Synthesis of CaWO4 as a Photocatalyst for Degradation of Methylene Blue and Carmine under Ultraviolet Light Irradiation
Abstract
:1. Introduction
2. Experimental Methods
2.1. Chemicals
2.2. Preparation Catalyst
2.3. Catalyst Characterization
2.4. Photocatalytic Degradation of MB and CR
3. Result and Discussion
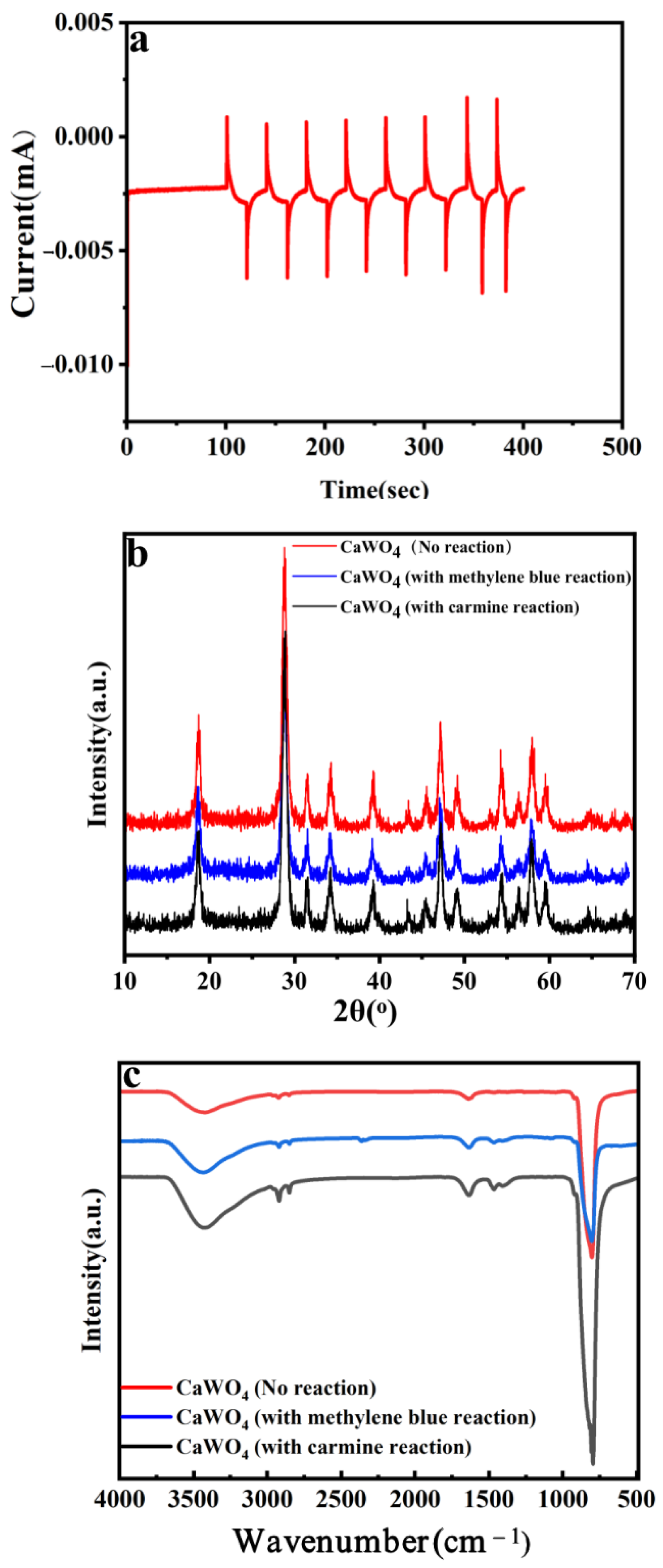
3.1. Characterization
3.2. Degradation of MB
3.2.1. Comparison Experiment and TOC Removal
3.2.2. Effect of Reaction Conditions
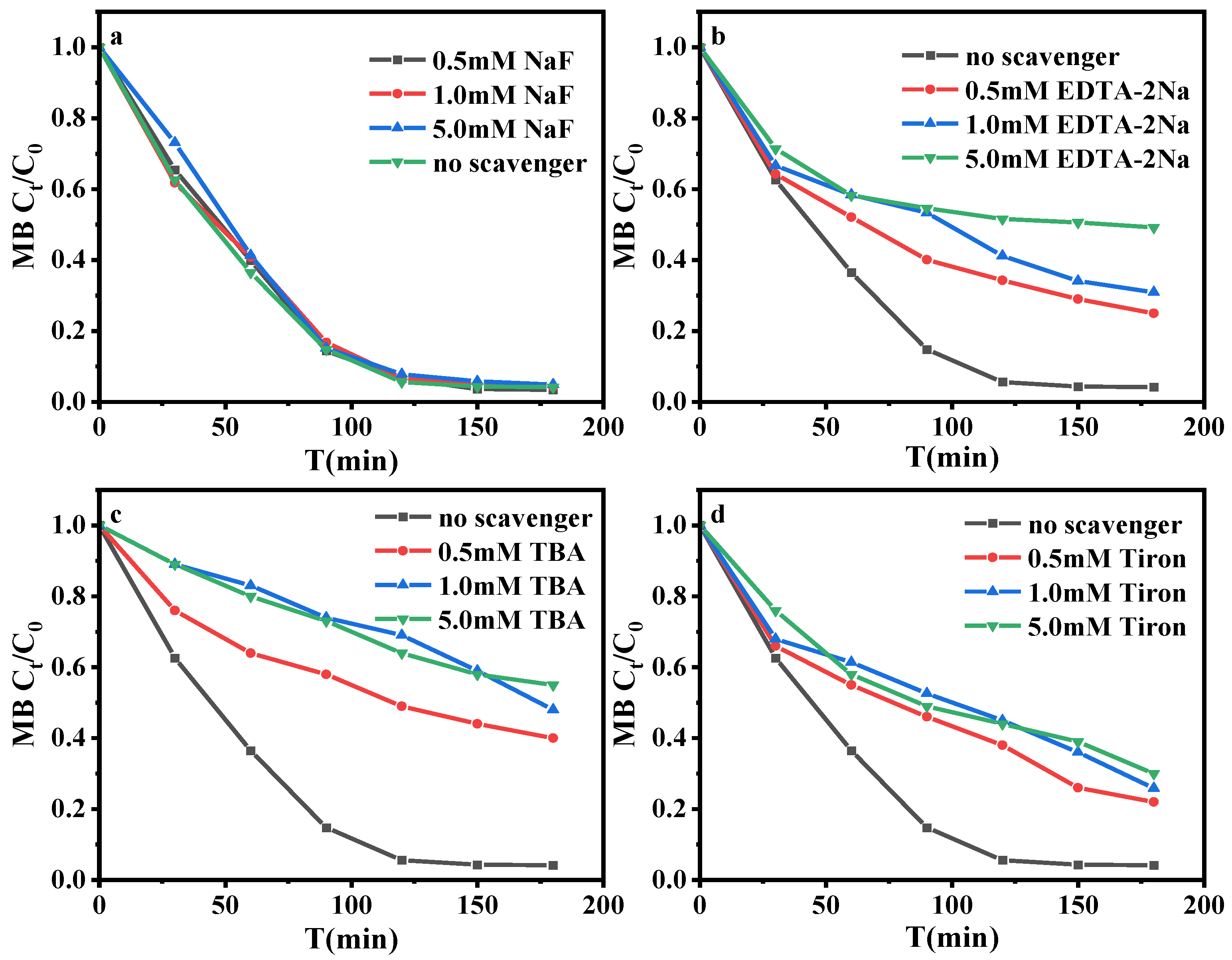
3.2.3. Identification of Reactive Species
3.3. Degradation of CR
3.3.1. Comparison Experiment and TOC Removal
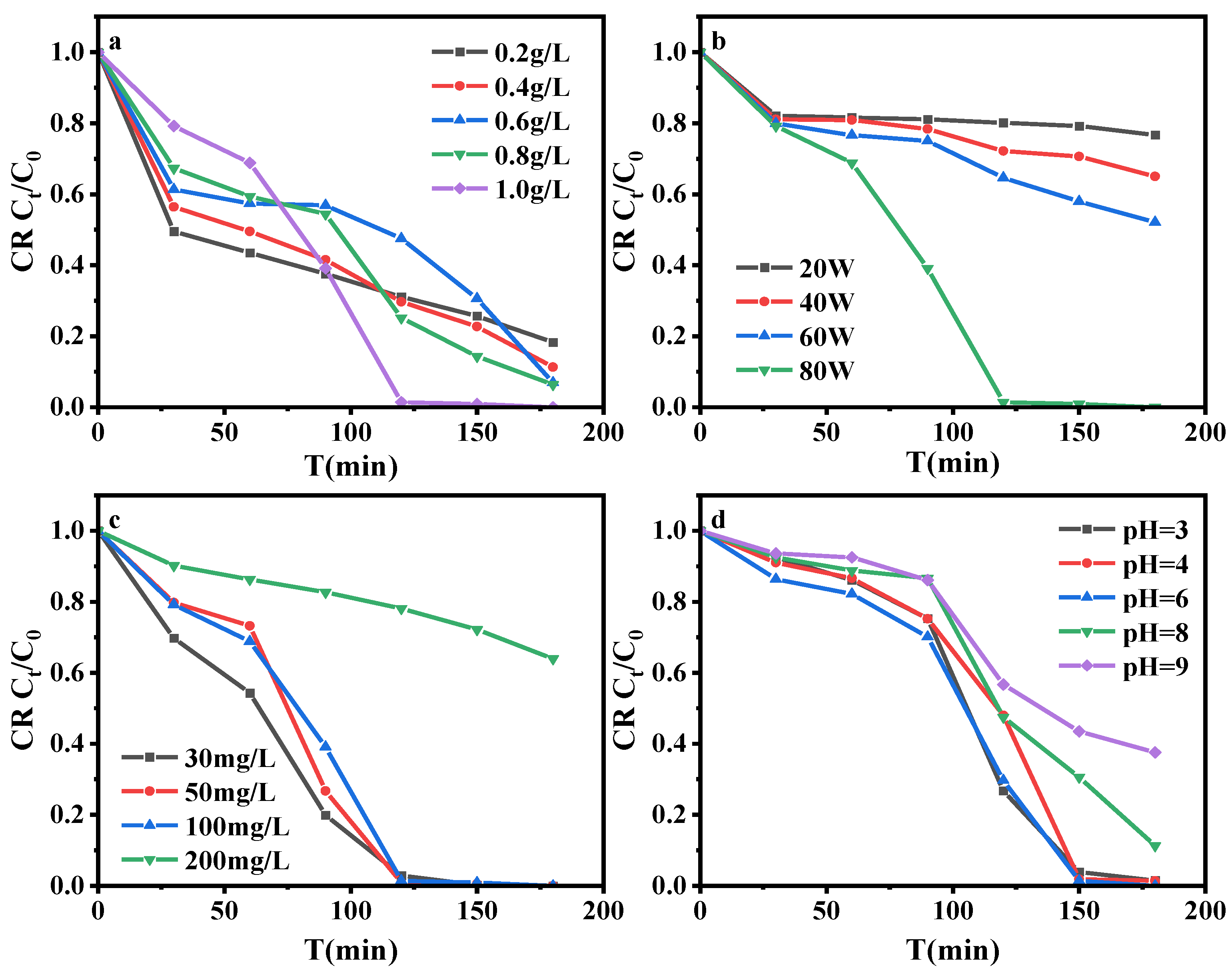
3.3.2. Effect of Reaction Conditions
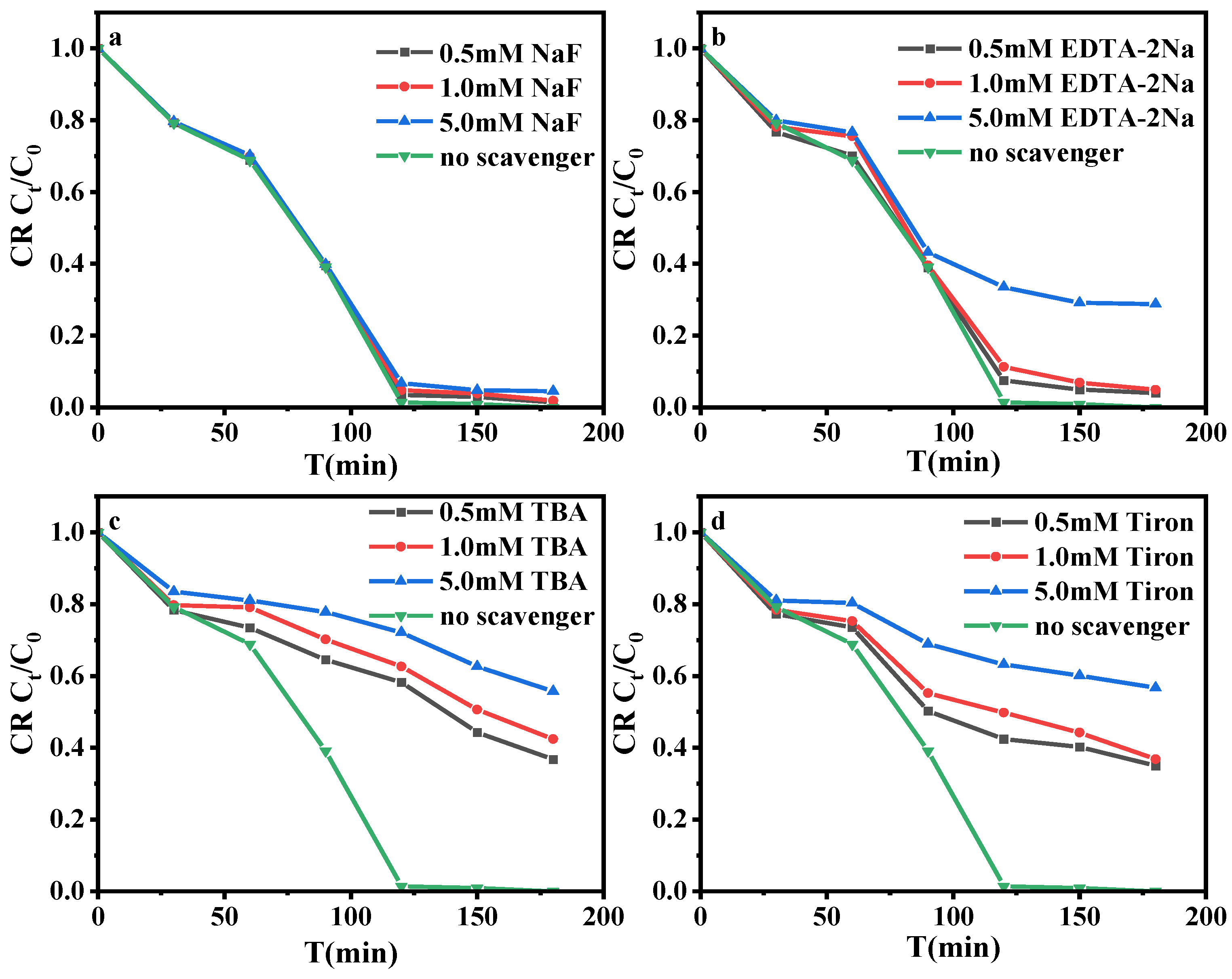
3.3.3. Identification of Reactive Species
3.4. Photocatalytic Activity
3.5. Study on Photocatalytic Mechanism
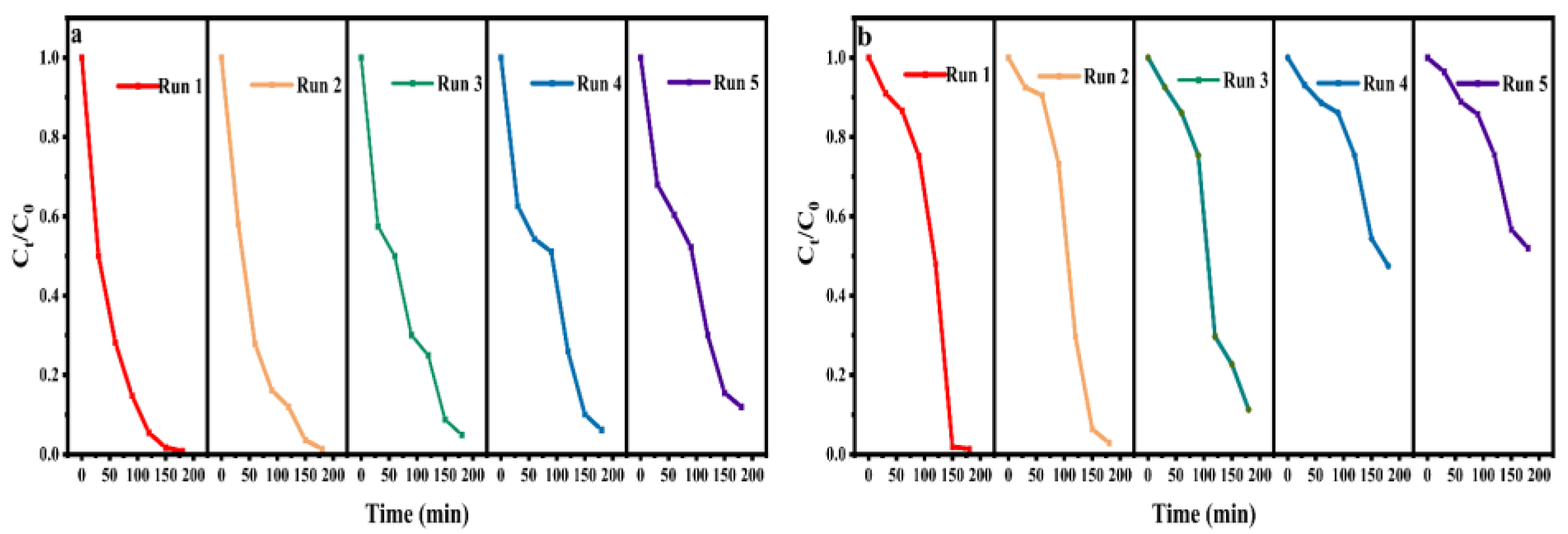
3.6. Reusability of CaWO4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garg, A.; Chopra, L. Dye Waste: A significant environmental hazard. Mater. Today 2022, 48, 1310–1315. [Google Scholar] [CrossRef]
- Verma, S.; Rao, B.T.; Singh, R.; Kaul, R. Photocatalytic degradation kinetics of cationic and anionic dyes using Au–ZnO nanorods: Role of pH for selective and simultaneous degradation of binary dye mixtures. Ceram. Int. 2021, 47, 34751–34764. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.; Jeon, B.; Park, Y. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Kruusma, J.; Mould, N.; Jurkschat, K.; Crossley, A.; Banks, C.E. Single walled carbon nanotubes contain residual iron oxide impurities which can dominate their electrochemical activity. Electrochem. Commun. 2007, 9, 2330–2333. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Huang, D.; Zeng, G.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; Xiong, W.; Wen, M.; et al. Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven. Appl. Catal. B Environ. 2018, 220, 202–210. [Google Scholar] [CrossRef]
- Baklanova, I.V.; Zhukov, V.P.; Krasil’nikov, V.N.; Gyrdasova, O.I.; Buldakova, L.Y.; Shalaeva, E.V.; Polyakov, E.V.; Kuznetsov, M.V.; Shein, I.R.; Vovkotrub, E.G. Fe and C doped TiO2 with different aggregate architecture: Synthesis, optical, spectral and photocatalytic properties, first-principle calculation. J. Phys. Chem. Solids 2017, 111, 473–486. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Xie, C.; Wang, Y.; Wei, L.; Yang, J. Synthesis and photocatalytic mechanism of visible-light-driven Ag/AgBr/(I/S) composites for organic dyes degradation. Opt. Mater. 2022, 123, 111947. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, Y.; Wang, Q.; Wang, Z.; Guan, R.; Shi, W. Potassium gluconate-cooperative pore generation based on g-C3N4 nanosheets for highly efficient photocatalytic hydrogen production and antibiotic degradation. J. Environ. Chem. Eng. 2022, 10, 107986. [Google Scholar] [CrossRef]
- Benz, D.; Bui, H.V.; Hintzen, H.T.; Kreutzer, M.T.; Ommen, J.R. Mechanistic insight into the improved photocatalytic degradation of dyes for an ultrathin coating of SiO2 on TiO2 (P25) nanoparticles. Chem. Eng. J. 2022, 10, 100288. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Xu, C.; Feng, L. Sonocatalysis and sono-photocatalysis in CaCu3Ti4O12 ceramics. Ceram. Int. 2022, 48, 11338–11345. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Song, J.; Zhang, G.; Li, C.; Zheng, S. Synthesis of novel ternary heterogeneous BiOCl/TiO2/sepiolite composite with enhanced visible-light-induced photocatalytic activity towards tetracycline. J. Colloid Interface Sci. 2019, 533, 238–250. [Google Scholar] [CrossRef]
- Jia, Z.; Li, T.; Zheng, Z.; Zhang, J.; Liu, J.; Li, R.; Wang, Y.; Zhang, X.; Wang, Y.; Fan, C. The BiOCl/diatomite composites for rapid photocatalytic degradation of ciprofloxacin: Efficiency, toxicity evaluation, mechanisms and pathways. Chem. Eng. J. 2020, 380, 122422. [Google Scholar] [CrossRef]
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Gao, H.; Wang, S.; Wang, Y.; Yang, H.; Fang, L.; Chen, X.; Yi, Z.; Li, D. Fabrication and Characterization of BaMoO4-Coupled CaWO4 Heterojunction Micro/Nanocomposites with Enhanced Photocatalytic Activity Towards MB and CIP Degradation. J. Electron. Mater. 2022, 51, 5230–5245. [Google Scholar] [CrossRef]
- Shimi, A.K.; Parvathiraj, C.; Kumari, S.; Dalal, J.; Kumar, V.; Wabaidur, S.M.; Alothman, Z.A. Green synthesis of SrO nanoparticles using leat extract of Albizia julibrissin and its recyclable photocatalytic activity: An eco-friendly approach for treatment of industrial wastewater. Environ. Sci. Adv. 2022, 5, 597–866. [Google Scholar]
- Chen, Z.; Gong, Q.; Zhu, J.; Yuan, Y.P.; Qian, L.W.; Qian, X.F. Controllable synthesis of hierarchical nanostructures of CaWO4 and SrWO4 via a facile low-temperature route. Mater. Res. Bull. 2009, 44, 45–50. [Google Scholar] [CrossRef]
- Duklan, N.; Chamoli, P.; Raina, K.K.; Shukla, R.K. Effect of UV light irradiation, pH and concentration on the dye sequestration efficiency of anionic surfactant based self-assembled aqueous mesophases. Surf. Interfaces 2022, 28, 101629. [Google Scholar] [CrossRef]
- Farsi, H.; Barzgari, Z.; Askari, S.Z. Sunlight-induced photocatalytic activity of nanostructured calcium tungstate for methylene blue degradation. Res. Chem. Intermed. 2015, 41, 5463–5474. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, R.; Zhang, Q.; Chen, Y.; Sharifi, O.; Leszczynska, D.; Zhang, R.; Dai, Q. Synthesis of CaWO4-biochar nanocomposites for organic dye removal. Mater. Res. Bull. 2019, 110, 169–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Kang, L.; Yang, M.; Zhang, K. A new CaWO4/alkali-activated blast furnace slag-based cementitious composite for production of hydrogen. Int. J. Hydrogen Energy 2017, 42, 3690–3697. [Google Scholar] [CrossRef]
- Wen, D.; Li, W.; Lv, J.; Qiang, Z.; Li, M. Methylene blue degradation by the VUV/UV/persulfate process: Effect of pH on the roles of photolysis and oxidation. J. Hazard. Mater. 2020, 391, 121855. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Fu, C.; Juang, R. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Wen, X.; Tang, L. One-dimensional copolymer nanostructures loaded with silver nanoparticles fabricated via metallogel template copolymerization and their pH dependent photocatalytic degradation of methylene blue. J. Mol. Catal. A Chem. 2015, 399, 86–96. [Google Scholar] [CrossRef]
- Kedves, E.; Bárdos, E.; Gyulavári, T.; Pap, Z.; Hernadi, K.; Baia, L. Dependence of cationic dyes’ adsorption upon α-MoO3 structural properties. Appl. Surf. Sci. 2022, 573, 151584. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Bi, Z.; Chen, X.; Hu, X.; Pan, W. Recent advances and perspectives of g-C3N4-based materials for photocatalytic dyes degradation. Chemosphere 2022, 295, 133834. [Google Scholar] [CrossRef]
- Sun, F.; Chen, T.; Liu, H.; Zou, X.; Zhai, P.; Chu, Z.; Shu, D.; Wang, H.; Chen, D. The pH-dependent degradation of sulfadiazine using natural siderite activating PDS: The role of singlet oxygen. Sci. Total Environ. 2021, 784, 147117. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D.; Pan, J.; Kong, X.; Liu, Y.; Sun, L.; Wang, L.; Li, D. Overexpression of a multiple stress-responsive gene, ZmMPK4, enhances tolerance to low temperature in transgenic tobacco. Plant Physiol. Biochem. 2012, 58, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Liu, H.; Wang, H.; Sun, F.; Zou, X.; Chen, T.; Chu, Z.; Chen, D.; Zhou, Y.; Xie, Q. Impact of the interaction between hematite and halloysite on environmental fate of organic pollutants. Appl. Clay Sci. 2021, 209, 106123. [Google Scholar] [CrossRef]
- Othman, I.; Mohamed, R.M.; Ibrahem, F.M. Study of photocatalytic oxidation of indigo carmine dye on Mn-supported TiO2. J. Photochem. Photobiol. A 2007, 189, 80–85. [Google Scholar] [CrossRef]
- Sahel, K.; Perol, N.; Dappozze, F.; Bouhent, M.; Derriche, Z.; Guillard, C. Photocatalytic degradation of a mixture of two anionic dyes: Procion Red MX-5B and Remazol Black 5 (RB5). J. Photochem. Photobiol. A 2010, 212, 107–112. [Google Scholar] [CrossRef]
- Barka, N.; Assabbane, A.; Nounah, A.; Ichou, Y.A. Photocatalytic degradation of indigo carmine in aqueous solution by TiO2-coated non-woven fibres. J. Hazard. Mater. 2008, 152, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.; Brillas, E.; Centellas, F.; Cabot, P.L.; Sirés, I. Electrochemical reactivity of Ponceau 4R (food additive E124) in different electrolytes and batch cells. Electrochim. Acta 2015, 173, 523–533. [Google Scholar] [CrossRef]
- Li, K.; Dong, C.; Zhang, Y.; Wei, H.; Zhao, F.; Wang, Q. Ag–AgBr/CaWO4 composite microsphere as an efficient photocatalyst for degradation of Acid Red 18 under visible light irradiation: Affecting factors, kinetics and mechanism. J. Mol. Catal. A Chem. 2014, 394, 105–113. [Google Scholar] [CrossRef]
- Jayakrishnan, C.; Sheeja, S.R.; Duraimurugan, J.; Prabhu, S.; Ramesh, R.; Kumar, G.S.; Subhash, K.G.; Shkir, M.; Pallavolu, M.R. Synthesis of dumbbell-shaped ZnO nanostructures for energy storage and photocatalytic dye degradation applications. Mate Technol. 2022, 37, 3006–3016. [Google Scholar] [CrossRef]
- Sampath, S.; Rohini, V.; Chinnasamy, K.; Ponnusamy, P.; Thangarasau, S.; Kim, W.K.; Reddy Minnam Reddy, V.; Shkir, M.; Maiz, F. Solvothermal synthesis of magnetically separable Co–ZnO nanowires for visible light driven photocatalytic applications. Phys. B 2023, 652, 414654. [Google Scholar] [CrossRef]
- Atran, A.A.; Ibrahim, F.A.; Awwad, N.S.; Shkir, M.; Hamdy, M.S. Facial One-Pot Synthesis, Characterization, and Photocatalytic Performance of Porous Ceria. Catalysts 2023, 13, 240. [Google Scholar] [CrossRef]
- Ranjith, R.; Vignesh, S.; Balachandar, R.; Suganthi, S.; Raj, V.; Ramasundaram, S.; Sundar, J.K.; Shkir, M.; Oh, T.H. Construction of novel g-C3N4 coupled efficient Bi2O3 nanoparticles for improved Z-scheme photocatalytic removal of environmental wastewater contaminant: Insight mechanism. J. Environ. Manag. 2023, 330, 117134. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Z.; Li, X.; Yang, Y.; Gao, J.; Yu, R.; Wang, H.; Wang, N. Synergistically boosting sulfamerazine degradation via activation of peroxydisulfate by photocatalysis of Bi2O3-TiO2/PAC under visible light irradiation. Chem. Eng. J. 2022, 428, 132613. [Google Scholar] [CrossRef]
- Syed, A.; Al-Shwaiman, H.A.; Al Khulaifi, M.M.; Al Zahrani, R.R.; Almajhdi, F.N.; Elgorban, A.M. Integrating plasmonic effect and nano-heterojunction formation for boosted light harvesting and photocatalytic performance using CaWO4/Ag2MoO4 and its antibacterial applications. Mat. Sci. Semicond. Proc. 2021, 133, 105921. [Google Scholar] [CrossRef]
- Ayappan, C.; Palanivel, B.; Jayaraman, V.; Maiyalagan, T.; Mani, A. One-step hydrothermal synthesis of CaWO4/α-Ag2WO4 heterojunction: An efficient photocatalyst for removal of organic contaminants. Mat. Sci. Semicond. Proc. 2019, 104, 104693. [Google Scholar] [CrossRef]
- Mahendra, K.; Fernandes, J.M.; Fernandes, B.J.; Bindu, K.; Ramesh, K.P. Photocatalytic degradation of organic textile dyes using tellurium-based metal alloy. Vacuum 2022, 199, 110960. [Google Scholar]
- Zhai, P.; Liu, H.; Sun, F.; Chen, T.; Zou, X.; Wang, H.; Chu, Z.; Wang, C.; Liu, M.; Chen, D. Carbonization of methylene blue adsorbed on palygorskite for activating peroxydisulfate to degrade bisphenol A: An electron transfer mechanism. Appl. Clay Sci. 2022, 216, 106327. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Zhang, Y.; Geng, H.; Liu, Y.; Wang, H. Photo-assisted Fe2+ modified molybdenum disulfide activated potassium persulfate to degrade sulfadiazine: Insights into the degradation pathway and mechanism from density functional theory. Chem. Eng. J. 2022, 435, 134904. [Google Scholar] [CrossRef]


| Material | Doping | Oxidant | Efficiency |
|---|---|---|---|
| ZnO | undesired | undesired | 91% (5 mg/L MB) within 180 min |
| Co-ZnO | requisite | undesired | 93% (10 mg/L MO) within 180 min |
| g-C3N4/Bi2O3 | requisite | undesired | 88.5%(16.6 mg/L Rh B) within 180 min |
| CeO2 | undesired | undesired | 40% (10 mg/L MG) within 120 min |
| Bi2O3-TiO2 | requisite | PDS (1 g/L) | 97.96% (100 mg/L SMZ) within 120 min |
| CaWO4 | undesired | undesired | 98% (100 mg/L MB) within 180 min 99% (100 mg/L CR) within 180 min |
| CaWO4/Ag2MoO2 | requisite | undesired | 91.4% (40 mg/L MB) within 180 min |
| CaWO4/α-AgWO4 | requisite | undesired | 85.84%(200 mg/L MB) within 105 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Sun, F.; Liu, H.; Chen, T.; Chu, Z.; Wang, H.; Zou, X.; Zhai, P.; Chen, D. Synthesis of CaWO4 as a Photocatalyst for Degradation of Methylene Blue and Carmine under Ultraviolet Light Irradiation. Processes 2023, 11, 1050. https://doi.org/10.3390/pr11041050
Yuan C, Sun F, Liu H, Chen T, Chu Z, Wang H, Zou X, Zhai P, Chen D. Synthesis of CaWO4 as a Photocatalyst for Degradation of Methylene Blue and Carmine under Ultraviolet Light Irradiation. Processes. 2023; 11(4):1050. https://doi.org/10.3390/pr11041050
Chicago/Turabian StyleYuan, Cunyang, Fuwei Sun, Haibo Liu, Tianhu Chen, Ziyang Chu, Hanlin Wang, Xuehua Zou, Peixun Zhai, and Dong Chen. 2023. "Synthesis of CaWO4 as a Photocatalyst for Degradation of Methylene Blue and Carmine under Ultraviolet Light Irradiation" Processes 11, no. 4: 1050. https://doi.org/10.3390/pr11041050





