Untargeted LC-QTOF-MS Analysis of Metabolites Produced by Penicillium brevicompactum during the Bioconversion of Ganoderic Acid A
Abstract
:1. Introduction
2. Materials and Methods
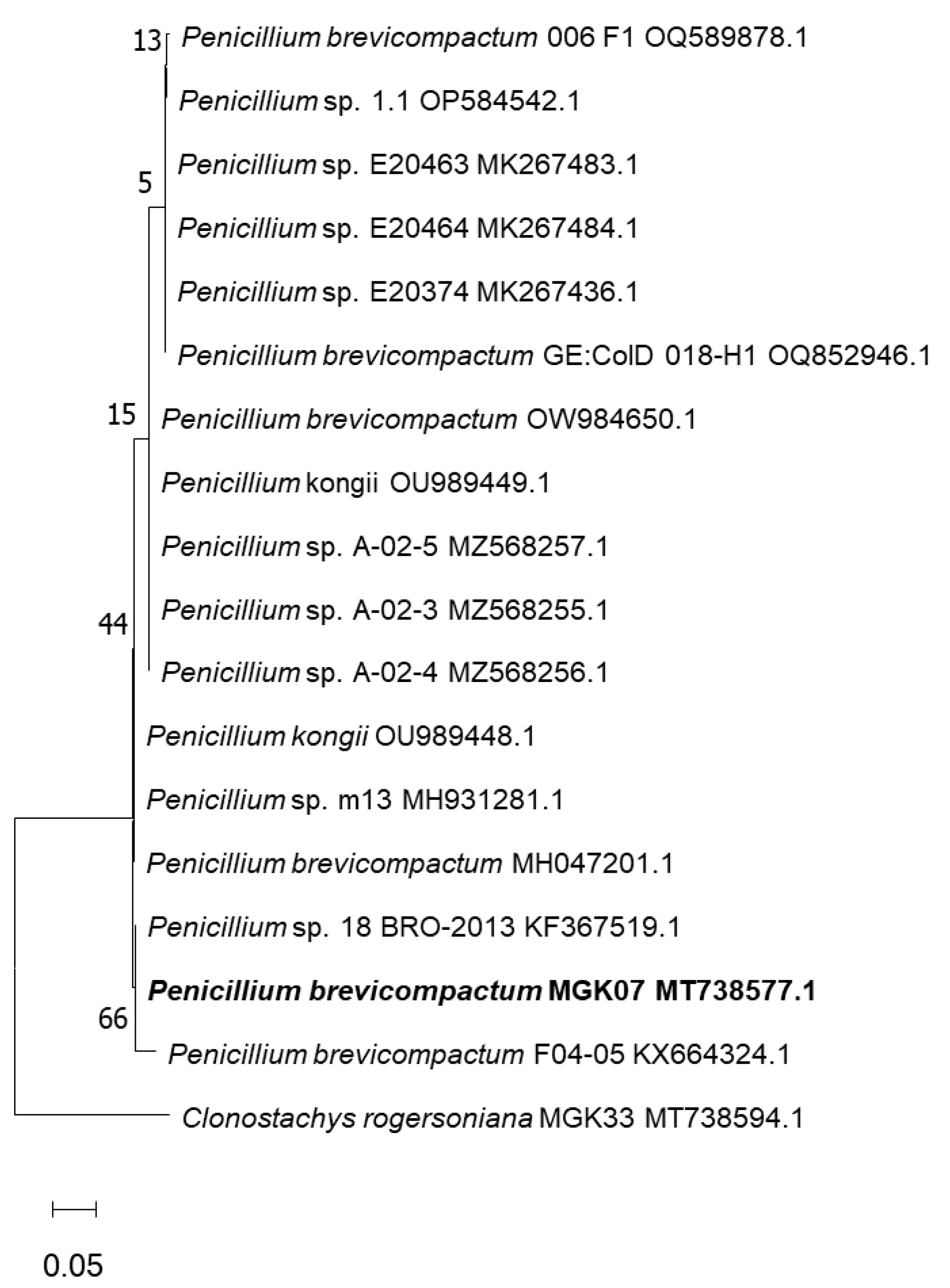
2.1. Isolation and Identification of the Fungal Organism
2.2. GAA Bioconversion
2.3. Untargeted Analysis of Metabolites Using LC-QTOF-MS
3. Results
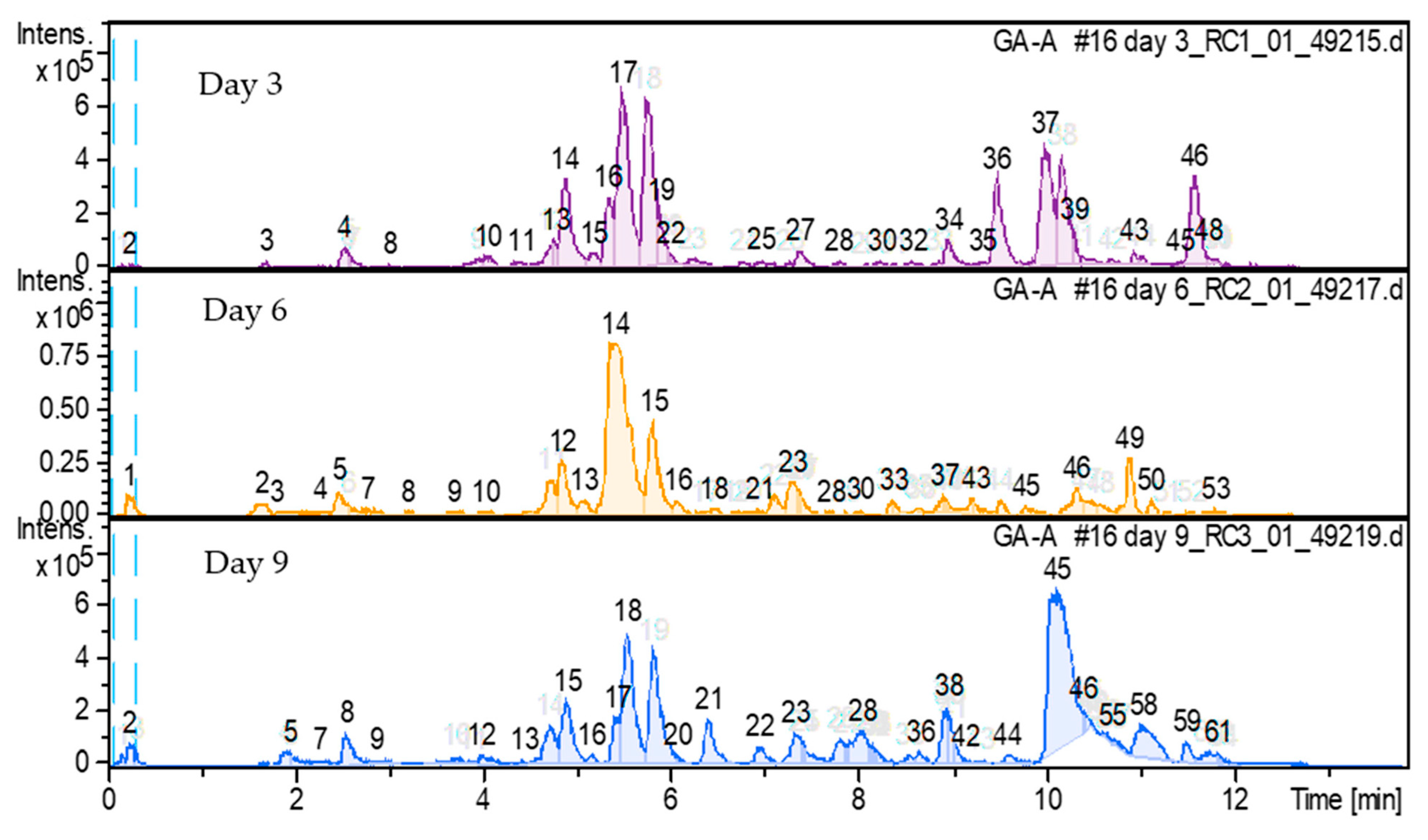
3.1. Bioconversion of Ganoderic Acid A and Detected Metabolites Using Penicillium brevicompactum MGK07
3.2. Metabolites Produced from GAA Bioconversion of Penicillium brevicompactum MGK07: Day 3
3.3. Metabolites Produced from GAA Bioconversion of Penicillium brevicompactum MGK07: Day 6
3.4. Metabolites Produced from GAA Bioconversion of Penicillium brevicompactum MGK07: Day 9
4. Discussion
4.1. Bioconversion of Ganoderic Acid A and Detected Metabolites Using Penicillium brevicompactum
4.2. Metabolites Produced from GAA Bioconversion of Penicillium brevicompactum: Day 3
4.3. Metabolites Produced from GAA Bioconversion of Penicillium brevicompactum: Day 6
4.4. Metabolites Produced from GAA Bioconversion of Penicillium brevicompactum: Day 9
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wachtel-Galor, S.J.; Yuen, J.A.; Buswell, J.A.; Benzie, I.F. Ganoderma lucidum (lingzhi or reishi): A medicinal mushroom. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hennicke, F.; Cheikh-Ali, Z.; Liebisch, T.; Maciá-Vicente, J.G.; Bode, H.B.; Piepenbring, M. Distinguishing commercially grown Ganoderma lucidum from ganoderma lingzhi from europe and east asia on the basis of morphology, molecular phylogeny, and triterpenic acid profiles. Phytochemistry 2016, 127, 29–37. [Google Scholar] [PubMed]
- Nahata, A. Ganoderma lucidum: A potent medicinal mushroom with numerous health benefits. Pharm. Anal. Acta 2013, 4, 1000e159. [Google Scholar]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A. Introductory chapter: Terpenes and terpenoids. In Terpenes and Terpenoids; IntechOpen: London, UK, 2018; Volume 1, pp. 1–12. [Google Scholar]
- Thurman, E.M. Chapter seven—Analysis of terpenes in hemp (Cannabis sativa) by gas chromatography/mass spectrometry: Isomer identification analysis. In Comprehensive Analytical Chemistry; Ferrer, I., Thurman, E.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–233. [Google Scholar]
- Cárdenas, P.D.; Almeida, A.; Bak, S. Evolution of structural diversity of triterpenoids. Front. Plant Sci. 2019, 10, 1523. [Google Scholar] [PubMed]
- Kubota, T.; Asaka, Y.; Miura, I.; Mori, H. Structures of ganoderic acid a and b, two new lanostane type bitter triterpenes from Ganoderma lucidum (fr.) karst. Helv. Chim. Acta 1982, 65, 611–619. [Google Scholar]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids a, c2, d, f, dm, x and y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar]
- Yang, Y.; Zhou, H.; Liu, W.; Wu, J.; Yue, X.; Wang, J.; Quan, L.; Liu, H.; Guo, L.; Wang, Z.; et al. Ganoderic acid a exerts antitumor activity against mda-mb-231 human breast cancer cells by inhibiting the janus kinase 2/signal transducer and activator of transcription 3 signaling pathway. Oncol. Lett. 2018, 16, 6515–6521. [Google Scholar]
- Wang, M.L.; Lu, C.H.; Xu, Q.Y.; Song, S.Y.; Hu, Z.Y.; Zheng, Z.H. Four new citrinin derivatives from a marine-derived penicillium sp. Fungal strain. Molecules 2013, 18, 5723–5735. [Google Scholar] [CrossRef]
- Wang, X.; Sun, D.; Tai, J.; Wang, L. Ganoderic acid a inhibits proliferation and invasion, and promotes apoptosis in human hepatocellular carcinoma cells. Mol. Med. Rep. 2017, 16, 3894–3900. [Google Scholar] [CrossRef]
- Chang, T.-S. Isolation, bioactivity, and production of ortho-hydroxydaidzein and ortho-hydroxygenistein. Int. J. Mol. Sci. 2014, 15, 5699–5716. [Google Scholar] [CrossRef]
- Chang, T.-S.; Chiang, C.-M.; Wang, T.-Y.; Lee, C.-H.; Lee, Y.-W.; Wu, J.-Y. New triterpenoid from novel triterpenoid 15-o-glycosylation on ganoderic acid a by intestinal bacteria of zebrafish. Molecules 2018, 23, 2345. [Google Scholar] [CrossRef]
- Zeng, W.-L.; Li, W.-K.; Han, H.; Tao, Y.-Y.; Yang, L.; Wang, Z.-T.; Chen, K.-X. Microbial biotransformation of gentiopicroside by the endophytic fungus penicillium crustosum 2t01y01. Appl. Environ. Microbiol. 2014, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, X.P.; Zhao, J.; Gao, H.W.; Xu, Q.M.; Wang, J.W. Biotransformation of artemisinic acid to bioactive derivatives by endophytic penicillium oxalicum b4 from artemisia annual. Phytochemistry 2021, 185, 112682. [Google Scholar] [CrossRef] [PubMed]
- Tapfuma, K.I.; Nyambo, K.; Adu-Amankwaah, F.; Baatjies, L.; Smith, L.; Allie, N.; Keyster, M.; Loxton, A.G.; Ngxande, M.; Malgas-Enus, R.; et al. Antimycobacterial activity and molecular docking of methanolic extracts and compounds of marine fungi from saldanha and false bays, south africa. Heliyon 2022, 8, e12406. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. Mega11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Ström, K.; Sjögren, J.; Broberg, A.; Schnürer, J. Lactobacillus plantarum milab 393 produces the antifungal cyclic dipeptides cyclo(l-phe-l-pro) and cyclo(l-phe-trans-4-oh-l-pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 2002, 68, 4322–4327. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Rhee, K.H. Radioprotective effect of cyclo (l-phenylalanyl-l-prolyl) on irradiated rat lung. J. Microbiol. Biotechnol. 2008, 18, 369–376. [Google Scholar]
- Yousuf, M.; Jamil, W.; Mammadova, K. Microbial bioconversion: A regio-specific method for novel drug design and toxicological study of metabolites. Curr. Pharm. Biotechnol. 2019, 20, 1156–1162. [Google Scholar] [CrossRef]
- Kim, I.H.; Kim, S.-Y.; Park, N.-Y.; Wen, Y.; Lee, K.-W.; Yoon, S.-Y.; Jie, H.; Lee, K.-H.; Kim, K.-S. Cyclo-(l-phe-l-pro), a quorum-sensing signal of vibrio vulnificus, induces expression of hydroperoxidase through a toxr-leuo-hu-rpos signaling pathway to confer resistance against oxidative stress. Infect. Immun. 2018, 86, e00932-17. [Google Scholar] [CrossRef]
- Hirotani, M.; Ino, C.; Hatano, A.; Takayanagi, H.; Furuya, T. Ganomastenols a, b, c and d, cadinene sesquiterpenes, from ganoderma mastoporum. Phytochemistry 1995, 40, 161–165. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [PubMed]
- Jamadagni, P.S.; Pawar, S.D.; Jamadagni, S.B.; Chougule, S.; Gaidhani, S.N.; Murthy, S. Review of Holarrhena antidysenterica (L.) wall. Ex a. Dc.: Pharmacognostic, pharmacological, and toxicological perspective. Pharmacogn. Rev. 2017, 11, 141–144. [Google Scholar] [CrossRef]
- Sinha, S.; Sharma, A.; Reddy, P.H.; Rathi, B.; Prasad, N.; Vashishtha, A. Evaluation of phytochemical and pharmacological aspects of Holarrhena antidysenterica (wall.): A comprehensive review. J. Pharm. Res. 2013, 6, 488–492. [Google Scholar] [CrossRef]
- Neuzil, J. Vitamin e succinate and cancer treatment: A vitamin e prototype for selective antitumour activity. Br. J. Cancer 2003, 89, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Malafa, M.P.; Neitzel, L.T. Vitamin e succinate promotes breast cancer tumor dormancy. J. Surg. Res. 2000, 93, 163–170. [Google Scholar] [CrossRef]
- Liang, L.; Qiu, L. Vitamin e succinate with multiple functions: A versatile agent in nanomedicine-based cancer therapy and its delivery strategies. Int. J. Pharm. 2021, 600, 120457. [Google Scholar]
- Huang, X.; Neckenig, M.; Sun, J.; Jia, D.; Dou, Y.; Ai, D.; Nan, Z.; Qu, X. Vitamin e succinate exerts anti-tumour effects on human cervical cancer cells via the cd47-sirpɑ pathway both in vivo and in vitro. J. Cancer 2021, 12, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Guan, X. Vitamin e succinate inhibits the growth of human gastric cancer cells. Indian J. Pharm. Sci. 2021, 83, 346–353. [Google Scholar] [CrossRef]
- Qu, X.; Zou, Y.; He, C.; Zhou, Y.; Jin, Y.; Deng, Y.; Wang, Z.; Li, X.; Zhou, Y.; Liu, Y. Improved intestinal absorption of paclitaxel by mixed micelles self-assembled from vitamin e succinate-based amphiphilic polymers and their transcellular transport mechanism and intracellular trafficking routes. Drug Deliv. 2018, 25, 210–225. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Yen, G.-C. Chapter three—Ganoderic acid and lucidenic acid (triterpenoid). In The Enzymes; Bathaie, S.Z., Tamanoi, F., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 33–56. [Google Scholar]
- Kikuchi, T.; Matsuda, S.; Murai, Y.; Ogita, Z. Ganoderic acid g and i and ganolucidic acid a and b, new triterpenoids from Ganoderma lucidum. Chem. Pharm. Bull. 1985, 33, 2628–2631. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Sakamura, S. New terpenoids, ganolucidic acid d, ganoderic acid l, lucidone c and lucidenic acid g, from the fungus Ganoderma lucidum. Agric. Biol. Chem. 1986, 50, 809–811. [Google Scholar] [CrossRef]
- Huie, C.W.; Di, X. Chromatographic and electrophoretic methods for lingzhi pharmacologically active components. J. Chromatogr. B 2004, 812, 241–257. [Google Scholar] [CrossRef]
- Gao, L.W.; Li, W.Y.; Zhao, Y.L.; Wang, J.W. The cultivation, bioactive components and pharmacological effects of armillaria mellea. Afr. J. Biotechnol. 2009, 8, 7383–7390. [Google Scholar]
- Kato, S.; Motoyama, T.; Futamura, Y.; Uramoto, M.; Nogawa, T.; Hayashi, T.; Hirota, H.; Tanaka, A.; Takahashi-Ando, N.; Kamakura, T.; et al. Biosynthetic gene cluster identification and biological activity of lucilactaene from Fusarium sp. Rk97-94. Biosci. Biotechnol. Biochem. 2020, 84, 1303–1307. [Google Scholar] [CrossRef]
- Maharjan, S.; Lee, S.B.; Kim, G.J.; Cho, S.J.; Nam, J.-W.; Chin, J.; Choi, H. Isolation of unstable isomers of lucilactaene and evaluation of anti-inflammatory activity of secondary metabolites produced by the endophytic fungus Fusarium sp. Qf001 from the roots of scutellaria baicalensis. Molecules 2020, 25, 923. [Google Scholar] [CrossRef]
- Campbell, E.L. The total synthesis of lucilactaene and efforts towards the total synthesis of ceratamines a and b. ASSAY Drug Dev. Technol. 2009, 7, 325. [Google Scholar]
- Fukuda, T.; Uchida, R.; Inoue, H.; Ohte, S.; Yamazaki, H.; Matsuda, D.; Katagiri, T.; Tomoda, H. Fungal pyrrolidine-containing metabolites inhibit alkaline phosphatase activity in bone morphogenetic protein-stimulated myoblastoma cells. Acta Pharm. Sin. B 2012, 2, 23–27. [Google Scholar] [CrossRef]

| RT (s) | m/z [M + H] | Molecular Formula | Err (ppm) | Possible Identities | Source | CAS ID |
|---|---|---|---|---|---|---|
| 4.8 | 197.129 | C10H16N2O2 | 2.770 | Cyclo(L-Pro-L-Val) | Aspergillus fumigatus, Chromocleista sp. | 2854-40-2 |
| 5.3 | 211.144 | C11H18N2O2 | −0.493 | Cyclo(-Leu-Pro) | Aspergillus fumigatus | 2873-36-1 |
| 5.3–6.0 | 245.128 | C14H16N2O2 | −1.852 | Cyclo-(L-Phe-L-Pro) | Penicillium bilaii | 3705-26-8 |
| 8.97 | 183.101 | C10H14O3 | −3.118 | 4-deoxovermiculic acid | Talaromyces flavus | |
| 9.48 | 253.181 | C15H24O3 | 4.957 | Ganomastenol A/B/D | Ganoderma mastoproum | 168986-51-4 169107-02-2 |
| 10 | 291.244 | C18H30N2O | - | |||
| 10.17 | 304.299 | C21H37N | −2.882 | Aminopregnane | ||
| 11.7 | 332.331 | C23H41N | - | |||
| 11.8 | 531.407 | C33H54O5 | 4.891 | Vitamin E succinate |
| RT (s) | m/z [M + H] | Err (ppm) | Molecular Formula | Possible Identities | Source | CAS ID |
|---|---|---|---|---|---|---|
| 4.8 | 197.128 | −2.303 | C10H16N2O2 | Cyclo(L-Pro-L-Val) | Aspergillus fumigatus, Chromocleista sp. | 2854-40-2 |
| 5.4 | 212.118 | −1.056 | C13H13N3 | 7,8,9,10-Tetrahydro-6,10-methano-6H-pyrazino(2,3-h)(3)benzazepine | ||
| 5.8 | 245.128 | −1.852 | C14H16N2O2 | Cyclo-(L-Phe-L-Pro) | Penicillium bilaii | 3705-26-8 |
| 6.47 | 105.070 | 1.171 | C8H8 | - | ||
| 6.7–6.9 | 501.319 | −4.121 | C30H44O6 | Ganoderic acid beta Ganolucidic acid A Ganolucidic acid D | Ganoderma lucidum | 98665-21-5 102607-22-7 217476-76- |
| 8.3 | 194.118 | 2.292 | C11H15NO2 | - | ||
| 8.6 | 415.213 | 3.576 | C24H30O6 | Armillaripin | Armillaria mellea, Caesalpinia crista | 129741-56-6 |
| 8.9 | 286.075 | −11.221 | C9H11N5O6 | - | ||
| 10.9 | 190.112 | - | - |
| Retention Time (s) | Mass [M − H]+ | Err (ppm) | Molecular Formula | Possible Names | Source | CAS ID |
|---|---|---|---|---|---|---|
| 4.9 | 197.128 | −2.303 | C10H16N2O2 | Cyclo(L-Pro-L-Val) | Aspergillus fumigatus, Chromocleista sp. | 2854-40-2 |
| 5.5 | 211.144 | −0.493 | C11H18N2O2 | Cyclo(-Leu-Pro) | Aspergillus fumigatus | 2873-36-1 |
| 5.8 | 245.128 | −1.852 | C14H16N2O2 | Cyclo-(L-Phe-L-Pro) | Penicillium bilaii | 3705-26-8 |
| 6.4 | 164.107 | 0.061 | C10H13NO | - | ||
| 6.9 | 501.320 | −2.126 | C30H44O6 | Ganoderic acid beta Ganolucidic acid A Ganolucidic acid D | Ganoderma lucidum | 98665-21-5 102607-22-7 217476-76-1 |
| 7.8–8.5 | 402.190 | −2.770 | C22H27NO6 | Lucilactaene | Fusarium sp. RK97-94 | 386278-77-9 |
| 9.0 | 286.072 | C9H11N5O6 | - | |||
| 10.5 | 291.245 | C18H30N2O | - | |||
| 11.9 | 531.404 | −0.754 | C33H54O5 | Vitamin E succinate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidoo, V.; Mavumengwana, V.; Tapfuma, K.; Rasifudi, N.F.; Mekuto, L. Untargeted LC-QTOF-MS Analysis of Metabolites Produced by Penicillium brevicompactum during the Bioconversion of Ganoderic Acid A. Processes 2023, 11, 2963. https://doi.org/10.3390/pr11102963
Naidoo V, Mavumengwana V, Tapfuma K, Rasifudi NF, Mekuto L. Untargeted LC-QTOF-MS Analysis of Metabolites Produced by Penicillium brevicompactum during the Bioconversion of Ganoderic Acid A. Processes. 2023; 11(10):2963. https://doi.org/10.3390/pr11102963
Chicago/Turabian StyleNaidoo, Vizelle, Vuyo Mavumengwana, Kudzanai Tapfuma, Ndiwanga F. Rasifudi, and Lukhanyo Mekuto. 2023. "Untargeted LC-QTOF-MS Analysis of Metabolites Produced by Penicillium brevicompactum during the Bioconversion of Ganoderic Acid A" Processes 11, no. 10: 2963. https://doi.org/10.3390/pr11102963






