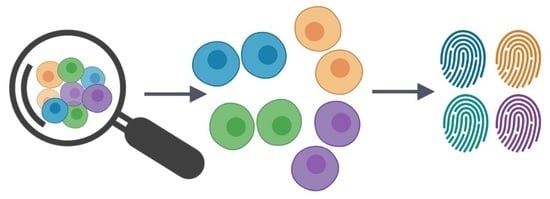
Single-Cell Techniques in Environmental Microbiology
Abstract
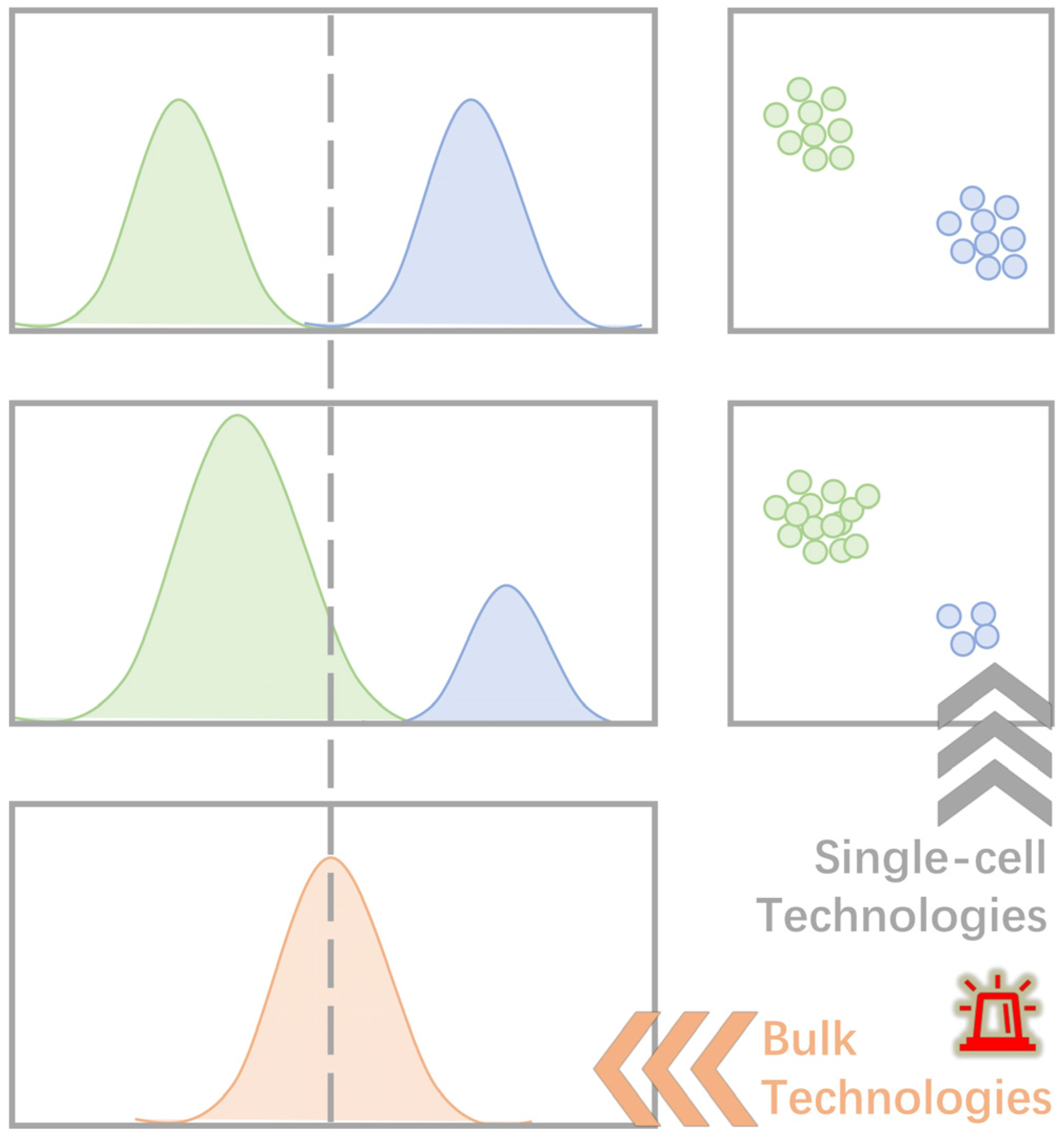
1. Introduction
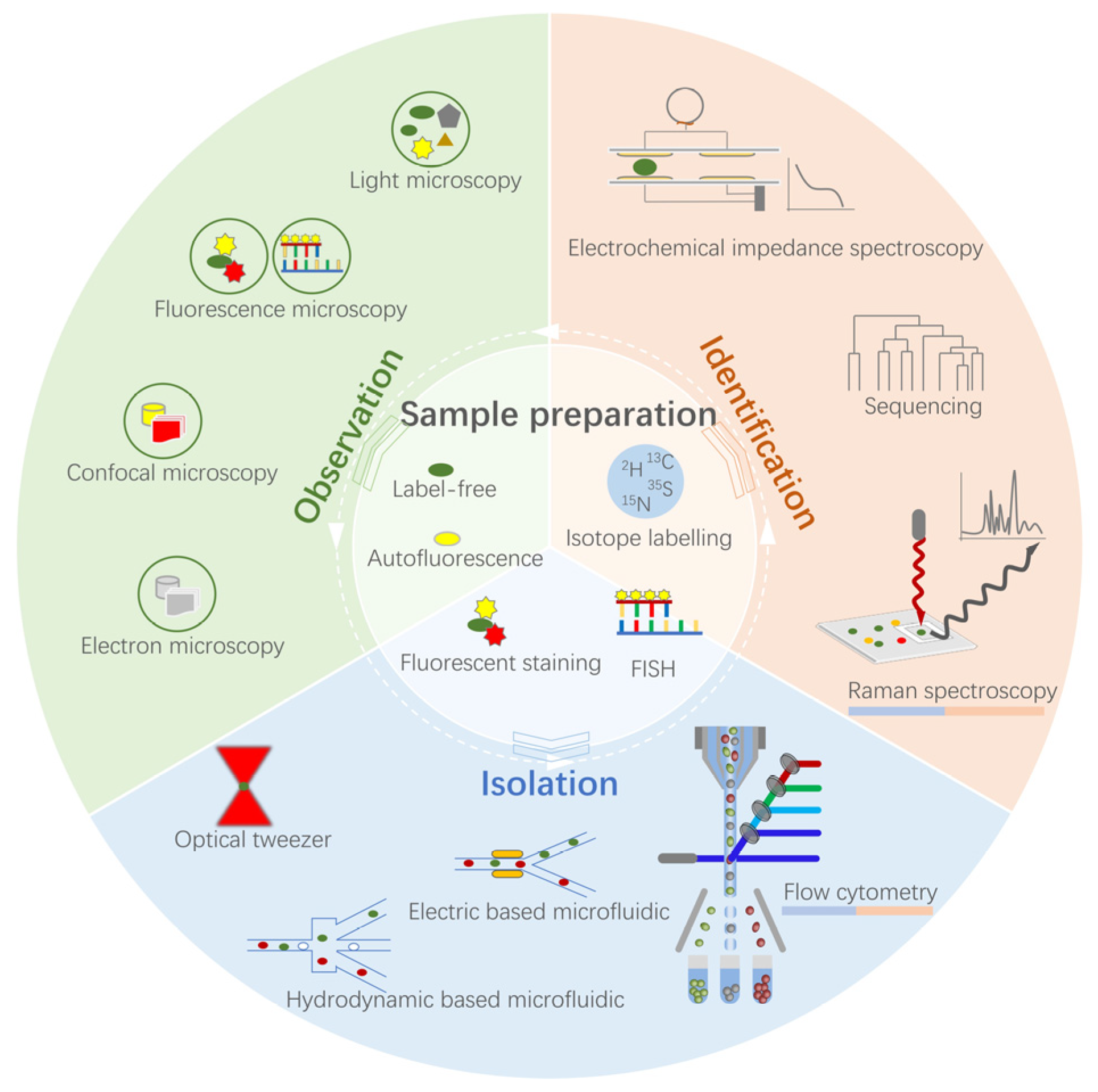
2. Single-Cell Techniques
2.1. Microscopic Observation
2.2. Sequencing Identification
2.3. Flow Cytometric Identification and Isolation
2.4. Raman Spectroscopy-Based Identification and Isolation
2.5. Integrated Microfluidic Single-Cell Techniques
3. Applications in Environmental Microbiology
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, N. The unseen world: Reflections on Leeuwenhoek (1677) ‘Concerning little animals’. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140344. [Google Scholar] [CrossRef] [PubMed]
- Hugerth, L.W.; Andersson, A.F. Analysing Microbial Community Composition through Amplicon Sequencing: From Sampling to Hypothesis Testing. Front. Microbiol. 2017, 8, 1561. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Eisen, J.A.; Salzberg, S.L. Microbial genome sequencing. Nature 2000, 406, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Newman, J.A.; Silverman, B.W.; Turner, S.L.; Lilley, A.K. The contribution of species richness and composition to bacterial services. Nature 2005, 436, 1157–1160. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Genet. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Khulan, A.; Kim, J. Development of a novel cultivation technique for uncultured soil bacteria. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Nichols, D.; Lewis, K.; Orjala, J.; Mo, S.; Ortenberg, R.; O’Connor, P.; Zhao, C.; Vouros, P.; Kaeberlein, T.; Epstein, S.S. Short Peptide Induces an “Uncultivable” Microorganism to Grow In Vitro. Appl. Environ. Microbiol. 2008, 74, 4889–4897. [Google Scholar] [CrossRef]
- Lane, D.J.; Pace, B.; Olsen, G.J.; Stahl, D.A.; Sogin, M.L.; Pace, N.R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc. Natl. Acad. Sci. USA 1985, 82, 6955–6959. [Google Scholar] [CrossRef]
- Stein, J.L.; Marsh, T.; Wu, K.Y.; Shizuya, H.; DeLong, E.F. Characterization of uncultivated prokaryotes: Isolation and analysis of a 40-kilobase-pair genome fragment from a planktonic marine archaeon. J. Bacteriol. 1996, 178, 591–599. [Google Scholar] [CrossRef]
- Props, R.; Kerckhof, F.-M.; Rubbens, P.; De Vrieze, J.; Sanabria, E.H.; Waegeman, W.; Monsieurs, P.; Hammes, F.; Boon, N. Absolute quantification of microbial taxon abundances. ISME J. 2017, 11, 584–587. [Google Scholar] [CrossRef]
- Paddock, S.W. Confocal Laser Scanning Microscopy. Biotechniques 1999, 27, 10. [Google Scholar] [CrossRef] [PubMed]
- Croix, C.M.S.; Shand, S.H.; Watkins, S.C. Confocal microscopy: Comparisons, applications, and problems. Biotechniques 2005, 39, S2–S5. [Google Scholar] [CrossRef]
- Da Costa, S.G.; Richter, A.; Schmidt, U.; Breuninger, S.; Hollricher, O. Confocal Raman microscopy in life sciences. Morphologie 2019, 103, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ang, R.B.Q.; Nisar, H.; Khan, M.B.; Tsai, C.-Y. Image segmentation of activated sludge phase contrast images using phase stretch transform. Microscopy 2019, 68, 144–158. [Google Scholar] [CrossRef]
- Campbell, K.; Wang, J.; Daniels, M. Assessing activated sludge morphology and oxygen transfer performance using image analysis. Chemosphere 2019, 223, 694–703. [Google Scholar] [CrossRef]
- De Jonge, N.; Ross, F.M. Electron microscopy of specimens in liquid. Nat. Nanotechnol. 2011, 6, 10. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, M.; Susanto, J.; Li, X.; Leung, W.; Xu, K. Transforming Rhodamine Dyes for (d)STORM Super-Resolution Microscopy via 1,3-Disubstituted Imidazolium Substitution. Angew. Chem. Int. Ed. 2022, 61, e202113612. [Google Scholar] [CrossRef]
- Mardis, E.R. A decade’s perspective on DNA sequencing technology. Nature 2011, 470, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Kulski, J.K. Next-Generation Sequencing—An Overview of the History, Tools, and “Omic” Applications. In Next Generation Sequencing—Advances, Applications and Challenges; Kulski, J.K., Ed.; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Woyke, T.; Doud, D.F.R.; Schulz, F. The trajectory of microbial single-cell sequencing. Nat. Methods 2017, 14, 1045–1054. [Google Scholar] [CrossRef]
- Zhao, N.; Cao, J.; Xu, J.; Liu, B.; Liu, B.; Chen, D.; Xia, B.; Chen, L.; Zhang, W.; Zhang, Y.; et al. Targeting RNA with Next- and Third-Generation Sequencing Improves Pathogen Identification in Clinical Samples. Adv. Sci. 2021, 8, 2102593. [Google Scholar] [CrossRef]
- Land, M.; Hauser, L.; Jun, S.-R.; Nookaew, I.; Leuze, M.R.; Ahn, T.-H.; Karpinets, T.; Lund, O.; Kora, G.; Wassenaar, T.; et al. Insights from 20 years of bacterial genome sequencing. Funct. Integr. Genom. 2015, 15, 141–161. [Google Scholar] [CrossRef]
- Greninger, A.L.; Naccache, S.N.; Federman, S.; Yu, G.; Mbala, P.; Bres, V.; Stryke, D.; Bouquet, J.; Somasekar, S.; Linnen, J.M.; et al. Rapid metagenomic identification of viral pathogens in clinical samples by real-time nanopore sequencing analysis. Genome Med. 2015, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Zhang, X.; Zhang, S.; Song, H.; Kong, Z. Insights into the bacterial species and communities of a full-scale anaerobic/anoxic/oxic wastewater treatment plant by using third-generation sequencing. J. Biosci. Bioeng. 2019, 128, 744–750. [Google Scholar] [CrossRef]
- Hutter, K.-J.; Eipel, H.E. Flow cytometric determinations of cellular substances in algae, bacteria, moulds and yeasts. Antonie Van Leeuwenhoek 1978, 44, 269–282. [Google Scholar] [CrossRef]
- Vives-Rego, J.; LeBaron, P.; Caron, G.N.-V. Current and future applications of flow cytometry in aquatic microbiology. FEMS Microbiol. Rev. 2000, 24, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Nebe-Von-Caron, G. Functional single-cell analyses: Flow cytometry and cell sorting of microbial populations and communities. FEMS Microbiol. Rev. 2010, 34, 554–587. [Google Scholar] [CrossRef]
- Wang, Y.; Hammes, F.; De Roy, K.; Verstraete, W.; Boon, N. Past, present and future applications of flow cytometry in aquatic microbiology. Trends Biotechnol. 2010, 28, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, N.; Hübschmann, T.; Schattenberg, F.; Kerckhof, F.-M.; Overmann, J.; Müller, S. Bacterial mock communities as standards for reproducible cytometric microbiome analysis. Nat. Protoc. 2020, 15, 2788–2812. [Google Scholar] [CrossRef]
- Liu, Z.; Müller, S. Bacterial Community Diversity Dynamics Highlight Degrees of Nestedness and Turnover Patterns. Cytom. Part A 2020, 97, 742–748. [Google Scholar] [CrossRef]
- Guo, Y.; Baumgart, S.; Stärk, H.-J.; Harms, H.; Müller, S. Mass Cytometry for Detection of Silver at the Bacterial Single Cell Level. Front. Microbiol. 2017, 8, 1326. [Google Scholar] [CrossRef]
- Guo, Y.; Cichocki, N.; Schattenberg, F.; Geffers, R.; Harms, H.; Müller, S. AgNPs Change Microbial Community Structures of Wastewater. Front. Microbiol. 2019, 9, 3211. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Harms, H.; Müller, S. Dynamics in the microbial cytome—Single cell analytics in natural systems. Curr. Opin. Biotechnol. 2014, 27, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Huber, K.J.; Bunk, B.; Overmann, J.; Harnisch, F. Trophic networks improve the performance of microbial anodes treating wastewater. Npj Biofilms Microbiomes 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.; Stärk, H.J.; Hause, G.; Schmidt, M.; Harms, H.; Wick, L.Y.; Müller, S. Heterogenic response of prokaryotes toward silver nanoparticles and ions is facilitated by phenotypes and attachment of silver aggregates to cell surfaces. Cytom. Part A 2017, 91, 775–784. [Google Scholar] [CrossRef]
- Liu, Z.; Cichocki, N.; Hübschmann, T.; Süring, C.; Ofiţeru, I.D.; Sloan, W.T.; Grimm, V.; Müller, S. Neutral mechanisms and niche differentiation in steady-state insular microbial communities revealed by single cell analysis. Environ. Microbiol. 2019, 21, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Faust, K.; Schumann, J.; Harms, H.; Raes, J.; Müller, S. Species-sorting and mass-transfer paradigms control managed natural metacommunities. Environ. Microbiol. 2016, 18, 4862–4877. [Google Scholar] [CrossRef] [PubMed]
- Besmer, M.D.; Weissbrodt, D.G.; Kratochvil, B.E.; Sigrist, J.A.; Weyland, M.S.; Hammes, F. The feasibility of automated online flow cytometry for in-situ monitoring of microbial dynamics in aquatic ecosystems. Front. Microbiol. 2014, 5, 265. [Google Scholar] [CrossRef]
- Buysschaert, B.; Vermijs, L.; Naka, A.; Boon, N.; De Gusseme, B. Online flow cytometric monitoring of microbial water quality in a full-scale water treatment plant. Npj Clean Water 2018, 1, 16. [Google Scholar] [CrossRef]
- Props, R.; Rubbens, P.; Besmer, M.; Buysschaert, B.; Sigrist, J.; Weilenmann, H.; Waegeman, W.; Boon, N.; Hammes, F. Detection of microbial disturbances in a drinking water microbial community through continuous acquisition and advanced analysis of flow cytometry data. Water Res. 2018, 145, 73–82. [Google Scholar] [CrossRef]
- Prest, E.; Hammes, F.; Kötzsch, S.; van Loosdrecht, M.; Vrouwenvelder, J. Monitoring microbiological changes in drinking water systems using a fast and reproducible flow cytometric method. Water Res. 2013, 47, 7131–7142. [Google Scholar] [CrossRef]
- Rubbens, P.; Props, R. Computational Analysis of Microbial Flow Cytometry Data. Msystems 2021, 6, e00895-20. [Google Scholar] [CrossRef]
- Bombach, P.; Hübschmann, T.; Fetzer, I.; Kleinsteuber, S.; Geyer, R.; Harms, H.; Müller, S. Resolution of Natural Microbial Community Dynamics by Community Fingerprinting, Flow Cytometry, and Trend Interpretation Analysis. High Resolut. Microb. Single Cell Anal. 2010, 124, 151–181. [Google Scholar] [CrossRef]
- Koch, C.; Fetzer, I.; Harms, H.; Müller, S. CHIC-an automated approach for the detection of dynamic variations in complex microbial communities. Cytom. Part A 2013, 83, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Harnisch, F.; Schröder, U.; Müller, S. Cytometric fingerprints: Evaluation of new tools for analyzing microbial community dynamics. Front. Microbiol. 2014, 5, 273. [Google Scholar] [CrossRef] [PubMed]
- Props, R.; Monsieurs, P.; Mysara, M.; Clement, L.; Boon, N. Measuring the biodiversity of microbial communities by flow cytometry. Methods Ecol. Evol. 2016, 7, 1376–1385. [Google Scholar] [CrossRef]
- Rogers, W.T.; Holyst, H.A. FlowFP: A Bioconductor Package for Fingerprinting Flow Cytometric Data. Adv. Bioinform. 2009, 1–11. [Google Scholar] [CrossRef]
- Rubbens, P.; Props, R.; Kerckhof, F.M.; Boon, N.; Waegeman, W. PhenoGMM: Gaussian mixture modelling of Cytometry Data Quantifies Changes in Microbial Community Structure. mSphere 2021, 6, e00530-20. [Google Scholar] [CrossRef]
- Ludwig, J.; zu Siederdissen, C.H.; Liu, Z.; Stadler, P.F.; Müller, S. FlowEMMi: An automated model-based clustering tool for microbial cytometric data. BMC Bioinform. 2019, 20, 643. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Fritz, Z.; Novik, E.M.; Yarmush, G.M.; Schloss, R.S.; Zahn, J.D.; Yarmush, M.L. Microfluidic flow cytometry: The role of microfabrication methodologies, performance and functional specification. Technology 2018, 6, 1–23. [Google Scholar] [CrossRef]
- Tsai, A.G.; Glass, D.R.; Juntilla, M.; Hartmann, F.J.; Oak, J.S.; Fernandez-Pol, S.; Ohgami, R.S.; Bendall, S.C. Multiplexed single-cell morphometry for hematopathology diagnostics. Nat. Med. 2020, 26, 408–417. [Google Scholar] [CrossRef]
- Schraivogel, D.; Kuhn, T.M.; Rauscher, B.; Rodríguez-Martínez, M.; Paulsen, M.; Owsley, K.; Middlebrook, A.; Tischer, C.; Ramasz, B.; Ordoñez-Rueda, D.; et al. High-speed fluorescence image–enabled cell sorting. Science 2022, 375, 315–320. [Google Scholar] [CrossRef]
- Park, L.M.; Lannigan, J.; Jaimes, M.C. OMIP-069: Forty-Color Full Spectrum Flow Cytometry Panel for Deep Immunophenotyping of Major Cell Subsets in Human Peripheral Blood. Cytom. Part A 2020, 97, 1044–1051. [Google Scholar] [CrossRef]
- Huang, W.E.; Li, M.; Jarvis, R.M.; Goodacre, R.; Banwart, S.A. Shining light on the microbial world: The application of Raman microspectroscopy. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 70, pp. 153–186. [Google Scholar] [CrossRef]
- Chakraborty, I.; Banik, S.; Biswas, R.; Yamamoto, T.; Noothalapati, H.; Mazumder, N. Raman spectroscopy for microplastic detection in water sources: A systematic review. Int. J. Environ. Sci. Technol. 2022, 1–14. [Google Scholar] [CrossRef]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech. Instrum. 2015, 2, 8. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef]
- García-Timermans, C.; Rubbens, P.; Heyse, J.; Kerckhof, F.-M.; Props, R.; Skirtach, A.G.; Waegeman, W.; Boon, N. Discriminating Bacterial Phenotypes at the Population and Single-Cell Level: A Comparison of Flow Cytometry and Raman Spectroscopy Fingerprinting. Cytom. Part A 2020, 97, 713–726. [Google Scholar] [CrossRef]
- García-Timermans, C.; Props, R.; Zacchetti, B.; Sakarika, M.; Delvigne, F.; Boon, N. Raman Spectroscopy-Based Measurements of Single-Cell Phenotypic Diversity in Microbial Populations. Msphere 2020, 5, e00806-20. [Google Scholar] [CrossRef]
- Huang, W.E.; Stoecker, K.; Griffiths, R.; Newbold, L.; Daims, H.; Whiteley, A.S.; Wagner, M. Raman-FISH: Combining stable-isotope Raman spectroscopy and fluorescence in situ hybridization for the single cell analysis of identity and function. Environ. Microbiol. 2007, 9, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kaster, A.-K.; Vollmers, J.; Song, Y.; Davison, P.A.; Frentrup, M.; Preston, G.M.; Thompson, I.P.; Murrell, J.C.; Yin, H.; et al. Single-cell genomics based on Raman sorting reveals novel carotenoid-containing bacteria in the Red Sea. Microb. Biotechnol. 2017, 10, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, P.; Gou, H.; Mou, C.; Huang, W.E.; Yang, M.; Xu, J.; Ma, B. Towards high-throughput microfluidic Raman-activated cell sorting. Analyst 2015, 140, 6163–6174. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.E.; Ward, A.; Whiteley, A. Raman tweezers sorting of single microbial cells. Environ. Microbiol. Rep. 2009, 1, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Fan, L.; Zhu, R.; Sun, D. Microfluidic Single-Cell Manipulation and Analysis: Methods and Applications. Micromachines 2019, 10, 104. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Kong, M.; Wang, Z.; Costa, K.D.; Li, R.A.; Sun, D. Enhanced cell sorting and manipulation with combined optical tweezer and microfluidic chip technologies. Lab Chip 2011, 11, 3656–3662. [Google Scholar] [CrossRef]
- Sinha, N.; Yang, H.; Janse, D.; Hendriks, L.; Rand, U.; Hauser, H.; Köster, M.; van de Vosse, F.N.; de Greef, T.F.A.; Tel, J. Microfluidic chip for precise trapping of single cells and temporal analysis of signaling dynamics. Commun. Eng. 2022, 1, 1–12. [Google Scholar] [CrossRef]
- FYu, F.B.; Blainey, P.C.; Schulz, F.; Woyke, T.; Horowitz, M.A.; Quake, S.R. Microfluidic-based mini-metagenomics enables discovery of novel microbial lineages from complex environmental samples. Elife 2017, 6, e26580. [Google Scholar] [CrossRef]
- Dusny, C.; Schmid, A. Microfluidic single-cell analysis links boundary environments and individual microbial phenotypes. Environ. Microbiol. 2015, 17, 1839–1856. [Google Scholar] [CrossRef]
- Martinez-Varela, A.; Casas, G.; Berrojalbiz, N.; Piña, B.; Dachs, J.; Vila-Costa, M. Polycyclic Aromatic Hydrocarbon Degradation in the Sea-Surface Microlayer at Coastal Antarctica. Front. Microbiol. 2022, 13, 907265. [Google Scholar] [CrossRef]
| Technologies | Examples in Achievements | Regression Analysis and Outlook |
|---|---|---|
| Microscope | Energy Dispersive X-ray Analysis (EDX) system combines with SEM or TEM to identify the elemental composition in a sample [35]. | Microscopic images are subject to a variety of factors, such as instrument limitations, signal intensity or image contrast, sample preparation, and experimental conditions. Regression analysis can be employed to examine the correlation variables to eliminate or control the discrepancies, leading to more precise and reliable measurements. The future of microscopy will focus on the application of super-resolution microscopy, multiphoton microscopy, and CRISPR-based microscopy to visualize specific DNA or RNA sequences within cells. |
| Confocal microscopy combined with Raman spectroscopy reveals the spatial distribution of the compounds within a sample [13]. | ||
| Flow cytometry | Investigate the dynamic community assembly in wastewater treatment plants to discover perturbation-associated symptoms for community control [37]. | Flow cytometry measurements are susceptible to several sources of variability, including instrument noise, variations in sample preparation, and different experimental conditions. Statistical regression analysis can be applied to flow cytometry data to account for these sources of errors. Therefore, regression analysis is required to correlate the fluorescence signal from the cell population with various independent variables, such as cell size, granularity, instrument gain, and protein expression levels. The future of flow cytometry looks promising, with advancements moving towards high-throughput analysis, imaging flow cytometry, and AI-powered data analysis. With these developments, the potential for this technology to revolutionize biological research is enormous. |
| Automatic online monitoring of the community changes as an early-warning tool to reflect/control drinking water processing operation [38,39,40]. | ||
| Automated approaches have been established for flow cytometric phenotypic diversification, including phenoflow [46], flow FP [47], PhenoGMM [48], and flowEMMi [49]. | ||
| Raman spectroscopy | By analyzing the Raman spectra of small particles in water samples, researchers have been able to identify and quantify microplastics, which pose a threat to marine life and ecosystems. | There are various sources of errors in Raman spectroscopy, including instrumental noise, sample heterogeneity, fluorescence, and solvent effects. To account for the error in Raman spectroscopy, regression analysis can be used to quantify the amount of a particular chemical in a sample based on the signal intensity of a Raman peak that is associated with the chemical, or to correct for interferences or background signals that may be present in the Raman spectrum to improve the accuracy of the quantitation method. The outlook for the development of Raman spectroscopy is to develop portable systems for in-field applications. And combining Raman with other techniques, such as infrared spectroscopy, surface-enhanced Raman spectroscopy (SERS), and fluorescence spectroscopy, can obtain more comprehensive information about samples. |
| Confocal Raman microscopy allows for three-dimensional imaging of samples with high spatial resolution [13]. | ||
| Tip-enhanced Raman spectroscopy combines scanning probe microscopy (SPM) with Raman spectroscopy to achieve high spatial resolution spectroscopy down to the nanometer level, which can investigate biological processes such as protein folding and DNA replication [54]. | ||
| Microfluidic single-cell techniques | The microfluidic device allows for real-time observations of apoptosis in intracellular signaling pathways in single cells [67]. | In microfluidic chips, the regression of error refers to the process of analyzing and quantifying the accuracy and precision of the device’s performance. This is typically achieved by comparing the results obtained from the chip to a known value or established standard. To accomplish regression of error in microfluidic chips, various statistical methods are used, such as linear regression and least-squares analysis. These methods allow researchers to determine the relationship between different variables and identify any sources of error in the system. The future of microfluidic chips is exciting and full of potential. Major advancements are expected in the development of miniaturized, easy-to-use, inexpensive, and highly integrated microfluidic systems. |
| Microfluidic devices can be used to isolate and analyze individual cells from environmental samples, such as soil or water, allowing for environmental metagenomics analysis at the single-cell level. This can provide a more accurate understanding of microbial diversity and function in complex environments [68]. | ||
| Microfluidic devices can mimic environmental stress conditions, such as changes in temperature, pH, or nutrient availability, allowing for the study of stress response phenotypes at the single-cell level [69]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Y.; Guo, Y.; Jiao, W.; Zeng, P. Single-Cell Techniques in Environmental Microbiology. Processes 2023, 11, 1109. https://doi.org/10.3390/pr11041109
Shan Y, Guo Y, Jiao W, Zeng P. Single-Cell Techniques in Environmental Microbiology. Processes. 2023; 11(4):1109. https://doi.org/10.3390/pr11041109
Chicago/Turabian StyleShan, Yongping, Yuting Guo, Wentao Jiao, and Ping Zeng. 2023. "Single-Cell Techniques in Environmental Microbiology" Processes 11, no. 4: 1109. https://doi.org/10.3390/pr11041109
APA StyleShan, Y., Guo, Y., Jiao, W., & Zeng, P. (2023). Single-Cell Techniques in Environmental Microbiology. Processes, 11(4), 1109. https://doi.org/10.3390/pr11041109