Bioactivities of Kenaf Biomass Extracts: A Review
Abstract
:1. Introduction
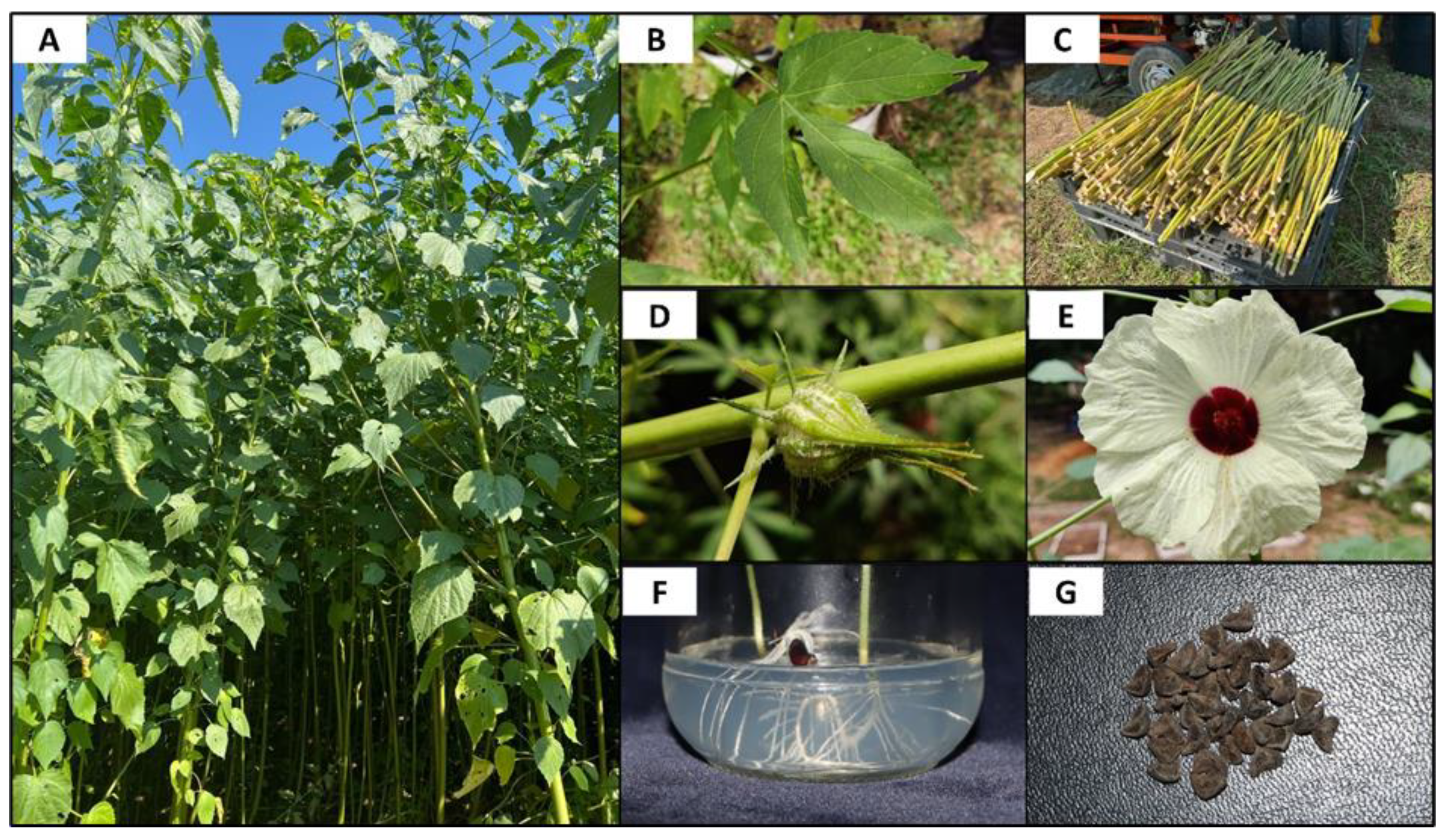
2. Taxonomy and Botanical Description
3. Current Status in Malaysia
4. Bioactivities of Kenaf Extracts
4.1. Anticancer Properties
Seed Extract
4.2. Antibacterial Properties
4.2.1. Seed Extract
4.2.2. Leaf Extract
4.3. Antifungal and Phytotoxic Properties
4.3.1. Seed Extract
4.3.2. Leaf Extract
4.4. Antithrombotic Properties
Seed Extract
4.5. Anti-Hypercholesterolemic Properties
Seed Extract
4.6. Anti-Hyperpigmentation, Skin Whitening and Anti-Aging Properties
4.6.1. Seed Extract
4.6.2. Leaf Extract
4.7. Antioxidant Properties
4.7.1. Seed Extract
4.7.2. Leaf Extract
4.7.3. Flower Extract
4.7.4. Stem Extract
4.8. Antihypertensive Properties
Seed Extract
4.9. Antidiabetic Properties
Leaf Extract
4.10. Immunomodulatory Effects
Leaf Extract
4.11. Phytochemistry of Kenaf Biomass Extracts
4.11.1. Seed
4.11.2. Leaves
4.11.3. Flowers and Stems
4.12. Extraction Methods for Kenaf Biomass Extracts
5. Kenaf-Based Products
6. Future Potential Applications
6.1. Pharmaceutical Applications
6.2. Food Applications
6.3. Cosmetic Applications
6.4. Nanotechnology Applications
7. Challenges and Limitations
7.1. Dependency on Harvesting Period
7.2. Inconsistent Plant Quality
7.3. Land Availability
7.4. Farmers’ Acceptance
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izran, K.; Mohd Zharif, T.; Beyer, G.; Mohamad Jani, S.; Noor Azrieda, A.R.; Yanti, A.K. Kenaf For Biocomposite: An Overview. J. Sci. Technol. 2014, 6, 41–66. [Google Scholar]
- Basri, M.H.A.; Abdu, A.; Junejo, N.; Hamid, H.A.; Ahmed, K. Journey of kenaf in Malaysia: A Review. Sci. Res. Essays 2014, 9, 458–470. [Google Scholar] [CrossRef] [Green Version]
- Ayadi, R.; Hanana, M.; Mzid, R.; Hamrouni, L.; Khouja, M.l.; Salhi Hanachi, A. Hibiscus cannabinus L.–Kenaf: A Review Paper. J. Nat. Fibers 2017, 14, 466–484. [Google Scholar] [CrossRef]
- Coetzee, R.; Labuschagne, M.T.; Hugo, A. Fatty acid and oil variation in seed from kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2008, 27, 104–109. [Google Scholar] [CrossRef]
- Adnan, M.; Oh, K.K.; Azad, M.O.K.; Shin, M.H.; Wang, M.H.; Cho, D.H. Kenaf (Hibiscus cannabinus L.) leaves and seed as a potential source of the bioactive compounds: Effects of various extraction solvents on biological properties. Life 2020, 10, 223. [Google Scholar] [CrossRef]
- Arulrajah, B.; Muhialdin, B.J.; Qoms, M.S.; Zarei, M.; Hussin, A.S.M.; Hasan, H.; Saari, N. Production of cationic antifungal peptides from kenaf seed protein as natural bio preservatives to prolong the shelf-life of tomato puree. Int. J. Food Microbiol. 2021, 359, 109418. [Google Scholar] [CrossRef]
- Ghafar, S.A.A.; Ismail, M.; Saiful Yazan, L.; Fakurazi, S.; Ismail, N.; Chan, K.W.; Md Tahir, P. Cytotoxic activity of kenaf seed oils from supercritical carbon dioxide fluid extraction towards human colorectal cancer (HT29) cell lines. Evid.-Based Complement. Altern. Med. 2013, 2013, 549705. [Google Scholar] [CrossRef] [Green Version]
- Hanumegowda, S.; Srinivasa, C.; Shivaiah, A.; Venkatappa, M.; Hanumanthappa, R.; Rangappa, R.; Laxmaiah, R.; Gonchigar, S.; Sannaningaiah, D. Protein extract of kenaf seed exhibits anticoagulant, antiplatelet and antioxidant activities. Asian Pac. J. Trop. Biomed. 2022, 12, 47–58. [Google Scholar] [CrossRef]
- Kai, N.S.; Nee, T.A.; Ling, E.L.C.; Ping, T.C.; Kamariah, L.; Lin, N.K. Anti-hypercholesterolemic effect of kenaf (Hibiscus cannabinus L.) seed on high-fat diet Sprague dawley rats. Asian Pac. J. Trop. Med. 2015, 8, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Kobaisy, M.; Tellez, M.R.; Webber, C.L.; Dayan, F.E.; Schrader, K.K.; Wedge, D.E. Phytotoxic and fungitoxic activities of the essential oil of kenaf (Hibiscus cannabinus L.) Leaves and its composition. J. Agric. Food Chem. 2001, 49, 3768–3771. [Google Scholar] [CrossRef]
- Goh, K.M.; Ng, S.Y.; Nyam, K.L. The infusion of goji berries and red dates ameliorates the overall qualities of kenaf leaves tea. Int. Food Res. J. 2021, 28, 1216–1222. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Nyam, K.L. Application of Hibiscus cannabinus L. (kenaf) leaves extract as skin whitening and anti-aging agents in natural cosmetic prototype. Ind. Crops Prod. 2021, 167, 113491. [Google Scholar] [CrossRef]
- Ryu, J.; Kwon, S.-J.; Ahn, J.-W.; Jo, Y.D.; Kim, S.H.; Jeong, S.W.; Lee, M.K.; Kim, J.-B.; Kang, S.-Y. Phytochemicals and antioxidant activity in the kenaf plant (Hibiscus cannabinus L.). J. Plant Biotechnol. 2017, 44, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Unfairtobacco. Kenaf in Malaysia; Unfairtobacco: Berlin, Germany, 2013; p. 2. [Google Scholar]
- Rymsza, T.A. Kenaf and Hemp—Identifying the Differences. Available online: http://www.visionpaper.com/PDF_speeches_papers/ (accessed on 13 November 2022).
- Sim, Y.Y.; Nyam, K.L. Hibiscus cannabinus L. (kenaf) studies: Nutritional composition, phytochemistry, pharmacology, and potential applications. Food Chem. 2021, 344, 128582. [Google Scholar] [CrossRef] [PubMed]
- Tahir, P.M.; Ahmed, A.B.; Azry, S.O.A.S.; Ahmed, Z. Retting process of some bast plant fibres and its effect on fibre quality: A review. BioResources 2011, 6, 5260–5281. [Google Scholar]
- Annual Report 2021: LKTN. Statistic Perangkaan 2021; LKTN: Kota Bharu, Malaysia, 2021; Volume 130102, pp. 1–43.
- Webber, C.L.I.; Bledsoe, V.K. Kenaf Yield Components and Plant Composition. In Trends in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2002; pp. 348–357. [Google Scholar]
- Arumingtyas, E.L. Kenaf: Its Prospect in Indonesia. Berk. Penelit. Hayati 2015, 20, 21–26. [Google Scholar] [CrossRef]
- Danalatos, N.G.; Archontoulis, S.V. Potential growth and biomass productivity of kenaf (Hibiscus cannabinus L.) under central Greek conditions: II. The influence of variety, sowing time and plant density. In Proceedings of the 2nd World Conference on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10–14 May 2004; pp. 10–14. [Google Scholar]
- LKTN. Soalan Lazim-Kenaf. Available online: https://www.lktn.gov.my (accessed on 7 October 2022).
- MPIC. One Stop Center for Kenaf & Tobacco. Available online: https://www.mpic.gov.my (accessed on 9 December 2022).
- Edeerozey, A.M.M.; Akil, H.M.; Azhar, A.B.; Ariffin, M.I.Z. Chemical modification of kenaf fibers. Mater. Lett. 2007, 61, 2023–2025. [Google Scholar] [CrossRef]
- Mossello, A.A.; Harun, J.; Tahir, P.M.; Resalati, H.; Ibrahim, R.; Fallah Shamsi, S.R.; Mohmamed, A.Z. A Review of Literatures Related of Using Kenaf for Pulp Production (Beating, Fractionation, and Recycled Fiber). Mod. Appl. Sci. 2010, 4, 21–22. [Google Scholar] [CrossRef] [Green Version]
- Kamaruddin, N.; Othman, M.S.H. Quantifying of farmers’ acceptance and perception in developing kenaf, hibiscus cannabinus, industry in Malaysia. Int. J. Green Econ. 2012, 6, 401–416. [Google Scholar] [CrossRef]
- myMetro. Tanaman Kenaf Akan Diperkasakan. Available online: https://www.hmetro.com.my (accessed on 25 October 2022).
- Yazan, L.S.; Abd Rahman, N.; Chan, K.W.; Wan Abd Ghani, W.N.H.; Tor, Y.S.; Foo, J.B. Phenolics-saponins rich fraction of defatted kenaf seed meal exhibits cytotoxicity towards cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Byeon, S.E.; Kim, J.Y.; Lee, J.Y.; Rhee, M.H.; Hong, S.; Wu, J.C.; Lee, H.S.; Kim, M.J.; Cho, D.H.; et al. Immunomodulatory effect of Hibiscus cannabinus extract on macrophage functions. J. Ethnopharmacol. 2007, 113, 62–71. [Google Scholar] [CrossRef]
- Ghafar, S.A.A.; Yazan, L.S.; Tahir, P.M.; Ismail, M. Kenaf seed supercritical fluid extract reduces aberrant crypt foci formation in azoxymethane-induced rats. Exp. Toxicol. Pathol. 2012, 64, 247–251. [Google Scholar] [CrossRef]
- Arulrajah, B.; Qoms, M.S.; Muhialdin, B.J.; Hasan, H.; Zarei, M.; Meor Hussin, A.S.; Chau, D.M.; Saari, N. Antibacterial and antifungal activity of kenaf seed peptides and their effect on microbiological safety and physicochemical properties of some food models. Food Control 2022, 140, 109119. [Google Scholar] [CrossRef]
- Birhanie, Z.M.; Xiao, A.; Yang, D.; Huang, S.; Zhang, C.; Zhao, L.; Liu, L.; Li, J.; Chen, A.; Tang, H.; et al. Polysaccharides, total phenolic, and flavonoid content from different kenaf (Hibiscus cannabinus l.) genotypes and their antioxidants and antibacterial properties. Plants 2021, 10, 1900. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Teoh, S.Y.; Sim, Y.Y.; Nyam, K.L. Optimization of ultrasonic extraction condition for maximal antioxidant, antimicrobial, and antityrosinase activity from Hibiscus cannabinus L. leaves by using the single factor experiment. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100321. [Google Scholar] [CrossRef]
- Cheong, A.M.; Jessica Koh, J.X.; Patrick, N.O.; Tan, C.P.; Nyam, K.L. Hypocholesterolemic Effects of Kenaf Seed Oil, Macroemulsion, and Nanoemulsion in High-Cholesterol Diet Induced Rats. J. Food Sci. 2018, 83, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.Y.; Tan, C.P.; Cheong, L.Z.; Nyam, K.L. Hibiscus cannabinus L. leaf and seed in cosmetic formulation: An integrated approach as antioxidant and melanogenesis inhibitor. Sustain. Mater. Technol. 2022, 33, e00457. [Google Scholar] [CrossRef]
- Chan, K.W.; Iqbal, S.; Khong, N.M.H.; Ooi, D.J.; Ismail, M. Antioxidant activity of phenolics-saponins rich fraction prepared from defatted kenaf seed meal. LWT Food Sci. Technol. 2014, 56, 181–186. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Hanafi, M.A.; Chay, S.Y.; Hussin, F.S.; Auwal, S.M.; Zarei, M.; Sarbini, S.R.; Wan Ibadullah, W.Z.; Karim, R.; Saari, N. Multifunctional hydrolysates from kenaf (Hibiscus cannabinus L.) seed protein with high antihypertensive activity in vitro and in vivo. J. Food Meas. Charact. 2020, 15, 652–663. [Google Scholar] [CrossRef]
- Elias, W. The Anti-Diabetic Effect of Hibiscus cannabinus Extract on the Submandibular Salivary Gland of Alloxan-Induced Diabetic Albino Rats. Int. J. Pharm. Res. Allied Sci. 2020, 2020, 195–202. [Google Scholar]
- Shaikh, S.; Joshi, Y.M.; Kadam, V. Comparative study of anti-inflammatory activity of aqueous and methanolic extracts of Hibiscus cannabinus leaf (Malvaceae). Int. J. Pharm. Pharm. Sci. 2016, 8, 64–68. [Google Scholar]
- Kumar, V.; Mahdi, F.; Khanna, A.K.; Singh, R.; Chander, R.; Saxena, J.K.; Mahdi, A.A.; Singh, R.K. Antidyslipidemic and antioxidant activities of hibiscus rosa sinensis root extract in alloxan induced diabetic rats. Indian J. Clin. Biochem. 2013, 28, 46–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, A.J.S.J.; Mohan, S.; Chellappan, D.K.; Kalusalingam, A.; Ariamuthu, S. Hibiscus vitifolius (Linn.) root extracts shows potent protective action against anti-tubercular drug induced hepatotoxicity. J. Ethnopharmacol. 2012, 141, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Nyam, K.L.; Tan, C.P.; Lai, O.M.; Long, K.; Che Man, Y.B. Physicochemical properties and bioactive compounds of selected seed oils. LWT 2009, 42, 1396–1403. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Application of response surface methodology for optimizing the deodorization parameters in chemical refining of kenaf seed oil. Sep. Purif. Technol. 2017, 184, 144–151. [Google Scholar] [CrossRef]
- Nesaretnam, K.; Wong, W.Y.; Wahid, M.B. Tocotrienols and cancer: Beyond antioxidant activity. Eur. J. Lipid Sci. Technol. 2007, 109, 445–452. [Google Scholar] [CrossRef]
- Rajaram, S. Health benefits of plant-derived α-linolenic acid. Am. J. Clin. Nutr. 2014, 100, 443S–448S. [Google Scholar] [CrossRef] [Green Version]
- Melgarejo, P.; Artés, F. Total lipid content and fatty acid composition of oilseed from lesser known sweet pomegranate clones. J. Sci. Food Agric. 2000, 80, 1452–1454. [Google Scholar] [CrossRef]
- Badal, S.; Delgoda, R. Pharmacognosy: Fundamentals, Applications and Strategy. In Chemotherapeutics; Shields, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Khandelwal, S.; Shidhaye, R.; Demonty, I.; Lakshmy, R.; Gupta, R.; Prabhakaran, D.; Reddy, S. Impact of omega-3 fatty acids and/or plant sterol supplementation on non-HDL cholesterol levels of dyslipidemic Indian adults. J. Funct. Foods 2013, 5, 36–43. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. Fungicidal mechanisms of the antimicrobial peptide Bac8c. Biochim. Biophys. Acta Biomembr. 2015, 1848, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, A.P.; Horst, H.; Sousa, E.D.; Pizzolatti, M.G.; Silva, F.R.M.B. Insulinomimetic effects of kaempferitrin on glycaemia and on 14C-glucose uptake in rat soleus muscle. Chem.-Biol. Interact. 2004, 149, 89–96. [Google Scholar] [CrossRef]
- Inikpi, E.; Lawal, O.A.; Ogunmoye, A.O.; Ogunwande, I.A. Volatile composition of the floral essential oil of Hibiscus sabdariffa L. from Nigeria. Am. J. Essent. Oils Nat. Prod. 2014, 2, 4–7. [Google Scholar]
- Sim, Y.Y.; Nyam, K.L. Effect of different drying methods on the physical properties and antioxidant activities of Hibiscus cannabinus leaves. J. Food Meas. Charact. 2019, 13, 1279–1286. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, Phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 20–31. [Google Scholar] [CrossRef]
- Toh, P.Y.; Leong, F.S.; Chang, S.K.; Khoo, H.E.; Yim, H.S. Optimization of extraction parameters on the antioxidant properties of banana waste. Acta Sci. Pol. Technol. Aliment. 2016, 15, 65–78. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, M.M.; Chen, P.L.; Cao, Y.Y.; Tan, X.L. Optimization of Ultrasonic-Assisted Enzymatic Hydrolysis for the Extraction of Luteolin and Apigenin from Celery. J. Food Sci. 2011, 76, 680–685. [Google Scholar] [CrossRef]
- Şahin, S.; Şamli, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Ultrasound-assisted extraction from defatted oat (Avena sativa L.) bran to simultaneously enhance phenolic compounds and β-glucan contents: Compositional and kinetic studies. J. Food Eng. 2018, 222, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Özkal, S.G.; Salgin, U.; Yener, M.E. Supercritical carbon dioxide extraction of hazelnut oil. J. Food Eng. 2005, 69, 217–223. [Google Scholar] [CrossRef]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]
- Chan, K.W.; Khong, N.M.H.; Iqbal, S.; Mansor, S.M.; Ismail, M. Defatted kenaf seed meal (DKSM): Prospective edible flour from agricultural waste with high antioxidant activity. LWT Food Sci. Technol. 2013, 53, 308–313. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Skin hyperpigmentation treatment using herbs: A review of clinical evidences. J. Cosmet. Laser Ther. 2018, 20, 123–131. [Google Scholar] [CrossRef]
- Adnan, M.; Azad, M.O.K.; Madhusudhan, A.; Saravanakumar, K.; Hu, X.; Wang, M.H.; Ha, C.D. Simple and cleaner system of silver nanoparticle synthesis using kenaf seed and revealing its anticancer and antimicrobial potential. Nanotechnology 2020, 31, 265101. [Google Scholar] [CrossRef]
- Adnan, M.; Oh, K.K.; Husen, A.; Wang, M.H.; Alle, M.; Cho, D.H. Microwave-Assisted Synchronous Nanogold Synthesis Reinforced by Kenaf Seed and Decoding Their Biocompatibility and Anticancer Activity. Pharmaceuticals 2022, 15, 111. [Google Scholar] [CrossRef]










| Category | Product | Description/Function | Pictures |
|---|---|---|---|
| Pet | Flea and tick powder | Control ticks, fleas and lice |  |
| Shampoo | Possesses antibacterial, anti-inflammatory and anodyne properties, which help eliminate ticks, fleas and lice |  | |
| Pet litter | Effectively and naturally absorbs and eradicates unpleasant odour |  | |
| Bedding | Reliable odour control with a good absorbent property |  | |
| Home living | Mattress | A long lasting, comfortable and toxin-free mattress |  |
| Air purifier | Made from kenaf core that can avoid bad odours naturally produced, such as in shoes and refrigerators |  | |
| Gardening | Compost | Can be mixed with plant medium such as top soil to improve soil structure and promote tree growth |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norhisham, D.‘A.; Saad, N.M.; Ahmad Usuldin, S.R.; Vayabari, D.A.G.; Ilham, Z.; Ibrahim, M.F.; Wan-Mohtar, W.A.A.Q.I. Bioactivities of Kenaf Biomass Extracts: A Review. Processes 2023, 11, 1178. https://doi.org/10.3390/pr11041178
Norhisham D‘A, Saad NM, Ahmad Usuldin SR, Vayabari DAG, Ilham Z, Ibrahim MF, Wan-Mohtar WAAQI. Bioactivities of Kenaf Biomass Extracts: A Review. Processes. 2023; 11(4):1178. https://doi.org/10.3390/pr11041178
Chicago/Turabian StyleNorhisham, Danial ‘Aizat, Norsharina Md Saad, Siti Rokhiyah Ahmad Usuldin, Diwiyaa A G Vayabari, Zul Ilham, Mohamad Faizal Ibrahim, and Wan Abd Al Qadr Imad Wan-Mohtar. 2023. "Bioactivities of Kenaf Biomass Extracts: A Review" Processes 11, no. 4: 1178. https://doi.org/10.3390/pr11041178
APA StyleNorhisham, D. ‘A., Saad, N. M., Ahmad Usuldin, S. R., Vayabari, D. A. G., Ilham, Z., Ibrahim, M. F., & Wan-Mohtar, W. A. A. Q. I. (2023). Bioactivities of Kenaf Biomass Extracts: A Review. Processes, 11(4), 1178. https://doi.org/10.3390/pr11041178









