Essential Oils of Two Portuguese Endemic Species of Lavandula as a Source of Antifungal and Antibacterial Agents
Abstract
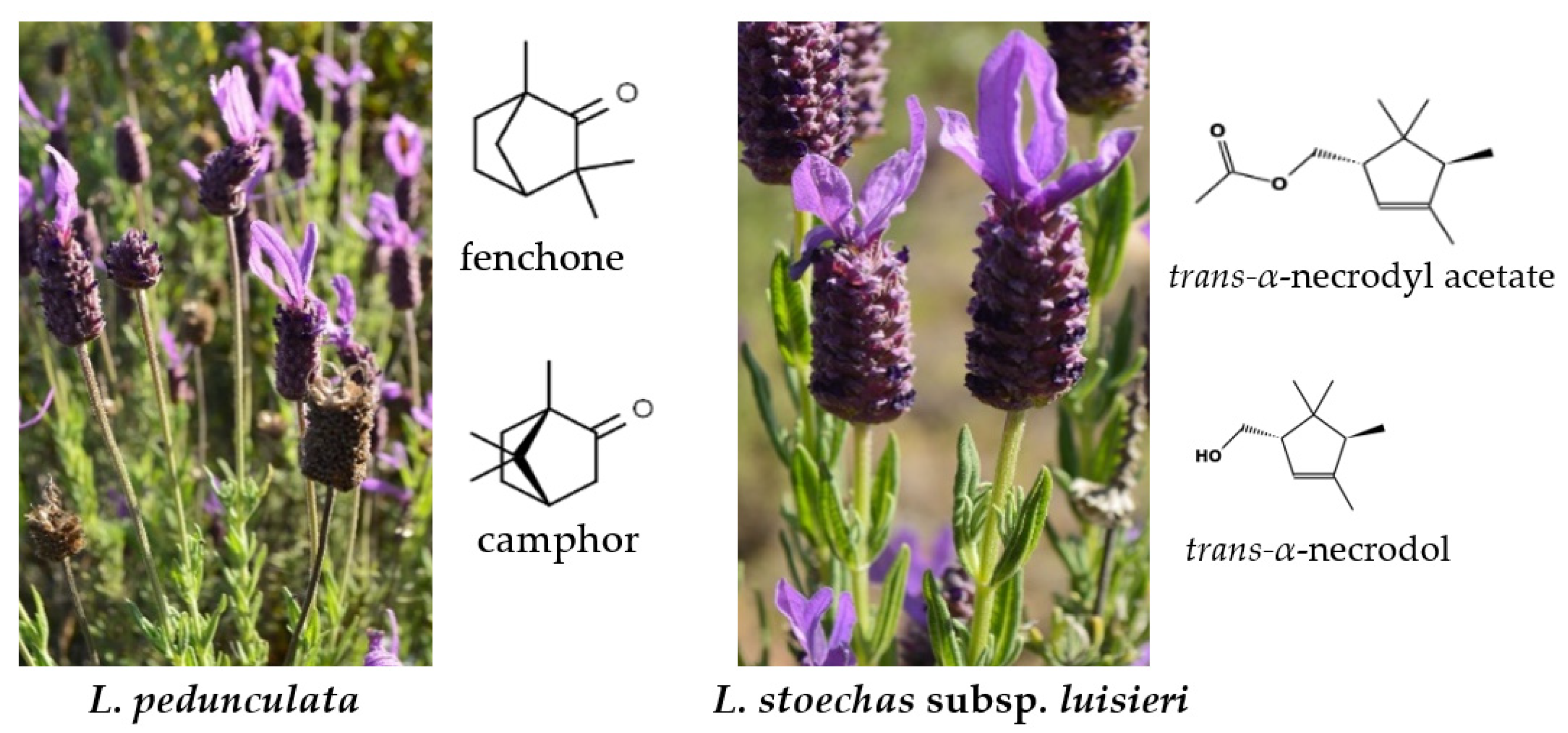
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Essential Oils
2.2. GC-MS Analysis
2.3. Microorganism Cultures
2.4. Microdilution Method for MIC and MFC/MBC Determination
2.5. Cell Viability
3. Results
3.1. Chemical Profile of the Essential Oils
3.2. Antifungal Activity of the Essential Oils
3.3. Antibacterial Activity of Essential Oils
3.4. Cytotoxicity of the Essential Oil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Upson, T.M.; Andrews, S. The Genus Lavandula; The Royal Botanical Gardens: London, UK, 2004.
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on Pharmaceutical Ethnobotany in Arrabida Natural Park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological Notes about Ancient Uses of Medicinal Plants in Trás-Os-Montes (Northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean Lavenders from Section Stoechas: An Undervalued Source of Secondary Metabolites with Pharmacological Potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Canhoto, J.; Vaz, S.; Pinto, E.; Salgueiro, L. Lavandula luisieri Essential Oil as a Source of Antifungal Drugs. Food Chem. 2012, 135, 1505–1510. [Google Scholar] [CrossRef]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Santos Pintado, C. Essential Oils of Lavandula stoechas Subsp. luisieri as Antifungal Agent against Fungi from Strawberry Tree Fruit. J. Pharm. Pharmacol. 2021, 9, 98–106. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants 2022, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Dinis, A.M.; Canhoto, J.M.; Salgueiro, L.R. Chemical Composition and Antifungal Activity of the Essential Oils of Lavandula pedunculata (Miller) Cav. Chem. Biodivers. 2009, 6, 1283–1292. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.F.; Romano, A. Metabolic Profile and Biological Activities of Lavandula pedunculata Subsp. lusitanica (Chaytor) Franco: Studies on the Essential Oil and Polar Extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils-A Review. Food Chem. Toxicol. 2008, 1, 446–475. [Google Scholar] [CrossRef]
- Karaca, N.; Demirci, B.; Demirci, F. Evaluation of Lavandula stoechas L. Subsp. stoechas L., Mentha spicata L. Subsp. spicata L. Essential Oils and Their Main Components against Sinusitis Pathogens. Z. Für Nat. C 2018, 73, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [Green Version]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Matos, F.; Miguel, M.G.; Duarte, J.; Venâncio, F.; Moiteiro, C.; Correia, A.I.D.; Figueiredo, A.C.; Barroso, J.; Pedro, L. Antioxidant Capacity of the Essential Oils from Lavandula luisieri, L. stoechas Subsp. lusitanica, L. stoechas Subsp. lusitanica x L. luisieri and L. viridis Grown in Algarve (Portugal). J. Essent. Oil Res. 2009, 21, 327–336. [Google Scholar] [CrossRef]
- Keskin, I.; Gunal, Y.; Ayla, S.; Kolbasi, B.; Sakul, A.; Kilic, U.; Gok, O.; Koroglu, K.; Ozbek, H. Effects of Foeniculum vulgare Essential Oil Compounds, Fenchone and Limonene, on Experimental Wound Healing. Biotech. Histochem. 2017, 92, 274–282. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Eisner, T.; Meinwald, J. Defensive Spray Mechanism of A Silphid Beetle (Necrodes surinamensis). Psyche 1982, 89, 357–367. [Google Scholar] [CrossRef]
- Kashima, Y.; Miyazawa, M. Chemical Composition and Aroma Evaluation of Essential Oils from Evolvulus alsinoides L. Chem. Biodivers. 2014, 11, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, Antioxidant and Anti-Proliferative Properties and Zinc Content of Five South Portugal Herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.; Baptista, R.; Ladeiras, D.; Madureira, A.M.; Teixeira, G.; Rosado, C.; Fernandes, A.S.; Ascensão, L.; Silva, C.O.; Reis, C.P.; et al. Production and Characterization of Nanoparticles Containing Methanol Extracts of Portuguese Lavenders. Meas. J. Int. Meas. Confed. 2015, 74, 170–177. [Google Scholar] [CrossRef]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic Composition and Bioactivity of Lavandula Pedunculata (Mill.) Cav. Samples from Different Geographical Origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council of Europe. European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- Domingues, J.; Goulão, M.; Coelho, M.T.; Gonçalves, J.C.; Pintado, C.S. Different postharvest storage conditions of Arbutus unedo L. fruits, and their physicochemical and microbiological characterisation. Int. Food Res. J. 2022, 29, 32–41. [Google Scholar] [CrossRef]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Teh, C.H.; Nazni, W.A.; Lee, H.L.; Fairuz, A.; Tan, S.B.; Sofian-Azirun, M. In vitro antibacterial activity and physicochemical properties of a crude methanol extract of the larvae of the blow fly Lucilia cuprina. Med. Vet. Entomol. 2013, 27, 414–420. [Google Scholar] [CrossRef]
- Santos, E.S.; Luís, Â.; Gonçalves, J.; Rosado, T.; Pereira, L.; Gallardo, E.; Duarte, A.P. Julbernardia paniculata and Pterocarpus angolensis: From ethnobotanical surveys to phytochemical characterization and bioactivities evaluation. Molecules 2020, 25, 1828. [Google Scholar] [CrossRef] [Green Version]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Baldovini, N.; Lavoine-Hanneguelle, S.; Ferrando, G.; Dusart, G.; Lizzani-Cuvelier, L. Necrodane Monoterpenoids from Lavandula luisieri. Phytochemistry 2005, 66, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Lavoine-Hanneguelle, S.; Casabianca, H. New Compounds from the Essential Oil and Absolute of Lavandula luisieri L. J. Essent. Oil Res. 2004, 16, 445–448. [Google Scholar] [CrossRef]
- González-Coloma, A.; Delgado, F.; Rodilla, J.M.; Silva, L.; Sanz, J.; Burillo, J. Chemical and Biological Profiles of Lavandula luisieri Essential Oils from Western Iberia Peninsula Populations. Biochem. Syst. Ecol. 2011, 39, 1–8. [Google Scholar] [CrossRef]
- Videira, R.; Castanheira, P.; Grãos, M.; Salgueiro, L.; Faro, C.; Cavaleiro, C. A Necrodane Monoterpenoid from Lavandula luisieri Essential Oil as a Cell-Permeable Inhibitor of BACE-1, the β-Secretase in Alzheimer’s Disease. Flavour Fragr. J. 2013, 28, 380–388. [Google Scholar] [CrossRef]
- Julio, L.F.; Martín, L.; Muñoz, R.; Mainar, A.M.; Urieta, J.S.; Sanz, J.; Burillo, J.; González-Coloma, A. Comparative Chemistry and Insect Antifeedant Effects of Conventional (Clevenger and Soxhlet) and Supercritical Extracts (CO2) of Two Lavandula luisieri Populations. Ind. Crops Prod. 2014, 58, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Rufino, A.T.; Ferreira, I.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Differential Effects of the Essential Oils of Lavandula luisieri and Eryngium duriaei Subsp. juresianum in Cell Models of Two Chronic Inflammatory Diseases. Pharm. Biol. 2015, 53, 1220–1230. [Google Scholar] [CrossRef] [Green Version]
- Arantes, S.; Candeias, F.; Lopes, O.; Lima, M.; Pereira, M.; Tinoco, T.; Cruz-Morais, J.; Martins, M.R. Pharmacological and Toxicological Studies of Essential Oil of Lavandula stoechas Subsp. luisieri. Planta Med. 2016, 82, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Pombal, S.; Rodrigues, C.F.; Araújo, J.P.; Rocha, P.M.; Rodilla, J.M.; Diez, D.; Granja, Á.P.; Gomes, A.C.; Silva, L.A. Antibacterial and Antioxidant Activity of Portuguese Lavandula luisieri (Rozeira) Rivas-Martinez and Its Relation with Their Chemical Composition. Springerplus 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, N.; Dias, M.C.; Cavaleiro, C.; Sousa, M.C.; Lima, N.; Machado, M. Oxygenated Monoterpenes-Rich Volatile Oils as Potential Antifungal Agents for Dermatophytes. Nat. Prod. Res. 2017, 31, 460–464. [Google Scholar] [CrossRef] [Green Version]
- Guitton, Y.; Nicolè, F.; Jullien, F.; Caissard, J.-C.; Saint-Marcoux, D.; Legendre, L.; Pasquier, B.; Moja, S. A comparative study of terpene composition in different clades of the genus Lavandula. Bot. Lett. 2018, 165, 494–505. [Google Scholar] [CrossRef]
- Vairinhos, J.; Miguel, M.G. Essential oils of spontaneous species of the genus Lavandula from Portugal: A brief review. Zeitschrift Naturforsch. 2020, 75, 233–245. [Google Scholar] [CrossRef]
- Özcan, M.M.; Starovic, M.; Aleksic, G.; Figueredo, G.; Juhaimi, F.A.; Chalchat, J.-C. Chemical Composition and Antifungal Activity of Lavender (Lavandula stoechas) Oil. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef] [Green Version]
- Baptista, R.; Madureira, A.M.; Jorge, R.; Adão, R.; Duarte, A.; Duarte, N.; Lopes, M.M.; Teixeira, G. Antioxidant and Antimycotic Activities of Two Native Lavandula Species from Portugal. Evid.-Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, G.; Correia, A.I.; Vasconcelos, T.; Duarte, A.; Oliveira, N.; Madureira, A.M. Lavandula stoechas Subsp. luisieri and L. pedunculata: Comparative Antibacterial Activity. J. Phyther. Pharmacol. 2012, 1, 11–15. [Google Scholar]
- Soro, N.K.; Majdouli, K.; Khabbal, Y.; Zair, T. Chemical Composition and Antibacterial Activity of Lavandula Species L. dentata L., L. pedunculata Mill and Lavandula abrialis Essential Oils from Morocco against Food-Borne and Nosocomial Pathogens. Int. J. Innov. Appl. Stud. 2014, 7, 774–781. [Google Scholar]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins–The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula stoechas Essential Oil from Morocco as Novel Source of Antileishmanial, Antibacterial and Antioxidant Activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Awaluddin, R.; Nugrahaningsih, D.A.A.; Solikhah, E.N.; Chabib, L. The Effect of Asiatic Acid and Metformin on The Viability Percentage of Mouse Macrophage Cell Lines RAW264. 7 and Mouse Fibroblast Cell Lines NIH3T3. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012021. [Google Scholar]
- Zuzarte, M.; Vitorino, C.; Salgueiro, L.; Girão, H. Plant Nanovesicles for Essential Oil Delivery. Pharmaceutics 2022, 14, 2581. [Google Scholar] [CrossRef] [PubMed]

| Cultures | Lab. Reference | Origin | Gram Staining 1 |
|---|---|---|---|
| Aeromonas hydrophila | SC-V-AP/2015 | Untreated water | - |
| Burkholderia sp. | B-AM-Pa-3F/2014 | Untreated water | - |
| Chromobacterium violaceum | SC-AF/2014 | Untreated water | - |
| Pseudomonas aeruginosa ATCC 27853 | ATCC 28753 | ATCC | - |
| Pseudomonas aeruginosa | SC-V-AP/2015 | Untreated water | - |
| Salmonella sp. | Food isolates | - | |
| Serratia marcescens | A-LO-596/2018 | Raw sheep’s milk | - |
| Bacillus cereus | A-FL-PB/2013 | Bread flour | + |
| Listeria monocytogenes | QD-LCP24/2014 | Raw goat’s milk | + |
| Coagulase-positive Staphylococcus | CB-QM-L7/11/2018 | Cheese | + |
| Compounds | Chemical Class a | RI b | RI c | % Peak Area | |
|---|---|---|---|---|---|
| LSL | LP | ||||
| α-Pinene | MH | 925 | 936 | 2.8 ± 0.1 | 7.0 ± 0.2 |
| Camphene | MH | 939 | 950 | 0.1 ± 0.0 | 1.1 ± 0.1 |
| β-Myrcene | MH | 982 | 989 | - | 0.1 ± 0.0 |
| p-Cymene | MH | 1016 | 1024 | 0.1 ± 0.0 | 0.4 ± 0.0 |
| Limonene | MH | 1020 | 1030 | 0.1 ± 0.0 | 2.1 ± 0.0 |
| 1,8-Cineole | OM | 1023 | 1032 | 3.2 ± 0.0 | 0.1 ± 0.0 |
| trans-β-Ocimene | MH | 1032 | 1038 | - | 0.7 ± 0.0 |
| cis-Linalool oxide | OM | 1068 | 1075 | 0.8 ± 0.0 | - |
| 3,4,4-Trimethyl-2-cyclohexene-1-one | OT | 1076 | 1055 * | 1.1 ± 0.0 | - |
| Fenchone | OM | 1084 | 1088 | 3.6 ± 0.0 | 50.5 ± 0.3 |
| Linalool | OM | 1099 | 1099 | 5.6 ± 0.1 | 0.9 ± 0.0 |
| Fenchol | OM | 1113 | 1115 | - | 0.6 ± 0.0 |
| α-Campholenal | OM | 1128 | 1124 | - | 0.3 ± 0.0 |
| Camphor | OM | 1146 | 1143 | 2.7 ± 0.0 | 30.0 ± 0.2 |
| trans-α-Necrodol | OM | 1151 | 1130 * | 10.4 ± 0.1 | - |
| Pinocarvone | OM | 1168 | 1161 | - | 0.1 ± 0.0 |
| Borneol | OM | 1171 | 1166 | - | 0.2 ± 0.0 |
| NI C L. luisieri | 1175 | 0.8 ± 0.0 | - | ||
| cis-α-Necrodol | OM | 1182 | 2.0 ± 0.0 | - | |
| 5-Methylene-2,3,4,4-tetramethylcyclopenten-2-enone | OT | 1198 | 1160 ** | 0.8 ± 0.0 | - |
| Terpinen-4-ol | OM | 1185 | 1177 | - | 0.2 ± 0.0 |
| p-Cymen-8-ol | OM | 1194 | 1184 | - | 0.4 ± 0.0 |
| α-Terpineol | OM | 1200 | 1190 | - | 0.2 ± 0.0 |
| Myrtenal | OM | 1206 | 1192 | - | 0.1 ± 0.0 |
| Verbenone | OM | 1220 | 1206 | - | 0.5 ± 0.0 |
| Fenchyl acetate | OM | 1233 | 1220 | - | 0.4 ± 0.0 |
| trans-α-Necrodyl acetate | OM | 1296 | 1265 * | 40.2 ± 0.1 | - |
| Bornyl acetate | OM | 1305 | 1284 | - | 0.8 ± 0.0 |
| Lavandulyl acetate | OM | 1312 | 1289 | 11.0 ± 0.1 | 0.2 ± 0.0 |
| cis-α-Necrodyl acetate | OM | 1324 | 1.7 ± 0.0 | - | |
| NI D L. luisieri | 1333 | 1.1 ± 0.0 | - | ||
| Valencene | SH | 1498 | 1492 | - | 0.3 ± 0.0 |
| Caryophyllene oxide | OS | 1602 | 1581 | 0.4 ± 0.0 | - |
| Viridiflorol | OS | 1610 | 1591 | 2.2 ± 0.1 | - |
| Isovalencenol | OS | 1788 | 1782 | - | 1.5 ± 0.1 |
| NI E L. luisieri | 1818 | 1.5 ± 0.1 | - | ||
| NI F L. luisieri | 1821 | 1.1 ± 0.1 | - | ||
| Yield (%, v/w) | 0.8 ± 0.1 | 2.1 ± 0.2 | |||
| Identification (%) | 88.8 | 98.7 | |||
| Monoterpene hydrocarbons (%) | 3.1 | 11.4 | |||
| Oxygenated monoterpenes (%) | 81.2 | 85.5 | |||
| Sesquiterpene hydrocarbons (%) | 0.0 | 0.3 | |||
| Oxygenated sesquiterpenes (%) | 2.6 | 1.5 | |||
| Others (%) | 1.9 | - | |||
| Fungi | Lavandula sp. | Essential Oil Concentration (µL/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 74.7 | 37.3 | 18.7 | 9.3 | 4.7 | 2.3 | 1.2 | 0.6 | 0.3 | ||
| Alternaria section Alternaria (ESA.M.11) | LSL | MFC> | MIC | |||||||
| LP | MFC> | MIC | ||||||||
| Aspergillus brasiliensis ATCC 16404 | LSL | MFC> | MIC | |||||||
| LP | MFC> | MIC | ||||||||
| Aspergillus niger (ESA.M.45) | LSL | MIC MFC | ||||||||
| LP | MIC MFC | |||||||||
| Aspergillus tubingensis (ESA.M.38) | LSL | MIC MFC | ||||||||
| LP | MIC MFC | |||||||||
| Aureobasidium sp. (ESA.M.57) | LSL | MFC | MIC | |||||||
| LP | MFC | MIC | ||||||||
| Candida albicans (ESALD/2016) | LSL | MBC | MIC | |||||||
| LP | MBC | MIC | ||||||||
| Hanseniaspora sp. (ESA.M.99) | LSL | MFC | MIC | |||||||
| LP | MIC MFC | |||||||||
| Meyerozyma guilliermondii (ESA.M.47) | LSL | MFC | MIC | |||||||
| LP | MFC | MIC | ||||||||
| Penicillium crustosum (ESA.M.48) | LSL | MFC | MIC | |||||||
| LP | MFC | MIC | ||||||||
| Penicillium glabrum (ESA.M.54) | LSL | MFC> | MIC | |||||||
| LP | MFC> | MIC | ||||||||
| Penicillium simile (ESA.M.13) | LSL | MIC MFC | ||||||||
| LP | MFC> | MIC | ||||||||
| Saccharomyces cerevisiae ATCC 9763 | LSL | MFC | MIC | |||||||
| LP | MFC | MIC | ||||||||
| Bacteria | Lavandula spp. | Essential Oil Concentration (µL/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 149.3 | 74.7 | 37.3 | 18.7 | 9.3 | 4.7 | 2.3 | 1.2 | 0.6 | ||
| Aeromonas hydrophila (SC-V-AP/2015) | LSL | MBC | MIC | |||||||
| LP | MIC MBC | |||||||||
| Burkholderia sp. (B-AM-Pa-3F) | LSL | MIC MBC | ||||||||
| LP | MIC MBC | |||||||||
| Chromobacterium violaceum (SC-AF/2014) | LSL | MIC MBC | ||||||||
| LP | MIC MBC | |||||||||
| Pseudomonas aeruginosa (ATCC 27853) | LSL | MIC MBC | ||||||||
| LP | MIC MBC | |||||||||
| Pseudomonas aeruginosa (SC-V-AP/2015) | LSL | MBC | MIC | |||||||
| LP | MBC> | MIC | ||||||||
| Salmonella sp. | LSL | MBC | MIC | |||||||
| LP | MBC | MIC | ||||||||
| Serratia marcescens (A-LO-596/2018) | LSL | MBC | MIC | |||||||
| LP | MIC MBC | |||||||||
| Bacillus cereus (A-FL-PB/2013) | LSL | MBC> | MIC | |||||||
| LP | MBC> | MIC | ||||||||
| Listeria monocytogenes (QD-LCP24/2014) | LSL | MIC MBC | ||||||||
| LP | MBC | MIC | ||||||||
| Coagulase-positive Staphylococcus (CB-QM-L7/11/2018) | LSL | MIC MBC | ||||||||
| LP | MIC MBC | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues, J.; Goulão, M.; Delgado, F.; Gonçalves, J.C.; Gonçalves, J.; Pintado, C.S. Essential Oils of Two Portuguese Endemic Species of Lavandula as a Source of Antifungal and Antibacterial Agents. Processes 2023, 11, 1165. https://doi.org/10.3390/pr11041165
Domingues J, Goulão M, Delgado F, Gonçalves JC, Gonçalves J, Pintado CS. Essential Oils of Two Portuguese Endemic Species of Lavandula as a Source of Antifungal and Antibacterial Agents. Processes. 2023; 11(4):1165. https://doi.org/10.3390/pr11041165
Chicago/Turabian StyleDomingues, Joana, Manuela Goulão, Fernanda Delgado, José Carlos Gonçalves, Joana Gonçalves, and Cristina Santos Pintado. 2023. "Essential Oils of Two Portuguese Endemic Species of Lavandula as a Source of Antifungal and Antibacterial Agents" Processes 11, no. 4: 1165. https://doi.org/10.3390/pr11041165
APA StyleDomingues, J., Goulão, M., Delgado, F., Gonçalves, J. C., Gonçalves, J., & Pintado, C. S. (2023). Essential Oils of Two Portuguese Endemic Species of Lavandula as a Source of Antifungal and Antibacterial Agents. Processes, 11(4), 1165. https://doi.org/10.3390/pr11041165








