HPLC-UV Analysis of Chrysophanol in Senna occidentalis Extract Obtained by Using the RSM-Optimized Ultrasonic Extraction Process
Abstract
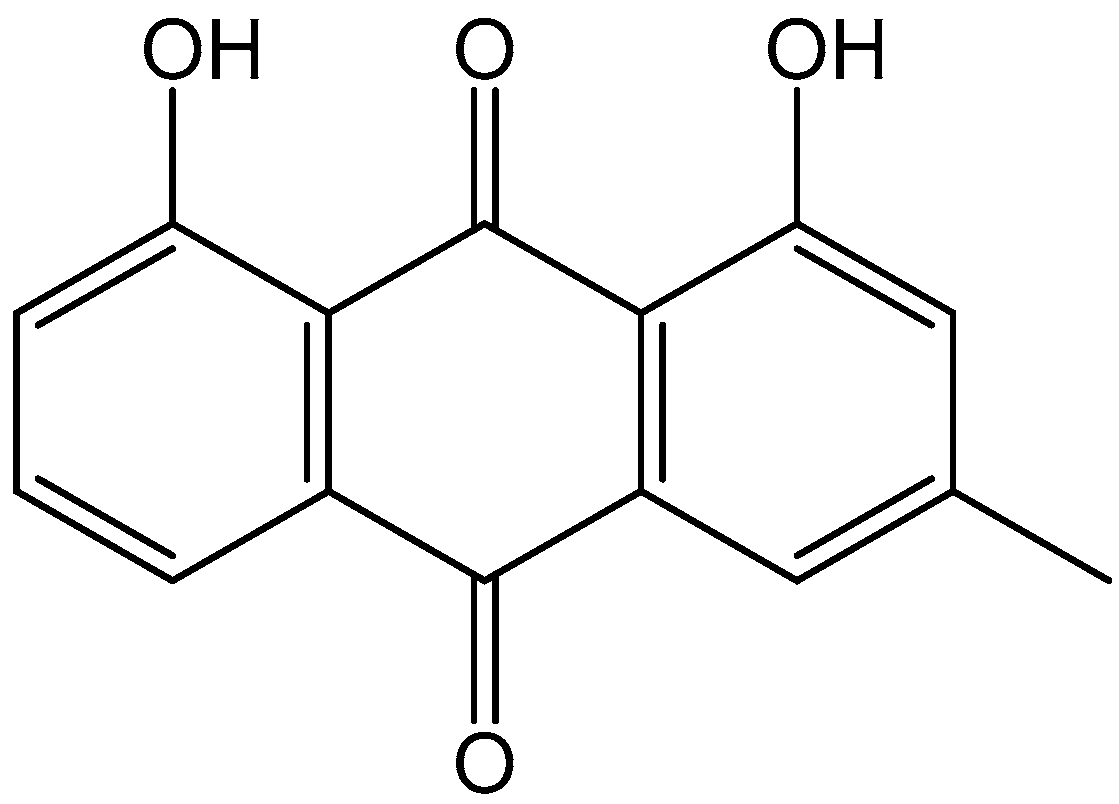
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Apparatus and Reagents
2.3. Extraction Process
2.3.1. Ultrasound-Assisted Extraction of S. occidentalis Aerial Parts
2.3.2. Conventional Solvent Extraction (CSE)
2.4. BBD Experimental Design
2.4.1. Single Factor Experimental Design
2.4.2. Optimization of UAE Parameters Using BBD Method and Method Validity Testing
2.5. HPLC-UV Analysis of Chrysophanol in the Optimized Extract of S. occidantalis
2.6. Statistical Analysis
3. Results
3.1. Single Extraction Factor Effect on Chrysophanol Content
3.2. BBD Optimization of Extraction Conditions
3.2.1. Model Fitting
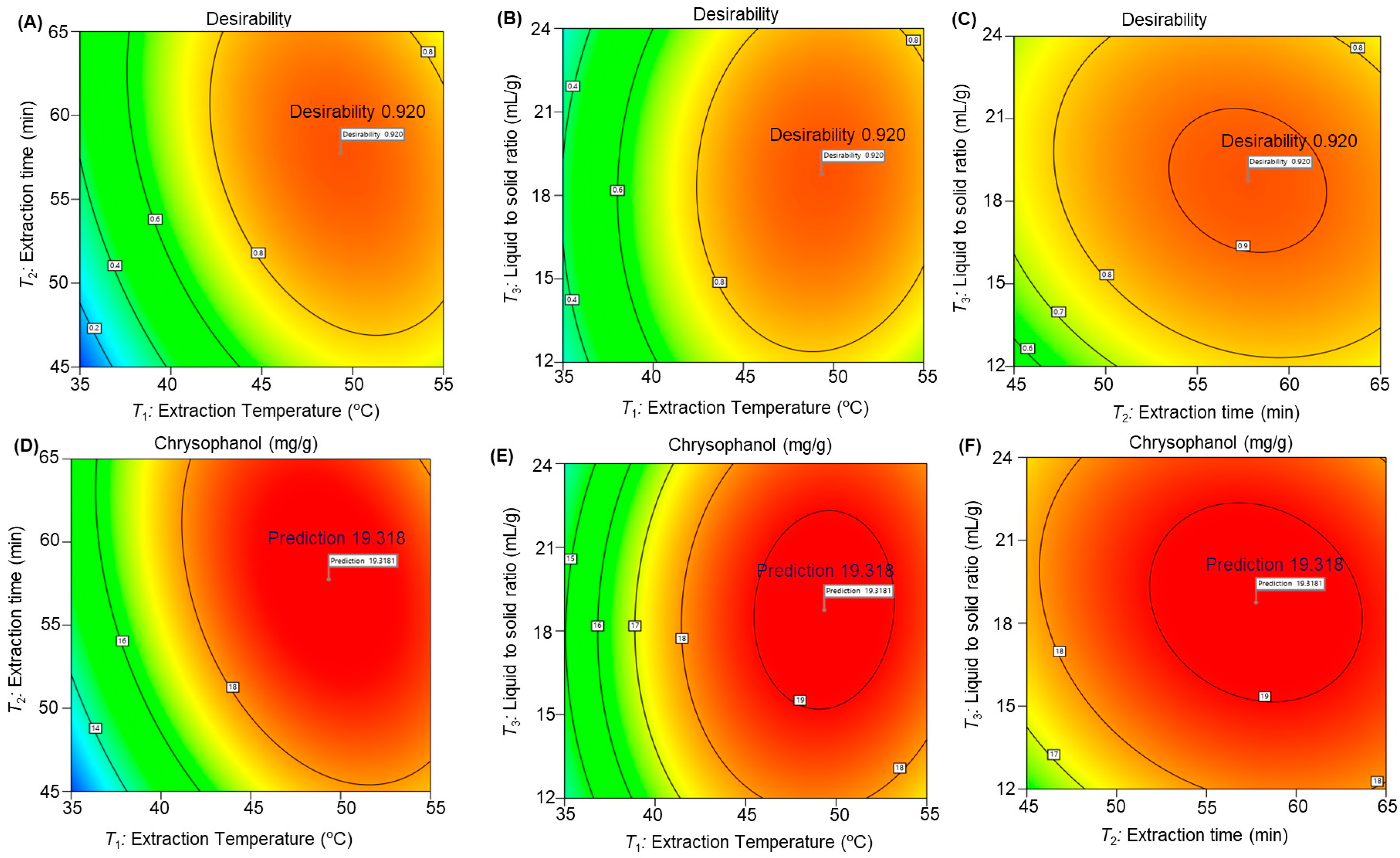
3.2.2. Influence of Extraction Parameters on Chrysophanol Extraction
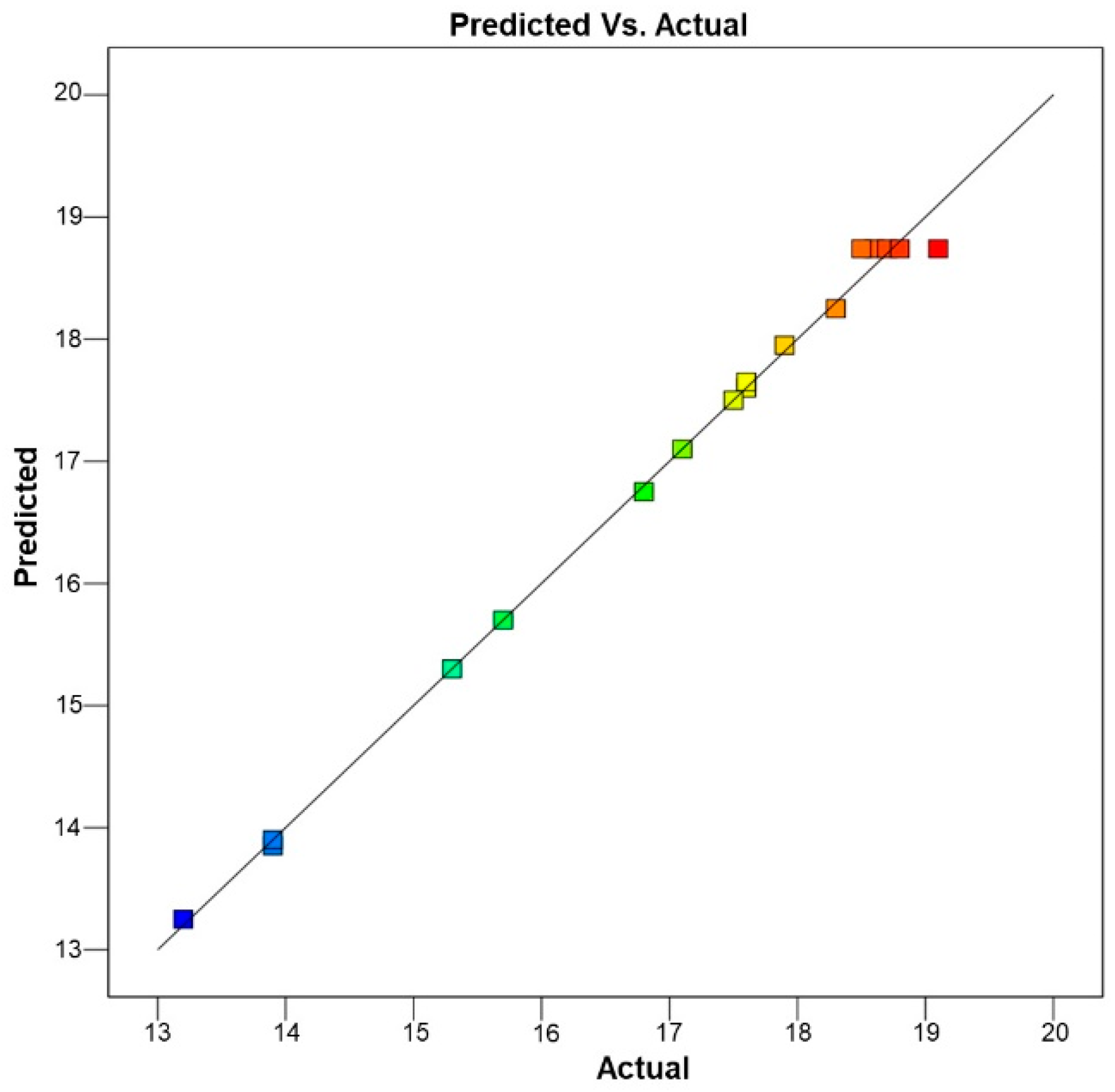
3.2.3. BBD Method Validation
3.2.4. Optimization of Extraction Conditions and Verification of the Predictive Model
3.2.5. HPLC-UV Analysis of Chrysophanol in the Optimized Extract of S. occidantalis
3.2.6. Comparison of UAE with CSE Methods of Extraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, J.P.; Arya, V.; Yadav, S.; Panghal, M.; Kumar, S.; Dhankhar, S. Cassia occidentalis L.: A review on its ethnobotany, phytochemical and pharmacological profile. Fitoterapia 2010, 81, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kolhapure, S.A.; Mitra, W.S. Meta-analysis of 50 phase III clinical trials in evaluation of efficacy and safety of Liv. 52 in infective hepatitis. Med. Update 2004, 12, 51–61. [Google Scholar]
- Humphry, C.; Clegg, M.S.; Keen, C.; Grivetti, L.E. Food diversity & drought survival—The Hausa example. Int. J. Food. Sci. Nutr. 1993, 44, 1–16. [Google Scholar]
- Warrier, P.K.; Nambiar, V.P.K. Indian Medicinal Plants: A Compendium of 500 Species; Orient Blackswan: Hyderabad, India, 1994; Volume 2, p. 21. [Google Scholar]
- Hatano, T.S.; Mizuta, S.; Ito, H.; Yoshida, T. C-glycosidic flavonoids from Cassia occidentalis. Phytochemistry 1999, 52, 1379–1383. [Google Scholar] [CrossRef]
- Rai, P.M.; Shok, M. Anthraquinone glycosides from plant parts of Cassia occidentalis. Ind. J. Pharm. Sci. 1983, 45, 87–88. [Google Scholar]
- Cowans, M.M. Plant materials as antimicrobial agents. Chem. Med. Rev. 1999, 12, 564–582. [Google Scholar]
- Xie, L.; Tang, H.; Song, J.; Long, J.; Zhang, L.; Li, X. Chrysophanol: A review of its pharmacology, toxicity and pharmacokinetics. J. Pharm. Pharmacol. 2019, 71, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, C.; Liu, J. HPLC determination of emodin and chrysophanol in Ganweiqitong tablets. Chin. J. Pharm. Anal. 2006, 26, 689–691. [Google Scholar]
- Jiao, Y.; Zuo, Y. Ultrasonic extraction and HPLC determination of anthraquinones, aloe-emodine, emodine, rheine, chrysophanol and physcione, in roots of Polygoni multiflori. Phytochem. Anal. 2009, 20, 272–278. [Google Scholar] [CrossRef]
- Genovese, S.; Tammaro, F.; Menghini, L.; Carlucci, G.; Epifano, F.; Locatelli, M. Comparison of three different extraction methods and HPLC determination of the anthraquinones aloe-emodine, emodine, rheine, chrysophanol and physcione in the bark of Rhamnus alpinus L. (Rhamnaceae). Phytochem. Anal. 2010, 21, 261–267. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, Y.; Liu, F.; Chen, J.; Tan, T.; Guo, L. Simultaneous determination of seven anthraquinones in Cassiae semen by natural deep eutectic solvent extraction. Phytochem. Anal. 2022, 33, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, H.; Wu, L.; Xing, J.; Poh, Y.; Cai, H.; Cai, B.C. Simultaneous quantification of chrysophanol and physcion in rat plasma by ultra-fast liquid chromatography-tandem mass spectrometry and application of the technique to comparative pharmacokinetic studies of Radix et Rhei Rhizoma extract alone and Dahuang Fuzi Decoction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 980, 88–93. [Google Scholar]
- Chen, Q.; He, H.; Luo, S.; Xiong, L.; Li, P. A novel GC-MS method for determination of chrysophanol in rat plasma and tissues: Application to the pharmacokinetics, tissue distribution and plasma protein binding studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 973C, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.C.; Nian, H.C.; Chiu, K.H.; Wang, A.Y.; Wu, B.Z. Rapid and efficient purification of chrysophanol in Rheum Palmatum LINN by supercritical fluid extraction coupled with preparative liquid chromatography in tandem. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 893–894, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Lin, C.C. Comparison of technique for extraction of isoflavones from the root of Radix Puerariae: Ultrasonic and pressurised solvent extraction. Food Chem. 2007, 105, 223–228. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Alam, P.; Alrehaily, A.J.; Alqahtani, A.S.; Akhtar, A.; Alhowiriny, T.A.; Almarfadi, O.M.; Mothana, R.A. Optimization of ultrasound-assisted parthenolide extraction from Tarchonanthus camphoratus leaves using response surface methodology: HPTLC and cytotoxicity analysis. Arab. J. Chem. 2021, 14, 103194. [Google Scholar] [CrossRef]
- Alam, P.; Siddiqui, N.A.; Alqahtani, A.S.; Haque, A.; Basudan, O.A.; Alqasoumi, S.I.; AL-Mishari, A.A.; Khan, M.U. Response surface methodology-based optimization of ultrasound-assisted extraction of β-sitosterol and lupeol from Astragalus atropilosus (roots) and validation by HPTLC method. Asian Pac. J. Trop. Biomed. 2020, 10, 281–292. [Google Scholar] [CrossRef]
- Alam, P.; Noman, O.M.; Herqash, R.N.; Almarfadi, O.M.; Akhtar, A.; Alqahtani, A.S. Efficient Extraction of an Anthraquinone Physcion Using Response Surface Methodology (RSM) Optimized Ultrasound-Assisted Extraction Method from Aerial Parts of Senna occidentalis and Analysis by HPLC-UV. Separations 2022, 9, 142. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 541927. [Google Scholar] [CrossRef]
- Zhao, L.C.; Liang, J.; Li, W.; Cheng, K.M.; Xia, X.; Deng, X.; Yang, G.L. The use of response surface methodology to optimize the ultrasound-assisted extraction of five anthraquinones from Rheum palmatum L. Molecules 2011, 16, 5928–5937. [Google Scholar] [CrossRef]
- Ruan, N.; Jiao, Z.; Tang, L. Response surface methodology to optimize supercritical carbon dioxide extraction of Polygonum cuspidatum. J. AOAC Int. 2022, 105, 272–281. [Google Scholar] [CrossRef] [PubMed]



| Independent Variable | Factor Level | Dependent Variables | Goal | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Extraction temperature (°C) (T1) | 35 | 45 | 55 | Chrysophanol yield (mg/g) (R1) | Maximized |
| Extraction time (min) (T2) | 45 | 55 | 65 | ||
| Liquid-to-solid ratio (mL/g) (T3) | 12 | 18 | 24 | ||
| Run | Factor (Coded) | Actual Variables | Chrysophanol Yield (R) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (T1) (°C) | (T2) (min) | (T3) (mL/g) | (T1) (°C) | (T2) (min) | (T3) (mL/g) | Experimental Value (mg/g) | Predicted Value (mg/g) | Residual | |
| 1 | 0 | −1 | −1 | 45 | 45 | 12 | 15.7 ± 0.61 | 15.70 | 0.00 |
| 2 | 0 | 0 | 0 | 45 | 55 | 18 | 19.1 ± 0.81 | 18.74 | 0.36 |
| 3 | 0 | 0 | 0 | 45 | 55 | 18 | 18.7 ± 0.83 | 18.74 | −0.04 |
| 4 | −1 | 1 | 0 | 35 | 65 | 18 | 15.3 ± 0.73 | 15.30 | 0.00 |
| 5 | 0 | 0 | 0 | 45 | 55 | 18 | 18.8 ± 0.92 | 18.74 | 0.06 |
| 6 | −1 | 0 | 1 | 35 | 55 | 24 | 13.9 ± 0.43 | 13.90 | 0.00 |
| 7 | 1 | 0 | −1 | 55 | 55 | 12 | 17.1 ± 0.87 | 17.10 | 0.00 |
| 8 | 1 | −1 | 0 | 55 | 45 | 18 | 17.6 ± 0.94 | 17.60 | 0.00 |
| 9 | 0 | −1 | 1 | 45 | 45 | 24 | 16.8 ± 0.95 | 16.75 | 0.05 |
| 10 | −1 | 0 | −1 | 35 | 55 | 12 | 13.9 ± 0.78 | 13.85 | 0.05 |
| 11 | 1 | 1 | 0 | 55 | 65 | 18 | 18.3 ± 0.97 | 18.25 | 0.05 |
| 12 | 1 | 0 | 1 | 55 | 55 | 24 | 17.9 ± 0.95 | 17.95 | −0.05 |
| 13 | 0 | 0 | 0 | 45 | 55 | 18 | 18.6 ± 0.86 | 18.74 | −0.14 |
| 14 | 0 | 1 | −1 | 45 | 65 | 12 | 17.6 ± 0.93 | 17.65 | −0.05 |
| 15 | 0 | 0 | 0 | 45 | 55 | 18 | 18.5 ± 0.94 | 18.74 | −0.24 |
| 16 | 0 | 1 | 1 | 45 | 65 | 24 | 17.5 ± 0.99 | 17.50 | 0.00 |
| 17 | −1 | −1 | 0 | 35 | 45 | 18 | 11.5 ± 0.61 | 11.91 | −0.47 |
| Dependent Variables | Source | R2 | Adjusted R2 | Predicted R2 | SD | Sequential p-Value | Lack of Fit p-Value | |
|---|---|---|---|---|---|---|---|---|
| R1 | Linear | 0.5496 | 0.4456 | 0.2919 | 1.59 | 0.0133 | 0.0005 | |
| 2FI | 0.5898 | 0.3437 | −0.1314 | 1.73 | 0.8060 | 0.0003 | ||
| Quadratic | 0.9846 | 0.9648 | 0.7955 | 0.40 | <0.0001 | 0.0628 | Suggested | |
| Cubic | 0.9971 | 0.9883 | 0.23 | 0.0628 |
| Analysis of Variance (ANOVA) | |
|---|---|
| F-value (model) | 49.69 |
| p-value (model) | <0.0001 s |
| F-value (lack of fit) | 5.71 |
| p-value (lack of fit) | 0.0628 ns |
| CV(%) | 2.37 |
| Adeq. Precision | 22.05 |
| Residual | 1.12 |
| Pure error | 0.21 |
| Dependent Variables | Independent Variables | SS a | DF b | MS c | F-Value | p-Value d |
|---|---|---|---|---|---|---|
| R | Linear effects | |||||
| T1 | 33.21 | 1 | 33.21 | 207.66 | <0.0001 | |
| T2 | 6.30 | 1 | 6.30 | 39.40 | 0.0004 | |
| T3 | 0.4050 | 1 | 0.4050 | 2.53 | 0.1556 ns | |
| Interaction effects | ||||||
| T1T2 | 2.40 | 1 | 2.40 | 15.02 | 0.0061 | |
| T1T3 | 0.1600 | 1 | 0.1600 | 1 | 0.3505 ns | |
| T2T3 | 0.3600 | 1 | 0.3600 | 2.25 | 0.1772 ns | |
| Quadratic effects | ||||||
| T12 | 19.15 | 1 | 19.15 | 119.73 | <0.0001 | |
| T22 | 3.66 | 1 | 3.66 | 22.89 | 0.002 | |
| T32 | 3.47 | 1 | 3.47 | 21.68 | 0.002 | |
| Response Variable | Optimum Extraction Condition | Maximum Value | |||
|---|---|---|---|---|---|
| Chrysophanol yield (mg/g) (R) | T1 (°C) | T2 (min) | T3 (mL/g) | Experimental value (mg/g) | Predicted value (mg/g) |
| 49.3 | 57.7 | 18.7 | 20.47 ± 0.77 | 19.31 | |
| Extraction Method | Extraction Temp. (°C) | Extraction Time (min) | Methanol (mL/g) | Chrysophanol Yield (mg/g) |
|---|---|---|---|---|
| UAE | 49.3 | 57.7 | 18.7 | 20.47 ± 0.77 |
| CSE | 60 | 60 | 25 | 14.17 ± 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamoud, G.A.; Alam, P.; Fantoukh, O.I.; Hawwal, M.F.; Akhtar, A. HPLC-UV Analysis of Chrysophanol in Senna occidentalis Extract Obtained by Using the RSM-Optimized Ultrasonic Extraction Process. Processes 2023, 11, 1410. https://doi.org/10.3390/pr11051410
Al-Hamoud GA, Alam P, Fantoukh OI, Hawwal MF, Akhtar A. HPLC-UV Analysis of Chrysophanol in Senna occidentalis Extract Obtained by Using the RSM-Optimized Ultrasonic Extraction Process. Processes. 2023; 11(5):1410. https://doi.org/10.3390/pr11051410
Chicago/Turabian StyleAl-Hamoud, Gadah A., Perwez Alam, Omer I. Fantoukh, Mohammed F. Hawwal, and Ali Akhtar. 2023. "HPLC-UV Analysis of Chrysophanol in Senna occidentalis Extract Obtained by Using the RSM-Optimized Ultrasonic Extraction Process" Processes 11, no. 5: 1410. https://doi.org/10.3390/pr11051410







