Abstract
Microbial nitrification and denitrification are efficient technologies for the treatment of nitrogen-containing wastewater. However, these biotic technologies are inapplicable for the treatment of toxic substances such as heavy metals, polyaromatic hydrocarbons, adsorbable organic halogens, and polychlorinated biphenyls, which have an inhibitory effect on microbial metabolism. It is therefore necessary to develop abiotic nitrogen removal technology with comparable cost efficiency. Nitrogen contaminants are promising indirect fuel sources. The integration of electrocatalysis energy conversion with nitrogen contaminants could drive an entire electrochemical system to obtain nitrogen removal in a self-powered fashion. Research advances in the development of fuel cells have corroborated their promising application for nitrogen removal. This work aims to review the most recent advances in the utilization of ammonia and nitrate as fuels for self-powered nitrogen removal and demonstrate how close this technology is to integration with future applications. The mechanism of ammonia–oxygen fuel cells is first summarized, followed by an overview of recent research on self-powered systems based on various noble-metal-free catalysts. We then introduce different harvesting and conversion methods using nitrate with a desired power output and nitrogen removal efficiency. The final section demonstrates the shortcomings of research and future innovative perspectives for self-powered wastewater treatment.
1. Introduction
Ammonia, urea, amino acids, nitrate, and nitrite are common nitrogen-containing contaminants generated from industry, fertilizers, biofuel byproducts, hygiene waste, urine, etc. [1,2,3,4]. Over the past century, human intervention has doubled the amount of fixed nitrogen in the environment [5]. The discharge of excessive nitrogen contaminants into the natural environment can damage the ecological balance, resulting in acidification and eutrophication [6,7,8]. Removal of nitrogen-containing contaminants is an environmental issue of increasing concern. The aim of nitrogen removal is to convert the nitrogen present in compounds into harmless nitrogen gas. This target can be achieved cost-effectively via microbial methods, such as nitrification and denitrification [9]. However, the working conditions of microbes are greatly limited by several factors, such as the carbon/nitrogen ratio, pH value, temperature, contaminant concentration, and toxicity. In order to reduce nutrient emissions, a variety of methods has been utilized for nitrogen removal, including ion exchange, catalysis, reverse osmosis, and ozonation [10,11,12]. Compared to microbial methods, toxic byproducts can be generated with these technologies, and they consume a considerable amount of energy and materials. Therefore, it is vital to develop new nitrogen removal methods for this non-biodegradable effluent.
Nitrification and denitrification are electron transfer processes, in which ammonia nitrogen is used as an electron donor and nitrate nitrogen is used as an electron acceptor. Based on this concept, it is possible to develop an abiotic electrochemical system to simulate biological function. The electron transfer process can conduct directly on the electrode surface instead of the biological cells. Due to the avoidance of biological system use, it is applicable for non-biodegradable effluents. Electrochemical nitrogen removal technology has been applied in the treatment of landfill leachate, which commonly creates an inhibitory effect on biological processes due to certain toxic substances, such as heavy metals, polyaromatic hydrocarbons, adsorbable organic halogens, and polychlorinated biphenyls [13]. Ammonia-N is oxidized to nitrogen gas at a specific voltage. Nitrogen removal efficiency can be improved by optimizing the concentration of Cl−1 and Fe2+ [14].
Electrotreatment is a promising alternative method as it works with a wide operation window in terms of pH, concentration, and scale. The main shortcoming of this method is its high cost due to its heavy electricity consumption and electrode replacement technique. On the other hand, these nitrogen-containing contaminants are also energy-storing materials. The energy density of ammonia reaches 12.9 MJ L−1, which is 4.3 MJ L−1 higher than that of liquid hydrogen [15]. Urea has advantages such as an ideal energy density (16.9 MJ L−1) and high solubility (1079 g L−1, 20 °C) and safety, and has thus attracted extensive attention as a hydrogen storage material [16]. If the chemical energy in wastewater could be utilized, a great amount of electricity would be saved. Nowadays, scientists are making huge efforts to develop waste utilization technology allowing for the recovery of energy and resources. Self-powered nitrogen removal technologies with electricity recovery potential, such as ammonia fuel cells and nitrate fuel cells, have been recently reported. Several advanced electrocatalysts have been developed for electrooxidation reaction and electroreduction reaction, including Pt-based catalysts and noble-metal-free catalysts.
Energy shortage and environmental pollution present critical challenges in the 21st century. The development of advanced clean energy technology and the realization of the sustainable development of the ecological environment are thus hot research topics. Nitrogenous substances, such as urea, are both environmental pollutants and ideal substances for energy production. Landfill leachate and factory wastewater are rich in nitrates, ammonia nitrogen, urea, and other nitrogenous pollutants. If the chemical energy in wastewater could be utilized, a significant amount of power could be produced. By constructing fuel cells, the chemical energy stored in nitrogenous substances such as urea can be directly converted into electricity; the treatment of pollutants for this purpose has attracted the attention of many scientists.
Up to now, there has not been a systematic review on the research reports on self-generating nitrogen removal via abiotic electrochemical catalysis. In this review, we summarize state-of-the-art methods of nitrogen-containing pollutant utilization, and focus on the current research and applications of energy recovery technologies as well as the utilization of ammonia in wastewater and nitrate wastewater to provide a reference for subsequent related work.
2. Ammonia Wastewater Utilization for Energy Recovery
2.1. Electricity Generation via Low-Temperature Ammonia Fuel Cells
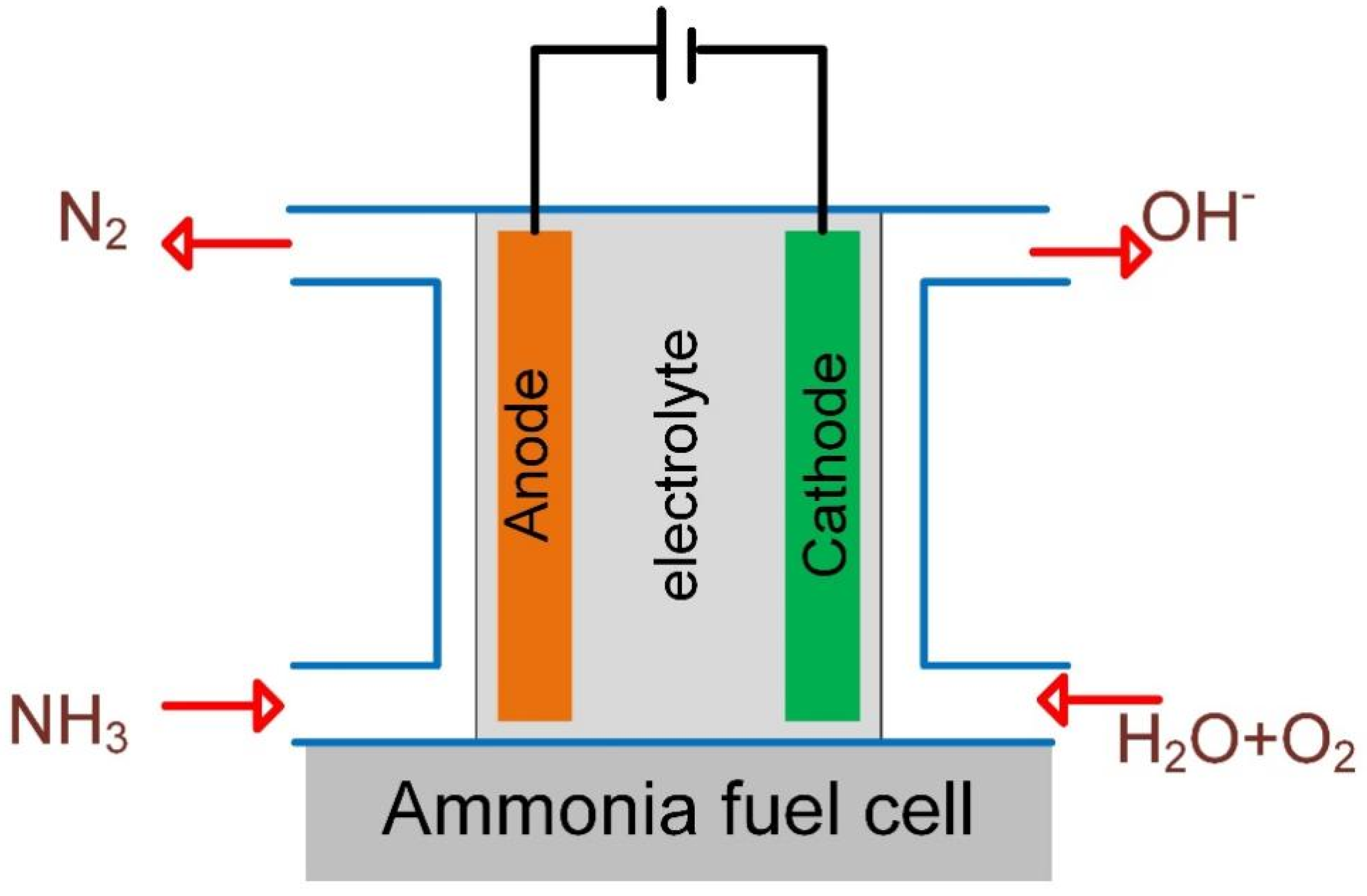
In low-temperature ammonia fuel cells, ammonia is converted into nitrogen gas via the ammonia oxidation reaction (AOR), and oxygen/air is reduced via the oxygen reduction reaction (ORR), as shown in Figure 1. Theoretically, the AOR potential is −0.77 V vs. SHE in alkaline conditions, and the ORR potential is 0.4 V vs. SHE, as shown in Reaction (1) and (2) [17,18]. There is a potential gap of 1.17 V between the ORR and AOR, indicating that it can be self-powered thermodynamically by coupling the AOR and ORR in an ammonia fuel cell. It is an energetically favorable process of removing nitrogen and producing electricity simultaneously via an ammonia fuel cell. However, there is a larger overpotential of the AOR, and the reaction rate is sluggish at room temperature. Hence, the fuel cell performance is unsatisfactory due to the lack of anode catalysts.

Figure 1.
Schematic diagram of ammonia fuel cell.
Pt-based electrocatalysts such as PtIr, PtNi, PtIrNi, PtIrZn, and PtSnO2 show a higher activity and lower onset potential for the AOR than other materials [19,20,21,22]. Yi Li et al. prepared a PtIrZn/SiO2-CNT-COOH AOR catalyst, achieving a compelling peak power density of 314 mW cm−2 at 95 °C using 7.0 M NH3 + 1.25 M KOH aqueous solution as the anode fuel (flow rate: 5.0 mL min−1) [21]. The outstanding cell performance was mainly due to the newly developed supporting material of SiO2-CNT-COOH. The charge transport and mass transport were greatly improved by the supporting material, SiO2-CNT-COOH. Pt-based catalysts can successfully achieve a large current density for the AOR with pure ammonia. However, the use of noble metals will increase the investment costs of waste treatment compared to biological technology. Additionally, wastewater usually has a complex composition, so Pt-based catalysts tend to be poisoned more easily in wastewater than in pure ammonia aqueous solution. So far, Ti-based metal oxides, boron-doped diamonds, and graphite have been applied in the electrochemical treatment of wastewater [23]. Desirable removal efficiency and electrochemical durability can be achieved using these electrode materials. However, a large voltage must be applied in order to reach the required current density as their electrocatalysis activity is much lower than that of Pt-based catalysts. Thus, robust and low-cost anode catalysts are needed to realize self-powered nitrogen removal with real wastewater.
2.2. Noble-Metal-Free Catalysts for the AOR
Recently, several newly designed noble-metal-free catalysts have been reported for an efficient AOR. Nickel–copper bimetal and double hydroxides have been reported to have exciting AOR activity with a synergistic effect [24,25]. Electrochemical characterization shows that the AOR current density with a NiCu catalyst is approximately seven times larger than that achieved with Ni catalysts. In alkaline conditions, Ni1−xCuxOOH is formed via an electrochemical reaction from Ni1−xCux(OH)2 and functions as the active species for the AOR. Additionally, the NiCu catalyst shows better durability than Pt/C. NiCu catalysts are a promising material for application in ammonia fuel cells. Mengfei Zhang et al. prepared alkaline membrane fuel cells using carbon-supported NiCu as the anode catalyst and α-MnO2/C as the cathode catalyst to remove ammonia from landfill leachate at room temperature [26]. The open-circuit voltage of the ammonia fuel cell reached 0.35 V with 0.1 M ammonia. After six hours of ammonia fuel cell operation, the concentration of ammonia in the landfill leachate dropped from 2711 ppm to 95 ppm, achieving removal rate of more than 96%. Huimin Zhang et al. developed a membrane-free microfluidic fuel cell with NiCu-based anode catalysts. In fuel cell tests, 50 wt% Ni50Cu50 supported on carbon nanotubes as an anode showed a 43% higher peak power density and 65% higher maximum current density than the Ni electrode [27]. When 2 M NaOH in 3 M NH4Cl was used as an anolyte, an OCV of 0.72 V and peak power density of 17.1 mW cm−2 were obtained in a microfluidic fuel cell with a core–shell NiCu@NiCuOOH 3D anode [28].
Ternary catalysts have been prepared to further improve catalysis activity. A NiCuFe catalyst showed excellent AOR activity and stability as compared to a NiCu electrode in alkaline solution [29,30]. Interestingly, the NiCuFe catalyst functioned well for both AOR and ORR. An OCV and power density of 0.62 V and 8.9 mW cm−2 °C, respectively, were obtained at 80 [29]. Huimin Zhang et al. demonstrated that sulfur doping and electrochemical tuning can effectively regulate the surface electrochemical reconfiguration of NiCu alloy nanoparticles, providing good external conditions for the adsorption of intermediates such as NH3 [31]. DFT calculation shows that Ni/Cu reduces the energy barrier of multi-step dehydrogenation and improves the catalytic activity of ammonia.
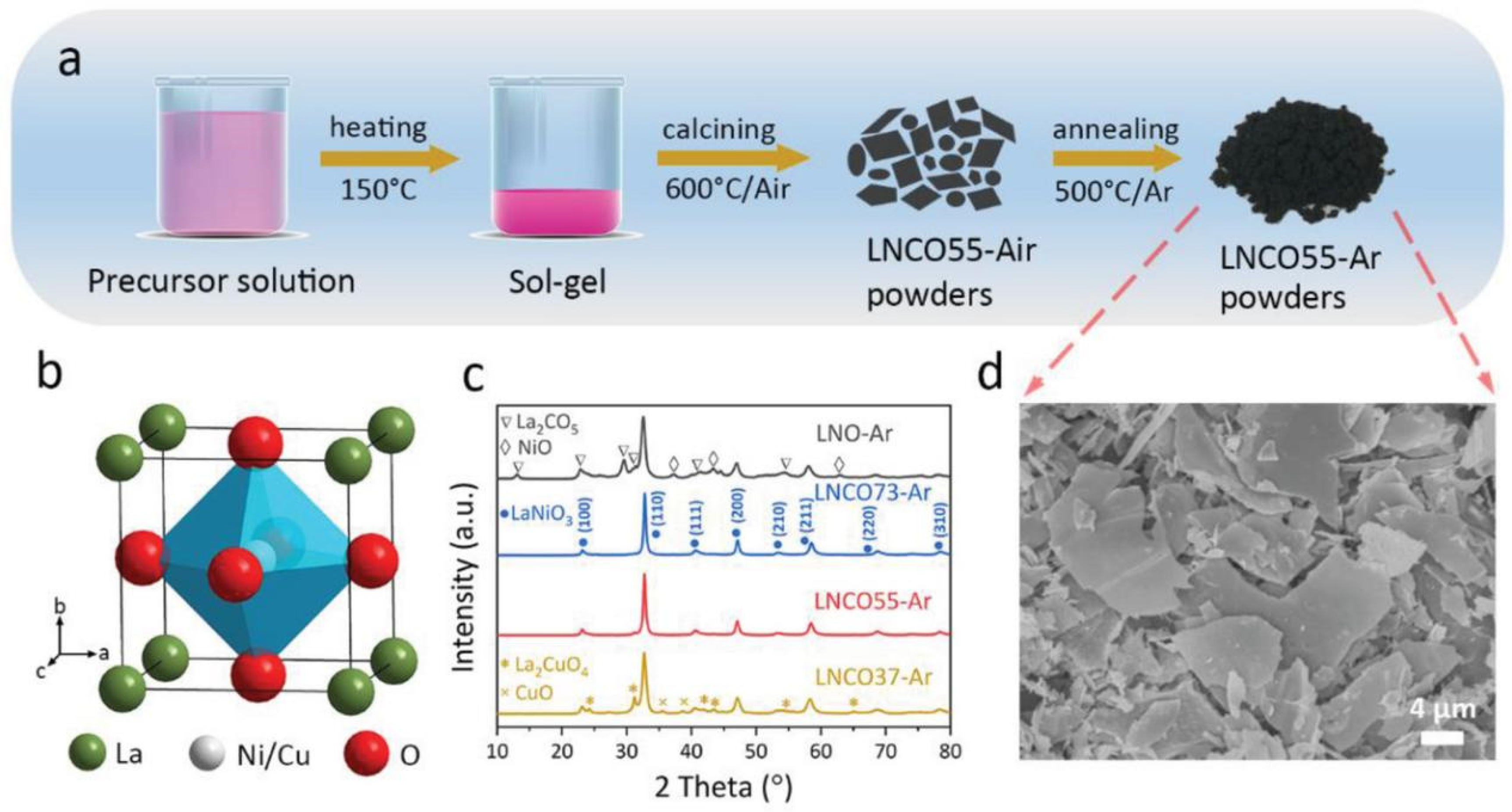
Perovskite oxides, generally ABO3, are emerging as a new class of AOR electrocatalysts due to their flexible composition and adjustable electronic structure. LaNi1-xCuxO3-𝛿 (LNCO) perovskite was synthesized via sol–gel melting and then reduced, as shown in Figure 2 [32]. When annealed in Ar gas, the LNCO catalyst possessed much higher AOR activity and stability in comparison with a commercial Pt/C catalyst. When the LNCO sample was fired in air, it was inactive toward the AOR. By doping Fe into LNCO and introducing A-site deficiencies, further enhanced oxidation performance and stability were achieved with a La0.9Ni0.6Cu0.4-xFexO3-δ (LNCF) catalyst [33]. The optimized activity of LNCF may be due to (i) the presence of iron, reducing the Gibbs free energy, and (ii) the presence of more oxygen vacancies, which may lead to greater surface exposure of active NiII and indirect enhancement of the AOR. A monolayer Ruddlesden–Popper oxide, La0.5Sr1.5Ni0.9Cu0.1O4-δ, was prepared via a modified Pechini method and subsequently annealed in Ar [34]. It could act as a robust AOR anode and achieved a current density of 13.4 mA cm−1 at a potential of 0.53 V versus Ag/AgCl in 0.5 M KOH with 0.055 M NH4Cl.
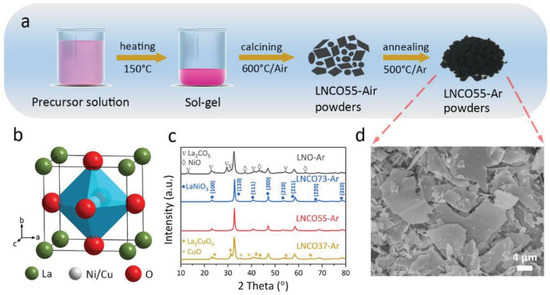
Figure 2.
(a) Schematic diagram of LNCO55-Ar powders production process (b) Perovskite structure. (c) XRD patterns of synthesized LNO-Ar, LNCO73-Ar, LNCO55-Ar, and LNCO37-Ar. (d) SEM image of LNCO55-Ar [32].
Cathode catalysts for ammonia fuel cells have also been developed. Perovskite oxides, such as LaCr0.25Fe0.25Co0.5O3-δ, SrFe0.8Cu0.1Nb0.1O3-δ, and SrCo0.8Cu0.1Nb0.1O3-δ [35,36,37], were proven to be promising cathode ORR catalysts. When LaCr0.25Fe0.25Co0.5O3-δ fired at 700 °C was employed as a cathode in a low-temperature ammonia–air fuel cell, an OCV of 0.72 V and a maximum current density of ~320 mA cm−2 were achieved, which were comparable to those achieved with Pt/C as the cathode [36]. Zijun Hu et al. prepared spinel Mn−Co−C via a hydrothermal method as cathode catalysts for low-temperature ammonia fuel cells. They displayed a good ORR performance with strong ammonia tolerance [38].
Several NiCu-based non-noble materials have been proved to be viable alternative AOR catalysts. Their use can remarkably reduce the cost of wastewater treatment. Experiments have indicated that ammonia can be removed from wastewater, such as landfill leachate, via the AOR [32,33,34]. By coupling the AOR with the ORR, self-powered nitrogen removal via an abiotic ammonia fuel cell can be realized [26].
3. Nitrate Wastewater Utilization for Energy Recovery
3.1. Ethanol–Nitrate Fuel Cell
The utilization of nitrate as an electron acceptor instead of O2 shows potential for self-powered removal via fuel cells. The standard redox potential of /N2 is 1.17 V vs. SHE in acidic conditions, which is close to 1.23 V of O2/H2O redox potential. Microbial fuel cells (MFCs) have been successfully developed as a self-powered technology for nitrate wastewater treatment [39,40,41]. However, high concentrations of nitrate and salt ions effectively reduce bacterial activity, which is the main shortcoming of the application of MFCs [42,43,44].
Recent progress has been made in the development of self-powered technology based on abiotic electrocatalysis of the nitrate reduction reaction (NRR). Kyeng-Bae Ma et al. attempted to replace O2 gas with nitrate at the cathode of an ethanol fuel cell (ethanol–nitrate fuel cell) [45]. Compared to the ethanol–O2 fuel cell, the ethanol–nitrate fuel cell exhibited improved the performance of OCV and the maximal power density owing to the low activation energy required for the NRR. Wei Xu et al. investigated the nitrogen removal process in ethanol–nitrate [46]. In order to improve the NRR rate and electrochemical selectivity of nitrate to N2 gas, carbon-supported PdCu was synthesized as a cathode catalyst. Electrochemical tests indicated that the reduction process of nitrate first involves the reduction of to and then to N2, in which the first step of formation is the rate-determining process. A maximum power density of 2.90 W m−2 with an OCV of 1.6 V was obtained at 20 °C when using a catholyte containing 300 ppm . After 24 h of cell operation, a nitrate removal efficiency of around 56% with a N2 selectivity of approximately 93% was achieved. The ethanol–nitrate fuel cell achieved successful nitrate removal with self-generated electricity.
3.2. Ammonia–Nitrate Fuel Cell
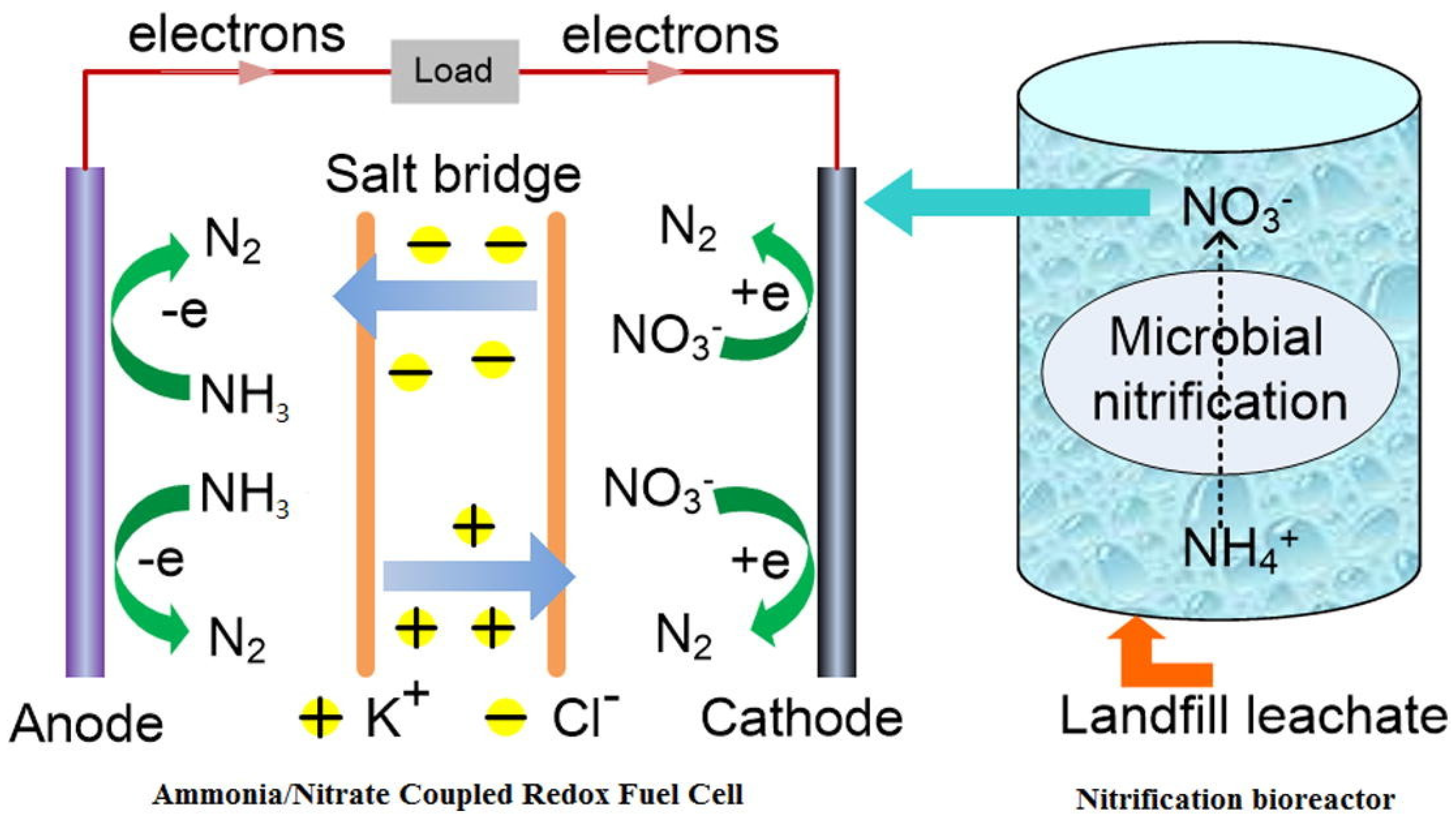
Novel fuel cells coupling the NRR with the AOR can engage in the nitrogen removal process at both anodes and cathodes simultaneously. Huimin Zhang et al. developed an ammonia–nitrate fuel cell, as shown in Figure 3, in which ammonia was catalytically oxidized at the anode and nitrate was reduced at the cathode to produce electricity [47]. A nitrate removal efficiency of 46.9% was achieved after a 18 h reaction, with 4.29 mM KNO3 in 0.1 M H2SO4 as the catholyte and 7.14 mM ammonia in 0.2 M KOH as the anolyte. Meanwhile, the maximum power density reached 170 mW m−2 when a Pd/C cathode was used as the catalyst. When NH4Cl–nitrate fuel cells and ammonia–nitrite fuel cells were applied, the removal efficiencies of N–NH4Cl and were 26.2% and 91.4%, respectively. The products of ammonia nitrification in leachate could be used as fuel cell anolyte. When using real leachate with the same initial NH3–N concentration, the nitrogen removal efficiency was 22.9%. A urea–nitrate fuel cell was invented by Senthilkumar Nangan et al. for the treatment of N-rich effluent to couple urea oxidation and nitrate reduction [48]. In order to achieve a higher cell performance, a hybrid system comprising alkaline urea and acid nitrate was applied and separated by a bipolar membrane. The bipolar membrane could function well as a separator to keep the pH gradient stable during the fuel cell discharge [49]. Nitrogen-doped carbon sheets supporting Ni@NiO-Cu@CuO composites were prepared and used as bifunctional catalysts for urea electrooxidation and nitrate reduction. The fabricated urea–nitrate fuel cell exhibited an enhanced fuel cell power density of 22.55 ± 2.3 mW cm−2 with urine and nitrate degraded at the anode and cathode, respectively.
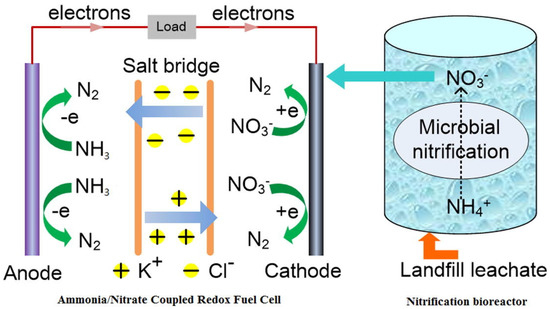
Figure 3.
Schematic diagram of NH3− fuel cell mechanism integrated with nitrification [47].
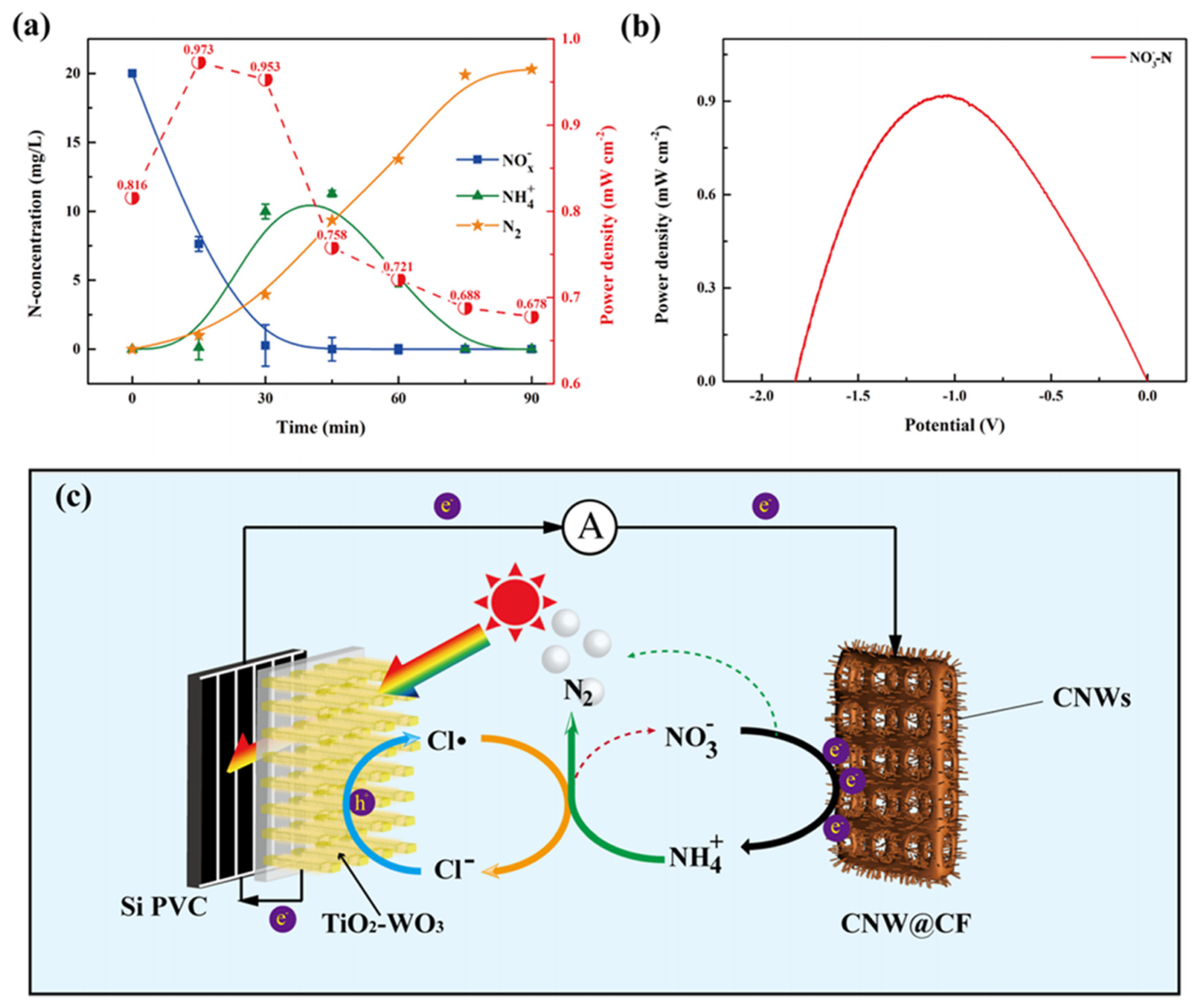
The main products of an NRR are ammonia and N2, and the selectivity of ammonia and N2 varies with the NRR catalyst and electrolyte conditions [50,51,52]. In order to achieve total nitrogen removal, ammonia products should be eliminated. Changhui Zhou et al. developed a novel denitrification fuel cell combining electrochemical catalysis of the NRR and photochemical catalysis of the AOR [53]. Instead of the direct reduction of nitrate to nitrogen gas, nitrate is first reduced to ammonium on a Cu foam cathode modified by a three-dimensional copper nanowire (CNW). Then, ammonium is oxidized to N2 by the highly oxidizing free chlorine radicals (Cl•) generated via photocatalysis in the TiO2−WO3 anode side. The electrode reactions are as follows.
As shown in Figure 4, the highest power density reached 0.973 mW cm−2 at pH = 5 with 0.03 M Cl− and a 1:7 ratio of to NO3−. Cl• plays a dominant role in the oxidation of , and quickly oxidized to mainly N2 and slight NO3−. Overoxidized NO3− was continually eliminated at the CNW@CF cathode, and the denitrification efficiency reached more than 99% via an exhaustive cycle.
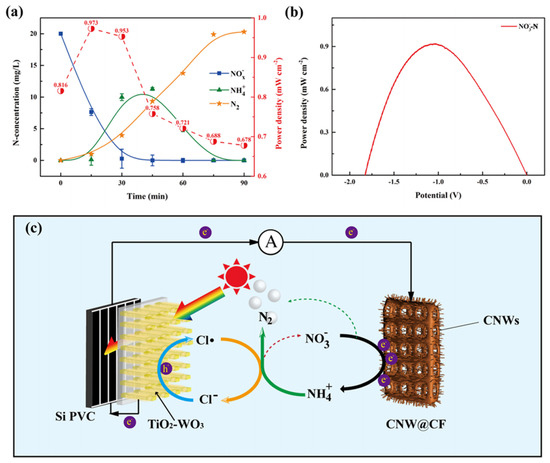
Figure 4.
(a) N concentration of NH4+, NOx−, and N2 (left panel, Y axis) and power density (right panel, Y axis) in the DFC system during the reaction. Under the conditions of 100 mW cm−2 solar energy, pH = 5, 0.03 M concentration of Cl−. (b) Power density at different potentials with the highest power density conditions of NO3−−N. (c) Schematic diagram of the NO3−−to N2−transformation in the DFC system [53].
4. Perspectives
Although relevant research has demonstrated the feasibility of self-powered nitrogen removal via abiotic electrochemical catalysis, there are still some shortcomings. First, the newly developed noble-metal-free catalysts are nickel-based catalysts, which achieve comparable activity to noble-metal catalysts only in alkaline conditions. However, nitrogen-containing wastewaters are mainly neutral or alkaline. Second, the AOR and NRR are multiple electron transfer reactions; thus, it is important to enhance the detection of products at each stage. Third, the degradation of the gas diffusion layer of fuel cells is a key factor in cell performance and stability. Recent work by Wang et al. reported that polytetrafluoroethylene (PTFE) content affects the antiaging performance via lattice Boltzmann simulation [54].
In addition to ammonia and urea, amino acids are a common nitrogenous substance. The fast growth of global energy needs and rise of environmental issues caused by the utilizations of conventional fossil fuel have prompted the search for extensive, cost-effective, and environmentally friendly renewable energy sources. Biomass is a promising resource due to its abundance and carbon neutrality. Biofuel production is predicted to reach several hundred or even over 1000 EJ yr−1 by 2050 [55]. This large-scale conversion process of biomass to biofuels will lead to a new environmental concern regarding protein bioresidue treatment.
Nowadays, biorefinery mainly focuses on utilizing compounds based on C, H, and O in the biomass to produce biofuel, such as lipid and carbohydrates. However, this approach overlooks the utilization of N-based compounds (protein) in the biomass since toxic constituents are produced when they are burned. Nitrogen comprises around 2~10 wt% of the biomass [56]. Additionally, protein comprises 3~13% of the non-food biomass [57]. For instance, approximately 9.1 million tons of protein byproducts were generated from corn ethanol production in 2010. However, these bioresidues can currently be used solely as animal feed, and consequently become greenhouse gases (N2O) eventually.
An innovative approach to producing biofuels from a protein-based biomass was proposed by Huo et al. [58]. They applied metabolic engineering in Escherichia coli to convert amino acids to carbon skeletons via deamination, and further transformed these organisms into biofuels or chemicals. However, several challenges exist in this approach. For example, deamination is highly reversible in thermodynamics, and this will largely restrict the reaction efficiency. Additionally, proteins contain 20 different amino acids with varying carbon skeletons. As a result, several products will be derived from the deamination process in a highly selective biosystem, complicating the further conversion of deamination products to biofuels. Furthermore, the products’ toxicity to microbes remains a pertinent issue.
Future innovative work, therefore, should aim to directly convert the chemical energy in amino acids to electricity via chemical fuel cells without the complex deamination process. Amino acids fuel cells can also provide a new approach to waste treatment with energy retrieval via directly using protein residues or a protein-based biomass from microalgae. Further, here, we propose an energy retrieval route via using microalgae culture as amino acids sources. Microalgae, the third-generation biomass, can potentially be cultivated via using N-rich municipal wastewater, such as human urine, for nitrogen removal and biomass production. Additionally, protein-rich microalgae are reported to have a faster biomass production rate than lipid-rich microalgae, because protein production does not require a nutrient starvation period, as is the case for lipid-rich microalgae. This indicates that it would be easier to achieve a large amount of biomass for energy production from microalgae when the target products are proteins rather than lipids. Accordingly, protein-rich microalgae represent a promising feedstock for proteins and amino acids.
Chemical fuel cell systems have advantages in terms of operating conditions. First, the pH of nonmicrobial systems can be adjusted to well above neutral. Thus, the amino acid concentration in anode feeding can become very high as the solubility of amino acids in alkaline conditions is much larger than in neutral conditions. Anode feeding with a high content of amino acids is necessary to increase the energy density of fuel cells. Second, alkaline fuel cells have the advantage of superior reaction kinetics in alkaline media with an enhancement of power output.
It is worth noting that amino acids will release ammonia, especially at high temperatures and alkaline pH. Even trace levels of ammonia (>0.1 ppm) will poison the conventional proton exchange membrane (PEM), which is widely used as a polymer electrolyte for fuel cells, leading to poor conductivity. Consequently, the power density of fuel cells will be largely limited due to the poor conductivity of the PEM. To avoid this, fuel cells with an anion exchange membrane (AEM) may be a rational system and can be applied to inspect energy retrieval ability.
Electrocatalysts based on transition-metal Ni showed a high catalytic activity of electrooxidation reactions toward organics containing a NH2 group. They have a wide range of applications in ammonia and urea electrooxidation as anode catalysts. Due to their high performance and low cost, nickel-based bimetals supported by carbon black may be promising substitutions for noble metals as anode catalysts for amino acid fuel cells (AAFCs) to directly convert the chemical energy in amino acids and proteins into electricity.
There are 20 amino acids, each with a different side chain group type, including glycine (aliphatic), proline (cyclic), serine (containing hydroxyl), methionine (containing sulfur), tyrosine and phenylalanine (aromatic), etc. In order to successfully produce electricity from protein-derived amino acids in AAFCs, anode selectivity is key due to the diversity of amino acids. If the anode catalyst of AAFCs has catalytic activity toward only one type of amino acid, the output processes will be challenging and costly, as the diverse range of amino acids must be purified. By contrast, if the catalyst presents high catalytic activity toward the electrooxidation of all 20 amino acid reactions, the energy production will be significantly reduced. Thus, it would be important to confirm whether catalysts demonstrate catalytic activity toward all these amino acids, and if they can serve as anode catalysts in the electrooxidation of amino acid (EAA) reactions.
5. Conclusions
In this work, we reviewed the recent research progress in self-powered nitrogen removal via abiotic electrochemical catalysis. Novel system designs of fuel cells coupled with functional nanomaterials enable nitrogen removal at both the anode and cathode. The innovative integration of energy collectors and pollutant degradation allow for a self-powered nitrogen removal system. The advances in self-powered nitrogen removal systems provide a new approach to the treatment of non-biodegradable wastewater.
Despite recent material developments in this area, many challenges need to be addressed before their practical application. First, these products’ selectivity, catalysis activity, and operation stability in real wastewater, especially wastewater with complex contaminants, need to be enhanced. Second, innovations in structural design, materials science, and systems integration are critical to making key components suitable for scaled-up manufacturing. Third, the combination of different environmental technologies and efficient power management strategies would enhance the applicability of self-powered nitrogen removal systems under complex conditions.
Author Contributions
Conceptualization, B.Y. and W.X.; investigation, B.Y. and W.X.; writing—original draft preparation, B.Y. and W.X.; writing—review and editing, B.Y. and W.X.; supervision, Y.J.; project administration, Y.J.; funding acquisition, B.Y. and Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by fund of National Natural Science Foundation of China (Grant Nos. 22202146, 2272115, 22202145, 22202147), Zhejiang Provincial Natural Science Foundation of China (Grant No. LGG21B070001), Key Research and Development Plan of Zhejiang Province (Grant No. 2021C03022) and National Innovation Training program for college students (Grant No. 202210350038).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Erisman, J.W. How ammonia feeds and pollutes the world. Science 2021, 374, 685–686. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.-X.; Wernick, D.G.; Liao, J.C. Toward nitrogen neutral biofuel production. Curr. Opin. Biotechnol. 2012, 23, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.E.; Allen, M.S.; Laser, M.; Lynd, L.R. Protein feeds coproduction in biomass conversion to fuels and chemicals. Biofuels Bioprod. Biorefining 2009, 3, 219–230. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.S.; Tao, S. Ammonia and related chemicals as potential indirect hydrogen storage materials. Interna-Tional J. Hydrog. Energy 2012, 37, 1482–1494. [Google Scholar] [CrossRef]
- Morrissy, J.G.; Currell, M.J.; Reichman, S.M.; Surapaneni, A.; Megharaj, M.; Crosbie, N.D.; Hirth, D.; Aquilina, S.; Ra-jendram, W.; Ball, A.S. Nitrogen contamination and bioremediation in groundwater and the environment: A review. Earth-Sci. Rev. 2021, 222, 103816. [Google Scholar] [CrossRef]
- Jauzein, C.; Couet, D.; Blasco, T.; Lemée, R. Uptake of dissolved inorganic and organic nitrogen by the benthic toxic di-noflagellate Ostreopsis cf. ovata. Harmful Algae 2017, 65, 9–18. [Google Scholar] [CrossRef]
- Jones, L.; Provins, A.; Holland, M.; Mills, G.; Hayes, F.; Emmett, B.; Hall, J.; Sheppard, L.; Smith, R.; Sutton, M.; et al. A review and application of the evidence for nitrogen impacts on eco-system services. Ecosyst. Serv. 2014, 7, 76–88. [Google Scholar] [CrossRef]
- Townsend, A.R.; Howarth, R.W.; Bazzaz, F.A.; Booth, M.S.; Cleveland, C.C.; Collinge, S.K.; Dobson, A.P.; Epstein, P.R.; Holland, E.A.; Keeney, D.R.; et al. Human health effects of a changing global nitrogen cycle. Front. Ecol. Environ. 2003, 1, 240–246. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, Y.; Huang, D.; Fan, J.; Du, R. Enhanced Nitrogen Removal from Domestic Wastewater by Par-tial-Denitrification/Anammox in an Anoxic/Oxic Biofilm Reactor. Processes 2022, 10, 109. [Google Scholar] [CrossRef]
- Chys, M.; Declerck, W.; Audenaert, W.T.M.; Van Hulle, S.W.H. UV/H2O2, O3 and (photo-) Fenton as treatment prior to granular activated carbon filtration of biologically stabilized landfill leachate. J. Chem. Technol. Biotechnol. 2015, 90, 525–533. [Google Scholar] [CrossRef]
- Prüsse, U.; Hähnlein, M.; Daum, J.; Vorlop, K.-D. Improving the catalytic nitrate reduction. Catal. Today 2000, 55, 79–90. [Google Scholar] [CrossRef]
- Samatya, S.; Kabay, N.; Yüksel, Ü.; Arda, M.; Yüksel, M. Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React. Funct. Polym. 2006, 66, 1206–1214. [Google Scholar] [CrossRef]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef]
- Fellah Jahromi, A.; Elektorowicz, M. Modified electrochemical processes for industrial-scale treatment of wastewater having high TKN content. Electrochim. Acta 2020, 354, 136724. [Google Scholar] [CrossRef]
- Jeerh, G.; Zhang, M.; Tao, S. Recent progress in ammonia fuel cells and their potential applications. J. Mater. Als Chem. A 2021, 9, 727–752. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Jones, J.; Dupont, V.; Twigg, M.V. Urea as a hydrogen carrier: A perspective on its potential for safe, sustainable and long-term energy supply. Energy Environ. Sci. 2011, 4, 1216–1224. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct Electrolytic Splitting of Seawater: Opportunities and Challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Estejab, A.; Daramola, D.A.; Botte, G.G. Mathematical model of a parallel plate ammonia electrolyzer for combined wastewater remediation and hydrogen production. Water Res. 2015, 77, 133–145. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Kou, Y.; Liu, Z.; Chen, X.; Li, Y.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Pt-Decorated highly porous flower-like Ni particles with high mass activity for ammonia electro-oxidation. J. Mater. Chem. A 2016, 4, 11060–11068. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Pillai, H.S.; Lattimer, J.; Mohd Adli, N.; Karakalos, S.; Chen, M.; Guo, L.; Xu, H.; Yang, J.; et al. Ternary PtIrNi Catalysts for Efficient Electrochemical Ammonia Oxidation. ACS Catal. 2020, 10, 3945–3957. [Google Scholar] [CrossRef]
- Li, Y.; Pillai, H.S.; Wang, T.; Hwang, S.; Zhao, Y.; Qiao, Z.; Mu, Q.; Karakalos, S.; Chen, M.; Yang, J.; et al. High-performance ammonia oxidation catalysts for anion-exchange membrane direct ammonia fuel cells. Energy Environ. Sci. 2021, 14, 1449–1460. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Leon, M.N.; Fernandes, C.M.; Antoniassi, R.M.; Alves, O.C.; Ponzio, E.A.; Silva, J.C.M. PtSnO2/C and Pt/C with preferential (100) orientation: High active electrocatalysts for ammonia electro-oxidation reaction. Appl. Catal. B Environ. 2020, 264, 118458. [Google Scholar] [CrossRef]
- Jing, H.; Yang, H.; Yu, X.; Hu, C.; Li, R.; Li, H. Treatment of organic matter and ammonia nitrogen in wastewater by elec-trocatalytic oxidation: A review of anode material preparation. Environ. Sci. Water Res. Technol. 2022, 8, 226–248. [Google Scholar] [CrossRef]
- Xu, W.; Lan, R.; Du, D.; Humphreys, J.; Walker, M.; Wu, Z.; Wang, H.; Tao, S. Directly growing hierarchical nickel-copper hydroxide nanowires on carbon fibre cloth for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2017, 218, 470–479. [Google Scholar] [CrossRef]
- Xu, W.; Du, D.; Lan, R.; Humphreys, J.; Miller, D.N.; Walker, M.; Wu, Z.; Irvine, J.T.S.; Tao, S. Electrodeposited NiCu bi-metal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2018, 237, 1101–1109. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, P.; Jeerh, G.; Chen, S.; Shields, J.; Wang, H.; Tao, S. Electricity Generation from Ammonia in Landfill Leachate by an Alkaline Membrane Fuel Cell Based on Precious-Metal-Free Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12817–12824. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, Y.F.; Kwok, Y.H.; Wu, Z.C.; Xia, D.H.; Leung, D.Y.C. A Direct Ammonia Microfluidic Fuel Cell using NiCu Nanoparticles Supported on Carbon Nanotubes as an Electrocatalyst. ChemSusChem 2018, 11, 2889–2897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, W.; Wang, H.; Tong, X.; Wang, Y.; Yang, X.; Wu, Z.; Liu, Z. A core-shell NiCu@NiCuOOH 3D electrode induced by surface electrochemical reconstruction for the ammonia oxidation reaction. Int. J. Hydrog. Energy 2022, 47, 16080–16091. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Jeerh, G.; Zou, P.; Sun, B.; Walker, M.; Xie, K.; Tao, S. A symmetric direct ammonia fuel cell using ternary NiCuFe alloy embedded in a carbon network as electrodes. J. Mater. Chem. A 2022, 10, 18701–18713. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Y.; Xi, S.; Diao, C.; Yu, Z.; Lee, W.S.V.; Xue, J. Deciphering NH3 Adsorption Kinetics in Ternary Ni–Cu–Fe Oxyhydroxide toward Efficient Ammonia Oxidation Reaction. Small 2021, 17, 2005616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Tong, X.; Zhou, L.; Yang, X.; Wang, Y.; Zhang, M.; Wu, Z. Sulfur induced surface reconfiguration of Ni1Cu3-S-T/CP anode for high-efficiency ammonia electro-oxidation. Chem. Eng. J. 2023, 452, 139582. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Duan, X.; Zou, P.; Jeerh, G.; Sun, B.; Chen, S.; Humphreys, J.; Walker, M.; Xie, K.; et al. An Efficient Symmetric Electrolyzer Based On Bifunctional Perovskite Catalyst for Ammonia Electrolysis. Adv. Sci. 2021, 8, 2101299. [Google Scholar] [CrossRef]
- Jeerh, G.; Zou, P.; Zhang, M.; Chen, S.; Humphreys, J.; Tao, S. Electrooxidation of ammonia on A-site deficient perov-skite oxide La0.9Ni0.6Cu0.35Fe0.05O3−δ for wastewater treatment. Sep. Purif. Technol. 2022, 297, 121451. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, P.; Jeerh, G.; Sun, B.; Walker, M.; Tao, S. Oxygen Vacancy-Rich La0.5Sr1.5Ni0.9Cu0.1O4–δ as a High-Performance Bifunctional Catalyst for Symmetric Ammonia Electrolyzer. Adv. Funct. Mater. 2022, 32, 2204881. [Google Scholar] [CrossRef]
- Zou, P.; Chen, S.; Lan, R.; Humphreys, J.; Jeerh, G.; Tao, S. Investigation of perovskite oxide SrFe0.8Cu0.1Nb0.1O3−δ as cathode for a room temperature direct ammonia fuel cell. Int. J. Hydrog. Energy 2019, 44, 26554–26564. [Google Scholar] [CrossRef]
- Jeerh, G.; Zou, P.; Zhang, M.; Tao, S. Perovskite oxide LaCr0.25Fe0.25Co0.5O3−δ as an efficient non-noble cathode for direct ammonia fuel cells. Appl. Catal. B Environ. 2022, 319, 121919. [Google Scholar] [CrossRef]
- Zou, P.; Chen, S.; Lan, R.; Tao, S. Investigation of Perovskite Oxide SrCo0.8Cu0.1Nb0.1O3–δ as a Cathode Material for Room Temperature Direct Ammonia Fuel Cells. ChemSusChem 2019, 12, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xiao, Q.; Xiao, D.; Wang, Z.; Gui, F.; Lei, Y.; Ni, J.; Yang, D.; Zhang, C.; Ming, P. Synthesis of Anti-poisoning Spinel Mn–Co–C as Cathode Catalysts for Low-Temperature Anion Exchange Membrane Direct Ammonia Fuel Cells. ACS Appl. Mater. Interfaces 2021, 13, 53945–53954. [Google Scholar] [CrossRef]
- Cucu, A.; Tiliakos, A.; Tanase, I.; Serban, C.E.; Stamatin, I.; Ciocanea, A.; Nichita, C. Microbial Fuel Cell for Nitrate Reduc-tion. Energy Procedia 2016, 85, 156–161. [Google Scholar] [CrossRef]
- Oon, Y.S.; Ong, S.A.; Ho, L.N.; Wong, Y.S.; Oon, Y.L.; Lehl, H.K.; Thung, W.E. Microbial fuel cell operation using nitrate as terminal electron acceptor for simultaneous organic and nutrient removal. Int. J. Environ. Sci. Technol. 2017, 14, 2435–2442. [Google Scholar] [CrossRef]
- Fang, C.; Min, B.; Angelidaki, I. Nitrate as an Oxidant in the Cathode Chamber of a Microbial Fuel Cell for Both Power Generation and Nutrient Removal Purposes. Appl. Biochem. Biotechnol. 2011, 164, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cheng, S.; Yang, J.; Li, C.; Sun, Y.; Cen, K. Effect of nitrate on electricity generation in single-chamber air cathode microbial fuel cells. Chem. Eng. J. 2018, 337, 661–670. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Yu, H.; Yi, X.; Wei, C. Simultaneous phenol removal, nitrification and denitrification using microbial fuel cell technology. Water Res. 2015, 76, 160–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Bai, J.; Li, X.; Shen, Z.; Xia, L.; Chen, S.; Xu, Q.; Zhou, B. Total organic carbon and total nitrogen removal and simultaneous electricity generation for nitrogen-containing wastewater based on the catalytic reactions of hydroxyl and chlorine radicals. Appl. Catal. B Environ. 2018, 238, 168–176. [Google Scholar] [CrossRef]
- Ma, K.-B.; Han, S.-B.; Kwon, S.-H.; Kwak, D.-H.; Park, K.-W. High-performance direct ethanol fuel cell using nitrate reduc-tion reaction. Int. J. Hydrog. Energy 2018, 43, 17265–17270. [Google Scholar] [CrossRef]
- Xu, W.; Yang, X.; Zhang, H.; Wu, Z. PdCu/C Catalyst with Electricity Self-Generation via a Fuel Cell for Electroreduction of Nitrate. ACS Appl. Energy Mater. 2022, 5, 10767–10775. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W.; Feng, D.; Liu, Z.; Wu, Z. Self-powered denitration of landfill leachate through ammonia/nitrate cou-pled redox fuel cell reactor. Bioresour. Technol. 2016, 203, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nangan, S.; Ding, Y.; Alhakemy, A.Z.; Liu, Y.; Wen, Z. Hybrid alkali-acid urea-nitrate fuel cell for degrading nitrogen-rich wastewater. Appl. Catal. B Environ. 2021, 286, 119892. [Google Scholar] [CrossRef]
- Giesbrecht, P.K.; Freund, M.S. Recent Advances in Bipolar Membrane Design and Applications. Chem. Mater. 2020, 32, 8060–8090. [Google Scholar] [CrossRef]
- Zhou, C.; Bai, J.; Zhang, Y.; Li, J.; Li, Z.; Jiang, P.; Fang, F.; Zhou, M.; Mei, X.; Zhou, B. Novel 3D Pd-Cu(OH)2/CF cathode for rapid reduction of nitrate-N and simultaneous total nitrogen removal from wastewater. J. Hazard. Mater. 2021, 401, 123232. [Google Scholar] [CrossRef]
- Su, L.; Li, K.; Zhang, H.; Fan, M.; Ying, D.; Sun, T.; Wang, Y.; Jia, J. Electrochemical nitrate reduction by using a novel Co3O4/Ti cathode. Water Res. 2017, 120, 1–11. [Google Scholar] [CrossRef] [PubMed]
- van Langevelde, P.H.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic Nitrate Reduction for Sustainable Ammonia Pro-duction. Joule 2021, 5, 290–294. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Zhang, Y.; Bai, J.; Li, L.; Mei, X.; Guan, X.; Zhou, B. Novel Denitrification Fuel Cell for Energy Recovery of Nitrate-N and TN Removal Based on NH4+ Generation on a CNW@CF Cathode. Environ. Sci. Technol. 2022, 56, 2562–2571. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; He, W.; Zhao, Y.; Wang, X. Lattice Boltzmann simulation of the structural degradation of a gas diffusion layer for a proton exchange membrane fuel cell. J. Power Sources 2023, 556, 232452. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A.; Gross, R. Global bioenergy resources. Nat. Clim. Chang. 2014, 4, 99–105. [Google Scholar] [CrossRef]
- Rittmann, B. Opportunities for renewable bioenergy using microorganisms. Biotechnol. Bioeng. 2008, 100, 203–212. [Google Scholar] [CrossRef]
- Haberl, H.; Beringer, T.; Bhattacharya, S.; Erb, K.; Hoogwijk, M. The global technical potential of bio-energy in 2050 considering sustainability constraints. Curr. Opin. Environ. Sustain. 2010, 2, 394–403. [Google Scholar] [CrossRef]
- Huo, Y.; Cho, K.; Rivera, J.; Monte, E.; Shen, C.; Yan, Y.; Liao, J. Conversion of proteins into biofuels by engineering nitrogen flux. Nat. Biotechnol. 2011, 29, 346–352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).