Dual Role of Sugarcane Waste in Benthic Microbial Fuel to Produce Energy with Degradation of Metals and Chemical Oxygen Demand
Abstract
: external resistance with 458
external resistance with 458  internal resistance. This demonstrates that electrons are effectively transported throughout the operation. The Bacillus strains are the most dominant bacterial community on the surface of the anode. This research’s mechanism, which involves metal ion degradation, is also explained. Finally, parameter optimization indicated that pH 7 works efficiently. In addition to that, there are some future perspectives and concluding remarks enclosed.
internal resistance. This demonstrates that electrons are effectively transported throughout the operation. The Bacillus strains are the most dominant bacterial community on the surface of the anode. This research’s mechanism, which involves metal ion degradation, is also explained. Finally, parameter optimization indicated that pH 7 works efficiently. In addition to that, there are some future perspectives and concluding remarks enclosed.1. Introduction
2. Methods and Experimentation
2.1. Chemical Materials
2.2. Benthic Microbial Fuel Cell Assembly and Process
 was the attached external resistance. We briefly utilized a threshold of 1000
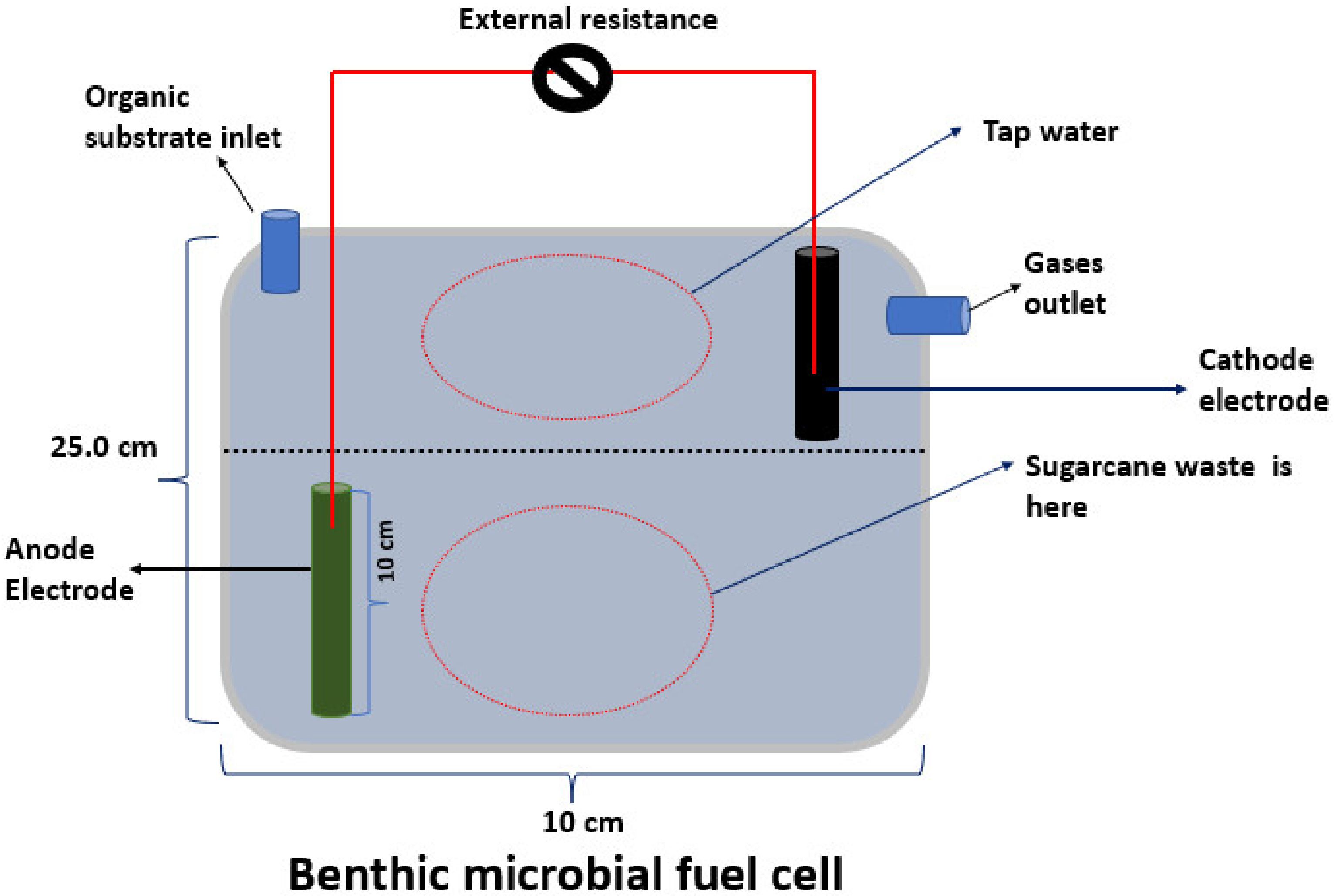
was the attached external resistance. We briefly utilized a threshold of 1000  while deciding on a certain external resistance for the first time. We observed a decrease in the voltage of the closed circuit relative to the voltage of the open circuit throughout this period. This voltage did, however, ultimately begin to rise steadily. In general terms, one should choose a greater resistance if there is no sign of a recovery and a lower resistance if there are no apparent changes. Furthermore, the experiment was conducted three times to ensure consistency of results. Figure 1 illustrates the pectoral BMFC presentation that was used in this research.
while deciding on a certain external resistance for the first time. We observed a decrease in the voltage of the closed circuit relative to the voltage of the open circuit throughout this period. This voltage did, however, ultimately begin to rise steadily. In general terms, one should choose a greater resistance if there is no sign of a recovery and a lower resistance if there are no apparent changes. Furthermore, the experiment was conducted three times to ensure consistency of results. Figure 1 illustrates the pectoral BMFC presentation that was used in this research.2.3. Electrochemical, Chemical, and Biological Analysis
 , 4000
, 4000  , 3000
, 3000  , 2000
, 2000  , 1000
, 1000  , 500
, 500  , and 100
, and 100  ). After the reaction had reached a condition that was deemed to be pseudo-steady, a study of the polarization curve was carried out. When investigating the polarization behavior, a variation time of at least half an hour is required, on average.
). After the reaction had reached a condition that was deemed to be pseudo-steady, a study of the polarization curve was carried out. When investigating the polarization behavior, a variation time of at least half an hour is required, on average.2.4. Parameter Optimization Study
3. Results and Their Significant Discussion
3.1. Electrochemical Results
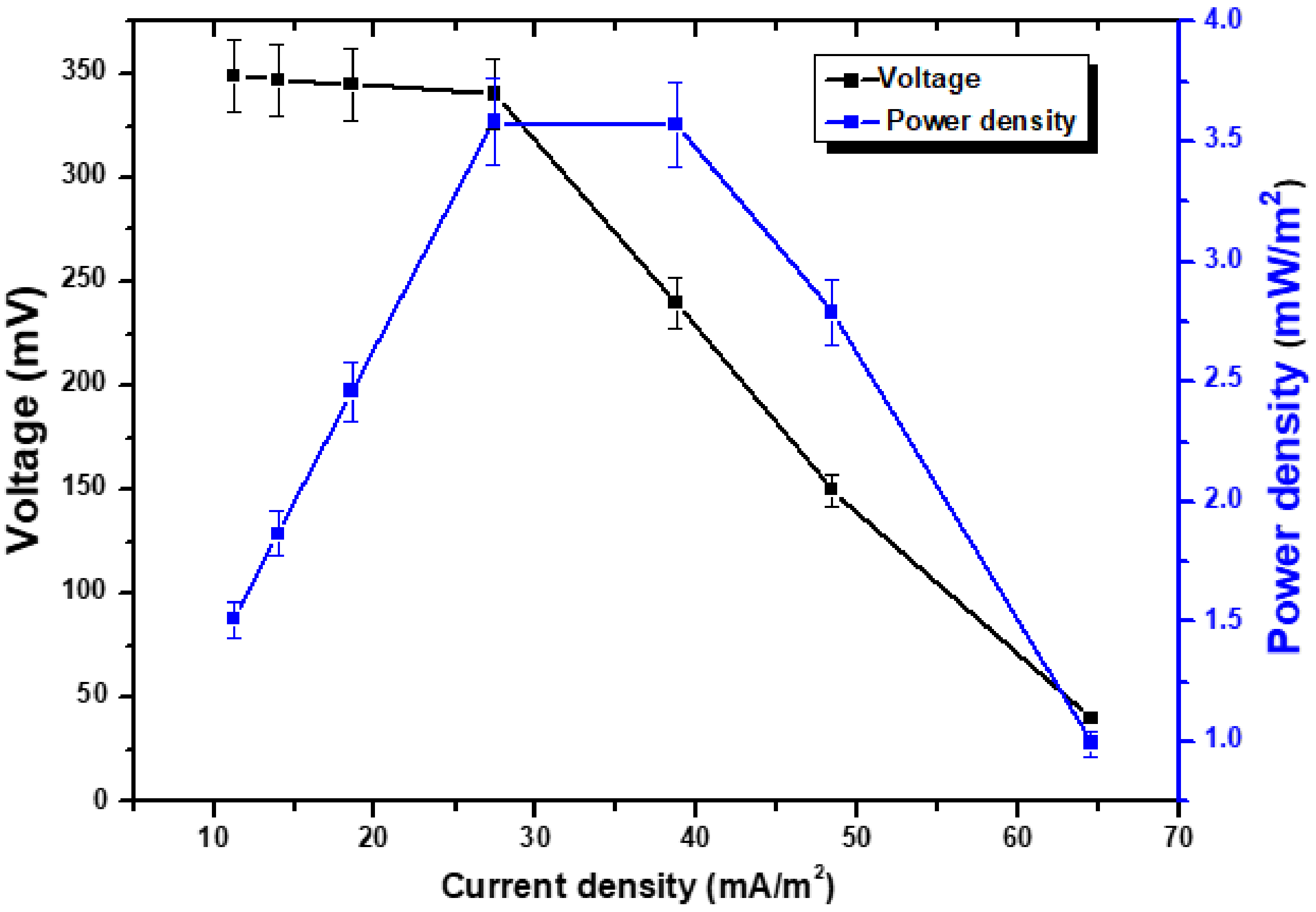
 resistance. On day 32, it was reported that the voltage reading had achieved its maximum level, which was measured at 300 mV. According to the data, the voltage generation began at a reasonable rate and gradually increased until it achieved its highest values on the 32nd day after the experiment began. After that, a downward trend in the voltage started to become apparent. However, after a few days it began increasing again, reaching 290 mV on day 40, and then after that it started an unstopped downward trend once again. The decline in the generation of voltage is an indication that the bacterial species are moving closer to the period of death. The observation after a few days showed that there was still a trend toward a lower voltage. This suggests that the organic substrate oxidation is continuing toward completion, since the exoelectrogens are unable to retake control of the process. This study found that day 32 was when the voltage reached its peak. Despite this, a few investigations have shown that the region with the maximum voltage is also a reliable predictor of a significant change in the metal’s state [22,23,24].
resistance. On day 32, it was reported that the voltage reading had achieved its maximum level, which was measured at 300 mV. According to the data, the voltage generation began at a reasonable rate and gradually increased until it achieved its highest values on the 32nd day after the experiment began. After that, a downward trend in the voltage started to become apparent. However, after a few days it began increasing again, reaching 290 mV on day 40, and then after that it started an unstopped downward trend once again. The decline in the generation of voltage is an indication that the bacterial species are moving closer to the period of death. The observation after a few days showed that there was still a trend toward a lower voltage. This suggests that the organic substrate oxidation is continuing toward completion, since the exoelectrogens are unable to retake control of the process. This study found that day 32 was when the voltage reached its peak. Despite this, a few investigations have shown that the region with the maximum voltage is also a reliable predictor of a significant change in the metal’s state [22,23,24]. , but only 1.510 mW/m2 at 5000
, but only 1.510 mW/m2 at 5000  . Both resistances (external and internal) must be equivalent for the electron transfer to be successful. The lower electron transportation shows the maximum internal environment’s resistance. The potential does not stabilize as soon as it would normally when the external resistance is low, but enough electrons are still produced and transferred. The increased electron mobility leads to voltage instability. The maximum was CD recorded was 64.51 mA/m2. The external oxygen supply enhanced the cathodic response even more, which helped to stabilize voltage production despite the high resistance. The internal resistance was 458
. Both resistances (external and internal) must be equivalent for the electron transfer to be successful. The lower electron transportation shows the maximum internal environment’s resistance. The potential does not stabilize as soon as it would normally when the external resistance is low, but enough electrons are still produced and transferred. The increased electron mobility leads to voltage instability. The maximum was CD recorded was 64.51 mA/m2. The external oxygen supply enhanced the cathodic response even more, which helped to stabilize voltage production despite the high resistance. The internal resistance was 458  . Several studies have shown electrochemical performance using this pattern [25,26,27].
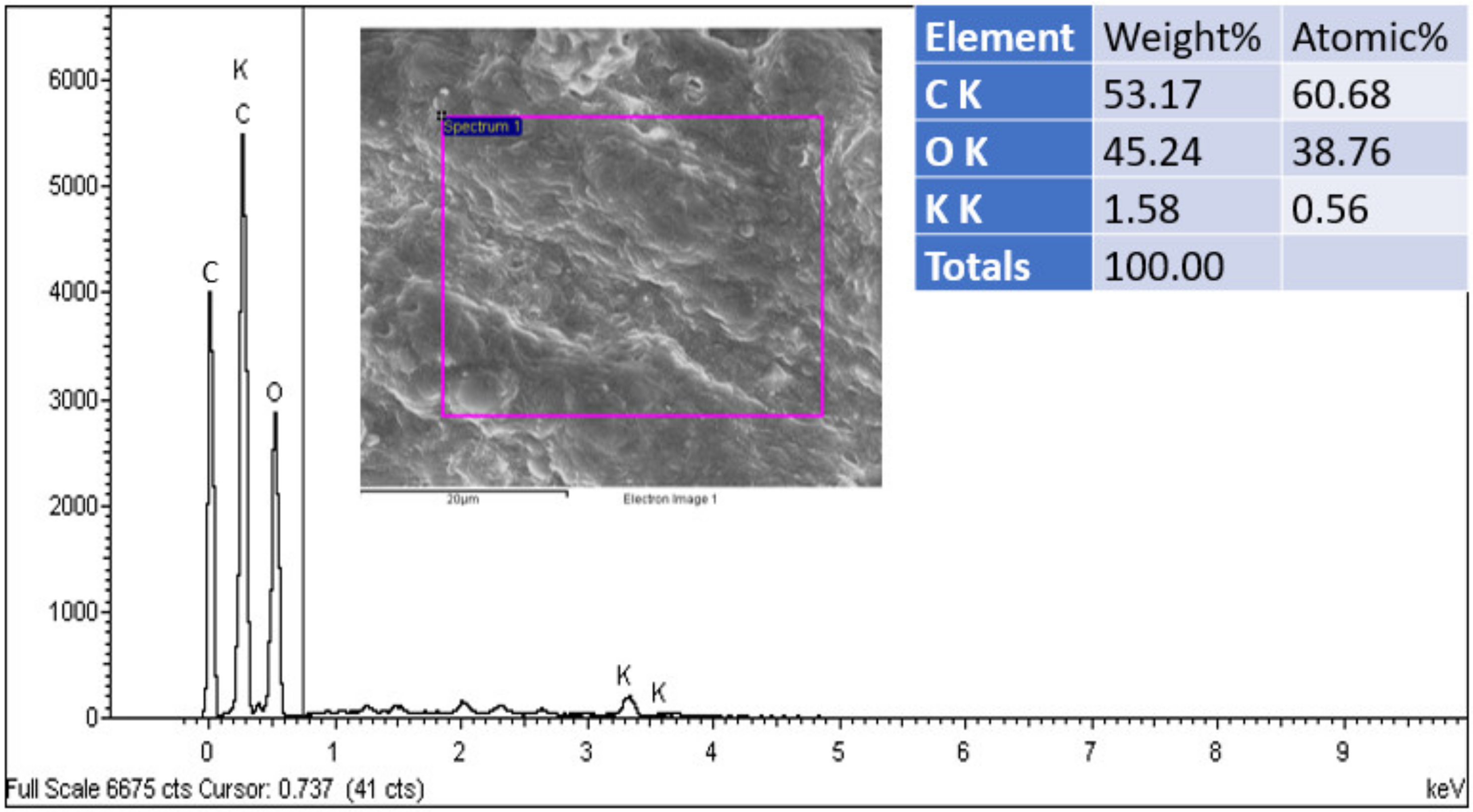
. Several studies have shown electrochemical performance using this pattern [25,26,27].3.2. Chemical Analysis Output of the Present Work
3.3. Biological Tests
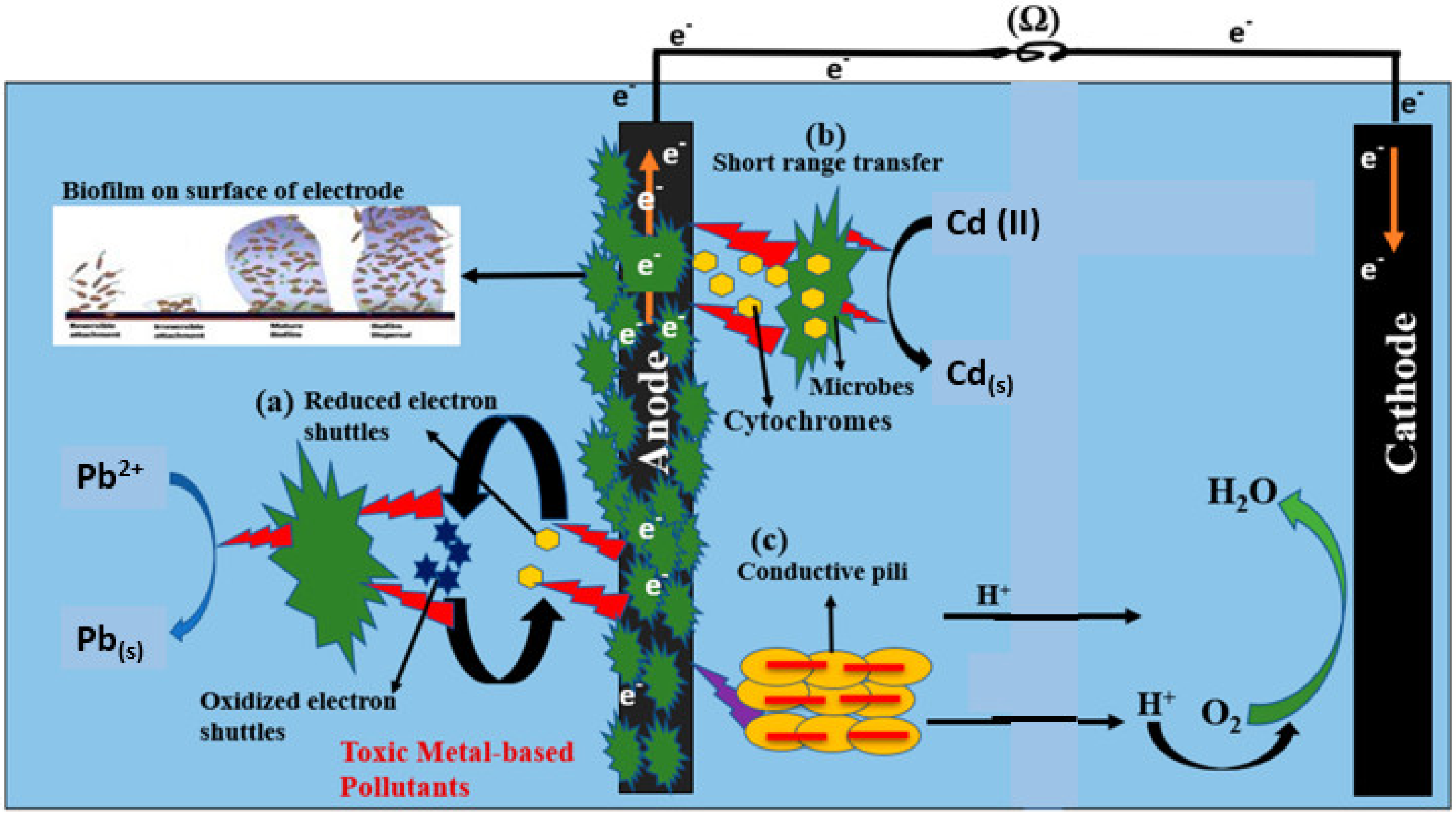
3.4. Sugarcane Waste Oxidation and Metal Reduction Mechanisms
4. Parameter Optimizations
5. Concluding Comments and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parveen, T.; Umar, K. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar]
- Hashim, R.; Song, T.H.; Muslim, N.Z.M.; Yen, T.P. Determination of heavy metal levels in fishes from the lower reach of the Kelantan River, Kelantan, Malaysia. Trop. Life Sci. Res. 2014, 25, 21. [Google Scholar]
- Razak, M.R.; Aris, A.Z.; Zakaria, N.A.C.; Wee, S.Y.; Ismail, N.A.H. Accumulation and risk assessment of heavy metals employing species sensitivity distributions in Linggi River, Negeri Sembilan, Malaysia. Ecotoxicol. Environ. Saf. 2021, 211, 111905. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.; Qari, H.A.; Umar, K. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar]
- Liu, Z.-h.; Dang, Z.; Yin, H.; Liu, Y. Making waves: Improving removal performance of conventional wastewater treatment plants on endocrine disrupting compounds (EDCs): Their conjugates matter. Water Res. 2021, 188, 116469. [Google Scholar] [CrossRef]
- Ahmad, A.; Alshammari, M.B. Advanced Technologies for Wastewater Treatment. Green Chem. Sustain. Water Purif. 2023, 1, 179–202. [Google Scholar] [CrossRef]
- Tian, J.-H.; Lacroix, R.; Bureau, C.; Midoux, C.; Desmond-Le Quéméner, E.; Bouchez, T. Study of a Pilot Scale Microbial Electrosynthesis Reactor for Organic Waste Biorefinery. Energies 2023, 16, 591. [Google Scholar] [CrossRef]
- Ahmad, A. Preparation, characterization, and application of modified carbonized lignin as an anode for sustainable microbial fuel cell. Process Saf. Environ. Prot. 2021, 155, 49–60. [Google Scholar]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Microbial fuel cells—Challenges and applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef]
- Umar, M.F.; Rafatullah, M.; Abbas, S.Z.; Mohamad Ibrahim, M.N.; Ismail, N. Advancement in Benthic Microbial Fuel Cells toward Sustainable Bioremediation and Renewable Energy Production. Int. J. Environ. Res. Public Health 2021, 18, 3811. [Google Scholar] [CrossRef] [PubMed]
- Umar, K.; Yaakop, A.S. Local fruit wastes driven benthic microbial fuel cell: A sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar]
- Daud, N.N.; Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K. The impact of biomass-derived electrodes on the electrochemical performance of microbial fuel cells (MFCs). In Microbial Fuel Cells: Emerging Trends in Electrochemical Applications; IOP Publishing: Bristol UK, 2022; Volume 1, p. 6-1. [Google Scholar]
- Serrà, A.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I. Utilizing biomass-based graphene oxide–polyaniline–ag electrodes in microbial fuel cells to boost energy generation and heavy metal removal. Polymers 2022, 14, 845. [Google Scholar]
- Ahmad, F.; Atiyeh, M.N.; Pereira, B.; Stephanopoulos, G.N. A review of cellulosic microbial fuel cells: Performance and challenges. Biomass Bioenergy 2013, 56, 179–188. [Google Scholar] [CrossRef]
- Flores, S.R.; Nazario-Naveda, R.; Betines, S.M.; De La Cruz–Noriega, M.; Cabanillas-Chirinos, L.; Valdiviezo-Dominguez, F. Sugar industry waste for bioelectricity generation. Environ. Res. Eng. Manag. 2021, 77, 15–22. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Membrane Contactors and Integrated Membrane Operations; Ke Xue Chu Ban She: Beijing, China, 2012. [Google Scholar]
- Umar, M.F.; Rafatullah, M.; Abbas, S.Z.; Ibrahim, M.N.M.; Ismail, N. Bioelectricity production and xylene biodegradation through double chamber benthic microbial fuel cells fed with sugarcane waste as a substrate. J. Hazard. Mater. 2021, 419, 126469. [Google Scholar] [CrossRef]
- Umar, M.F.; Rafatullah, M.; Abbas, S.Z.; Ibrahim, M.N.M.; Ismail, N. Enhanced benzene bioremediation and power generation by double chamber benthic microbial fuel cells fed with sugarcane waste as a substrate. J. Clean. Prod. 2021, 310, 127583. [Google Scholar] [CrossRef]
- Idris, M.O.; Kim, H.-C. Exploring the effectiveness of microbial fuel cell for the degradation of organic pollutants coupled with bio-energy generation. Sustain. Energy Technol. Assess. 2022, 52, 102183. [Google Scholar] [CrossRef]
- Rafatullah, M.; Yaakop, A.S. Utilization of biomass-derived electrodes: A journey toward the high performance of microbial fuel cells. Appl. Water Sci. 2022, 12, 99. [Google Scholar]
- Yang, Y.; Lu, Z.; Lin, X.; Xia, C.; Sun, G.; Lian, Y.; Xu, M. Enhancing the bioremediation by harvesting electricity from the heavily contaminated sediments. Bioresour. Technol. 2015, 179, 615–618. [Google Scholar] [CrossRef]
- Al-Zaqri, N.; Yaakop, A.S.; Umar, K. Potato waste as an effective source of electron generation and bioremediation of pollutant through benthic microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53, 102560. [Google Scholar]
- Bakar, M.A.B.A.; Kim, H.-C.; Ahmad, A.; Alshammari, M.B. Oxidation of food waste as an organic substrate in a single chamber microbial fuel cell to remove the pollutant with energy generation. Sustain. Energy Technol. Assess. 2022, 52, 102282. [Google Scholar]
- Sajana, T.; Ghangrekar, M.; Mitra, A. Effect of presence of cellulose in the freshwater sediment on the performance of sediment microbial fuel cell. Bioresour. Technol. 2014, 155, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Sajana, T.; Ghangrekar, M.; Mitra, A. Application of sediment microbial fuel cell for in situ reclamation of aquaculture pond water quality. Aquac. Eng. 2013, 57, 101–107. [Google Scholar] [CrossRef]
- Idris, M.O.; Noh, N.A.; Ibrahim, M.N.M. Sustainable microbial fuel cell functionalized with a bio-waste: A feasible route to formaldehyde bioremediation along with bioelectricity generation. Chem. Eng. J. 2023, 455, 140781. [Google Scholar] [CrossRef]
- Hong, Y.; Call, D.F.; Werner, C.M.; Logan, B.E. Adaptation to high current using low external resistances eliminates power overshot in microbial fuel cells. Biosens. Bioelectron. 2011, 28, 71–76. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Sharma, V. Bioelectricity production from various wastewaters through microbial fuel cell technology. J. Biochem. Technol. 2010, 2, 133–137. [Google Scholar]
- Lobato, J.; Cañizares, P.; Rodrigo, M.; Morales, F.F. Short-term effects of temperature and COD in a microbial fuel cell. Appl. Energy 2013, 101, 213–217. [Google Scholar]
- Zhang, X.; He, W.; Ren, L.; Stager, J.; Evans, P.J.; Logan, B.E. COD removal characteristics in air-cathode microbial fuel cells. Bioresour. Technol. 2015, 176, 23–31. [Google Scholar] [CrossRef]
- Singh, S.; Songera, D.S. A review on microbial fuel cell using organic waste as feed. CIBTech J. Biotechnol. 2012, 2, 17–27. [Google Scholar]
- Ahmad, A.; Ibrahim, M.N.M.; Yaqoob, A.A.; Setapar, S.H.M. Microbial Fuel Cells for Environmental Remediation; Springer Nature: Singapore, 2022; Volume 1, p. 450. [Google Scholar]
- Sepehri, A.; Sarrafzadeh, M.-H. Effect of nitrifiers community on fouling mitigation and nitrification efficiency in a membrane bioreactor. Chem. Eng. Process. Process Intensif. 2018, 128, 10–18. [Google Scholar] [CrossRef]
- Kumar, M.A.; Anandapandian, K.T.K.; Parthiban, K. Production and characterization of exopolysaccharides (EPS) from biofilm forming marine bacterium. Braz. Arch. Biol. Technol. 2011, 54, 259–265. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Yaqoob, A.A.; Ahmad, A. Microbial Fuel Cells: Emerging Trends in Electrochemical Applications; IOP Publishing: Oxford, UK, 2022; Volume 1, pp. 1–350. [Google Scholar]
- Asim, Y.; Fadzli, F. Benthic microbial fuel cells: A sustainable approach for metal remediation and electricity generation from sapodilla waste. Int. J. Environ. Sci. Technol. 2022, 20, 3927–3940. [Google Scholar]
- Ayangbenro, A.S.; Babalola, O.O. Genomic analysis of Bacillus cereus NWUAB01 and its heavy metal removal from polluted soil. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nimje, V.R.; Chen, C.-Y.; Chen, C.-C.; Jean, J.-S.; Reddy, A.S.; Fan, C.-W.; Pan, K.-Y.; Liu, H.-T.; Chen, J.-L. Stable and high energy generation by a strain of Bacillus subtilis in a microbial fuel cell. J. Power Sources 2009, 190, 258–263. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Umar, K.; Ahmad, A. Modified Graphene Oxide Anode: A Bioinspired Waste Material for Bioremediation of Pb2+ with Energy Generation through Microbial Fuel Cells. Chem. Eng. J. 2020, 417, 128052. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; AlAmmari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135. [Google Scholar] [CrossRef]
- Chuo, S.C.; Mohamed, S.F.; Mohd Setapar, S.H.; Ahmad, A.; Jawaid, M.; Wani, W.A.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Insights into the Current Trends in the Utilization of Bacteria for Microbially Induced Calcium Carbonate Precipitation. Materials 2020, 13, 4993. [Google Scholar] [CrossRef]
- Ahmad, A.; Alshammari, M.B. Basic principles and working mechanisms of microbial fuel cells. In Microbial Fuel Cells: Emerging Trends in Electrochemical Applications; IOP Publishing: Oxford, UK, 2022; pp. 1–30. [Google Scholar]
- Khatoon, A.; Mohd Setapar, S.H.; Umar, K.; Parveen, T. Outlook on the role of microbial fuel cells in remediation of environmental pollutants with electricity generation. Catalysts 2020, 10, 819. [Google Scholar]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Shakoori, F.R. Electrochemistry and microbiology of microbial fuel cells treating marine sediments polluted with heavy metals. RSC Adv. 2018, 8, 18800–18813. [Google Scholar] [CrossRef] [PubMed]
- Yaakop, A.S.; Ahmad, A. Application of microbial fuel cells energized by oil palm trunk sap (OPTS) to remove the toxic metal from synthetic wastewater with generation of electricity. Appl. Nanosci. 2021, 11, 1949–1961. [Google Scholar]
- Huang, L.; Chai, X.; Quan, X.; Logan, B.E.; Chen, G. Reductive dechlorination and mineralization of pentachlorophenol in biocathode microbial fuel cells. Bioresour. Technol. 2012, 111, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, B.; Zhou, S.; Zhong, S.; Zhuang, L. Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour. Technol. 2011, 102, 6887–6891. [Google Scholar] [CrossRef]
- Mohan, S.V.; Srikanth, S.; Raghuvulu, S.V.; Mohanakrishna, G.; Kumar, A.K.; Sarma, P. Evaluation of the potential of various aquatic eco-systems in harnessing bioelectricity through benthic fuel cell: Effect of electrode assembly and water characteristics. Bioresour. Technol. 2009, 100, 2240–2246. [Google Scholar]
- Mohan, S.V.; Mohanakrishna, G.; Chiranjeevi, P. Sustainable power generation from floating macrophytes based ecological microenvironment through embedded fuel cells along with simultaneous wastewater treatment. Bioresour. Technol. 2011, 102, 7036–7042. [Google Scholar] [CrossRef]
- Sharma, Y.; Li, B. The variation of power generation with organic substrates in single-chamber microbial fuel cells (SCMFCs). Bioresour. Technol. 2010, 101, 1844–1850. [Google Scholar] [CrossRef]
- Salvin, P.; Ondel, O.; Roos, C.; Robert, F. Energy harvest with mangrove benthic microbial fuel cells. Int. J. Energy Res. 2015, 39, 543–556. [Google Scholar] [CrossRef]
- Yamada, H.; Tanaka, R.; Sulaiman, O.; Hashim, R.; Hamid, Z.; Yahya, M.; Kosugi, A.; Arai, T.; Murata, Y.; Nirasawa, S. Old oil palm trunk: A promising source of sugars for bioethanol production. Biomass Bioenergy 2010, 34, 1608–1613. [Google Scholar] [CrossRef]





| Physiochemical Properties | DW | MSW |
|---|---|---|
| pH | 6.89 | 5.99 |
| Temperature | Room | Room |
| Color | Light yellowish | Dark yellowish |
| Electrical conductivity | 105 µS/cm | 350 µS/cm |
| Pb (II) | 0 mg/L | 200 mg/L |
| Cd (II) | 0 mg/L | 200 mg/L |
| Cr (III) | 0 mg/L | 200 mg/L |
| Days | Specific Capacitance (F/g) |
|---|---|
| 10 | 0.00001 |
| 20 | 0.00001 |
| 30 | 0.00007 |
| 40 | 0.00007 |
| 50 | 0.00003 |
| 60 | 0.00003 |
| 70 | 0.04 |
| Organic Substrate | Initial Concentration | Operational Days | Degradation % of Pb (II) | Degradation % of Cr (III) | Degradation % of Cd (II) |
|---|---|---|---|---|---|
| Sugarcane waste | 200 mg/L | 0 | 0 | 0 | 0 |
| 15 | 30.10 | 29.50 | 29.10 | ||
| 30 | 48.19 | 51.99 | 54.78 | ||
| 45 | 58.30 | 69.25 | 71.40 | ||
| 60 | 76.11 | 74.35 | 85.00 | ||
| 70 | 88.68 | 87.60 | 90.20 |
| Query Cover (%) | Identity (%) | Accession Number (16S rRNA Gene) | |
|---|---|---|---|
| Bacillus thuringiensis strain | 99 | 96.54 | NR_043403.1 |
| Bacillus sanguinis strain | 99 | 96.54 | NR_175555.1 |
| Bacillus cereus ATCC | 99 | 96.54 | NR_074540.1 |
| Rossellomorea marisflavi strain TF-11 | 99 | 93.54 | NR_118437.1 |
| Sutcliffiella halmapala strain | 99 | 93.56 | NR_026144.1 |
| Priestia flexa strain | 99 | 93.28 | NR_024691.1 |
| Peribacillus muralis strain | 99 | 93.02 | NR_042083.1 |
| Bacillus salis strain | 99 | 92.82 | NR_179406.1 |
| Cytobacillus kochii strain | 93 | 94.81 | NR_117050.1 |
| Neobacillus ginsengisoli strain | 95.35 | 92.00 | NR_109068.1 |
| Sutcliffiella cohnii strain | 99 | 93.18 | NR_113776.1 |
| Cytobacillus purgationiresistens strain | 94 | 95.20 | NR_108492.1 |
| Bacillus marcorestinctum strain | 91 | 96.14 | NR_117414.1 |
| Organic Substrate | Total days | Voltage (mV) | Degradation % of Pb (II) | Degradation % of Cr (III) | Degradation % of Cd (II) |
|---|---|---|---|---|---|
| Sugarcane waste | 10 | 55 | 25 | 24 | 24 |
| Commercial glucose | 10 | 28 | 11.20 | 12.99 | 13.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleid, G.M.; Alshammari, A.S.; Alomari, A.D.; A. Almukhlifi, H.; Ahmad, A.; Yaqoob, A.A. Dual Role of Sugarcane Waste in Benthic Microbial Fuel to Produce Energy with Degradation of Metals and Chemical Oxygen Demand. Processes 2023, 11, 1060. https://doi.org/10.3390/pr11041060
Aleid GM, Alshammari AS, Alomari AD, A. Almukhlifi H, Ahmad A, Yaqoob AA. Dual Role of Sugarcane Waste in Benthic Microbial Fuel to Produce Energy with Degradation of Metals and Chemical Oxygen Demand. Processes. 2023; 11(4):1060. https://doi.org/10.3390/pr11041060
Chicago/Turabian StyleAleid, Ghada Mohamed, Anoud Saud Alshammari, Asma D. Alomari, Hanadi A. Almukhlifi, Akil Ahmad, and Asim Ali Yaqoob. 2023. "Dual Role of Sugarcane Waste in Benthic Microbial Fuel to Produce Energy with Degradation of Metals and Chemical Oxygen Demand" Processes 11, no. 4: 1060. https://doi.org/10.3390/pr11041060








