.; Dulla, G.; Lindow, S. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. MPMI 2005, 18, 682–693. [Google Scholar] [CrossRef] [Green Version]
- Krishna, P.S.; Woodcock, S.D.; Pfeilmeier, S.; Bornemann, S.; Zipfel, C.; Malone, J.G. Pseudomonas syringae addresses distinct environmental challenges during plant infection through the coordinated deployment of polysaccharides. J. Exp. Bot. 2022, 73, 2206–2221. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Iacobellis, N.; De Marco, N.; Capasso, F.; Senatore, F. Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller Var. vulgare (Miller) EO. J. Agric. Food Chem. 2004, 52, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Kokoskova, B.; Pouvova, D.; Pavela, R. Effectiveness of plant EO against Erwinia amylovora, Pseudomona syringae pv syringae and associated saprophytic bacteria on/in host plants. J. Plant Pathol. 2011, 93, 133–139. [Google Scholar]
- Zhang, L.; Gao, F.; Ge, J.; Li, H.; Xia, F.; Bai, H.; Piao, X.; Shi, L. Potential of aromatic plant-derived essential oils for the control of foodborne bacteria and antibiotic resistance in animal production: A review. Antibiotics 2022, 11, 1673. [Google Scholar] [CrossRef] [PubMed]
- Rudilla, H.; Merlos, A.; Sans-Serramitjana, E.; Fuste, E.; Sierra, J.M.; Zalacain, A.; Vinuesa, T.; Vinas, M. New and old tools to evaluate new antimicrobial peptides. AIMS Microbiol. 2018, 4, 522–540. [Google Scholar] [CrossRef]
- Yourassowsky, E.; Van Der Linden, M.P.; Lismont, M.J.; Crokaert, F.; Glupczynski, Y. Correlation between growth curve and killing curve of Escherichia coli after a brief exposure to suprainhibitory concentrations of ampicillin and piperacillin. Antimicrob. Agents Chemother. 1985, 28, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Othman, A.S. Determination of the antibacterial effect of some natural products against some gram-positive and gram-negative bacteria. Egypt. Pharm. J. 2016, 15, 10–16. [Google Scholar] [CrossRef]
- Araya-Contreras, T.; Veas, R.; Escobar, C.A.; Machuca, P.; Bittner, M. Antibacterial effect of Luma apiculata (DC.) burret extracts in clinically important bacteria. Int. J. Microbiol. 2019, 10, 7803726. [Google Scholar] [CrossRef] [Green Version]
- Veldhuizen, T.J.A.; Tjeerdsma-van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural requirements for the antimicrobial activity of carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Donlan, R.D.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Funari, R.; Shen, A.Q. Detection and characterization of bacterial biofilms and biofilm-based sensors. ACS Sensors 2022, 7, 347–357. [Google Scholar] [CrossRef]
- Monier, J.M.; Lindow, S.E. Aggregates of resident bacteria facilitate survival of immigrant bacteria on leaf surfaces. Microb. Ecol. 2005, 49, 343–352. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control. 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Myszka, K.; Schmidt, M.T.; Majcher, M.; Juzwa, W.; Olkowicz, M.; Czaczyk, K. Inhibition of quorum sensing-related biofilm of Pseudomonas fluorescens KM121 by Thymus vulgare EO and its major bioactive compounds. Int. Biodeterior. Biodegrad. 2016, 114, 252–259. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin-An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Pandey, H.; Narvi, S.S.; Agarwal, V. Effect of cinnamon oil on quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa. PLoS ONE 2015, 10, e0135495. [Google Scholar] [CrossRef] [Green Version]
- Ariel, G.; Rabani, A.; Benisty, S.; Partridge, J.D.; Harshey, R.M.; Be’er, A. Swarming bacteria migrate by Lévy Walk. Nat. Commun. 2015, 6, 8396. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, F.; Yamamoto, M.; Ohnishi-Kameyama, M.; Iwaki, M.; Yoshida, M.; Ishii, T.; Konishi, T.; Ichinose, Y. Defects in flagellin glycosylation affect the virulence of Pseudomonas syringae pv tabaci 6605. Microbiology 2010, 156, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Datta, S.; Narayanan, K.B.; Narayanan Rajnish, K. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Tomihama, T.; Nishi, Y.; Arai, K. Biofilm formation and resistance to bactericides of Pseudomonas syringae pv theae. J. Gen. Plant Pathol. 2006, 73, 193–196. [Google Scholar] [CrossRef]









| Strains | Source | Accession N° | Reference |
|---|---|---|---|
| Pseudomonas syringae C13LS | Soybean | KJ569375 | [23] |
| Pseudomonas syringae EM1 | Soybean | KJ569377 | [23] |
| Pseudomonas syringae LS3 | Soybean | KJ569373 | [23] |
| Pseudomonas syringae Q | Soybean | KJ569372 | [23] |
| Compounds | Thymus vulgaris EO |
|---|---|
| α thujene | 1.7 |
| α pinene | 1.6 |
| α fenchene | 0.8 |
| β pinene | 1.1 |
| myrcene | 1.8 |
| 3-octanol | Tr |
| α phellandrene | Tr |
| 3-carene | Tr |
| α terpinene | 1.7 |
| p-cymene | 31.5 |
| 1,8-cineole | 2.4 |
| γ terpinene | 11.3 |
| terpinolene | 1.5 |
| para-cymenene | Tr |
| linalool | 3.5 |
| cis sabinene hydrate | Tr |
| camphor | Tr |
| borneol | 1 |
| 4-terpineol | 1.2 |
| p-cymen-8-ol | Tr |
| α terpineol | Tr |
| thymol methyl ether | 1.7 |
| geraniol | Tr |
| geranial | Tr |
| thymol | 1 |
| carvacrol | 29.5 |
| isobornyl acetate | Tr |
| α copaene | Tr |
| β bourbonene | Tr |
| longifolene | 3.6 |
| α cadinene | 1.1 |
| γ muurolene | Tr |
| γ cadinene | Tr |
| δ cadinene | Tr |
| cis calamenene 1S | Tr |
| oxide caryophyllene | 1.3 |
| 99.3 |
| Strains | Source | T. vulgaris (0.022–45.99 mg/mL) | |
|---|---|---|---|
| MIC | MBC | ||
| P. syringae C13LSa | Soybean | 11.5 * | 5.7 |
| P. syringae EM1 | Soybean | 11.5 * | 0.71 |
| P. syringae LS3 | Soybean | 11.5 * | 0.17 |
| P. syringae Q | Soybean | 11.5 * | 0.71 |
| P. savastanoi pv. glycinea B076 | Soybean | 5.8 ** | 0.17 |
| P. aeruginosa PAO1 | 11.5 | 23 | |
| Strains | Control | 23 | 11.5 | 5.7 | 2.9 |
| P. savastanoi pv. glycinea B076 | ++ | - | + | ++ | ++ |
| P. syringae Q | +++ | - | + | ++ | ++ |
| P. aeruginosa PAO1 | +++ | ++ | ++ | ++ | +++ |
| Strains | Control | 23 | 11.5 | 5.7 | 2.9 |
| P. savastanoi pv. glycinea B076 | +++ | - | + | ++ | ++ |
| P. syringae Q | +++ | + | + | + | ++ |
| P. aeruginosa PAO1 | +++ | + | + | ++ | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carezzano, M.E.; Paletti Rovey, M.F.; Sotelo, J.P.; Giordano, M.; Bogino, P.; Oliva, M.d.l.M.; Giordano, W. Inhibitory Potential of Thymus vulgaris Essential Oil against Growth, Biofilm Formation, Swarming, and Swimming in Pseudomonas syringae Isolates. Processes 2023, 11, 933. https://doi.org/10.3390/pr11030933
Carezzano ME, Paletti Rovey MF, Sotelo JP, Giordano M, Bogino P, Oliva MdlM, Giordano W. Inhibitory Potential of Thymus vulgaris Essential Oil against Growth, Biofilm Formation, Swarming, and Swimming in Pseudomonas syringae Isolates. Processes. 2023; 11(3):933. https://doi.org/10.3390/pr11030933
Chicago/Turabian StyleCarezzano, María Evangelina, María Fernanda Paletti Rovey, Jesica P. Sotelo, Melina Giordano, Pablo Bogino, María de las Mercedes Oliva, and Walter Giordano. 2023. "Inhibitory Potential of Thymus vulgaris Essential Oil against Growth, Biofilm Formation, Swarming, and Swimming in Pseudomonas syringae Isolates" Processes 11, no. 3: 933. https://doi.org/10.3390/pr11030933