Electrochemical Surface Treatment for Tailored Porous Structures
Abstract
:1. Introduction
2. Materials and Methods
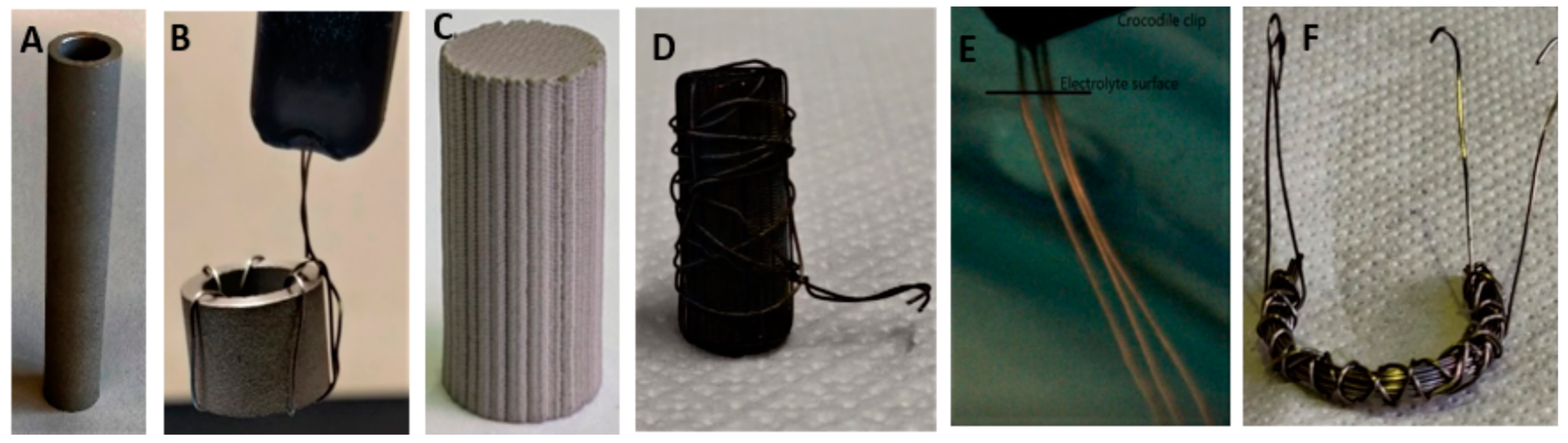
2.1. Materials
2.2. Experimentation
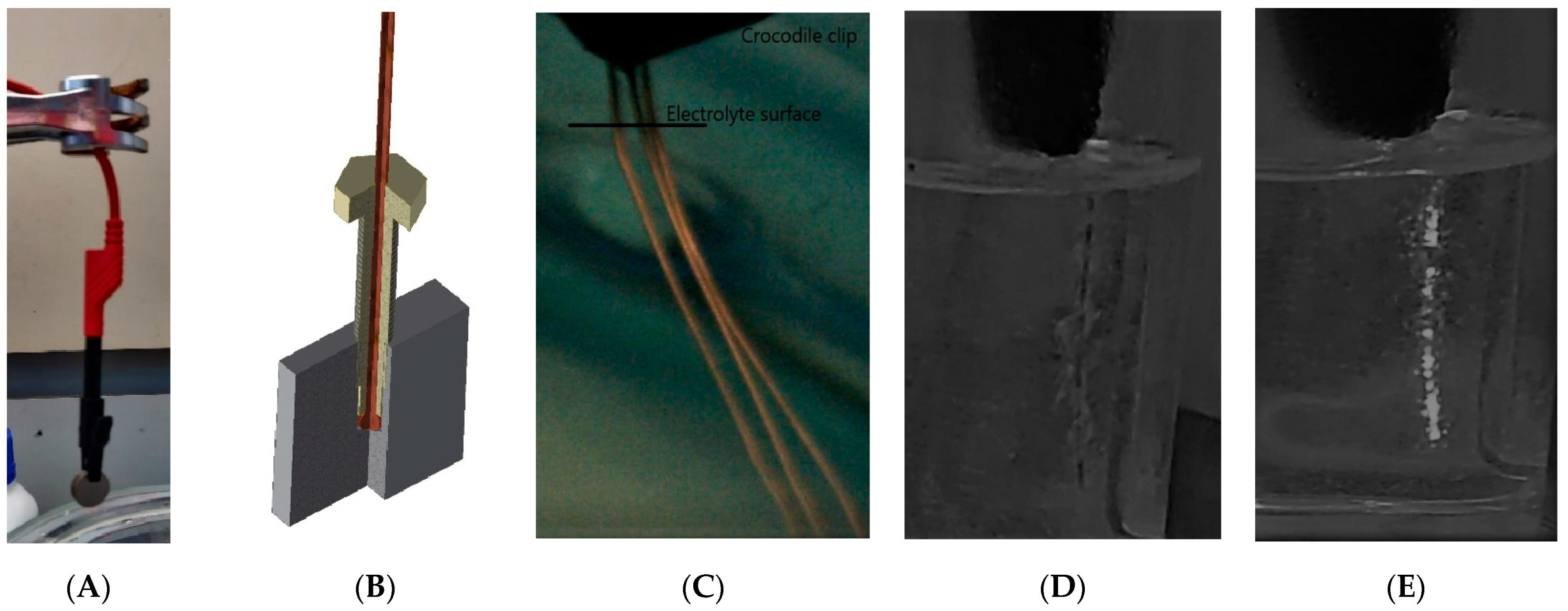
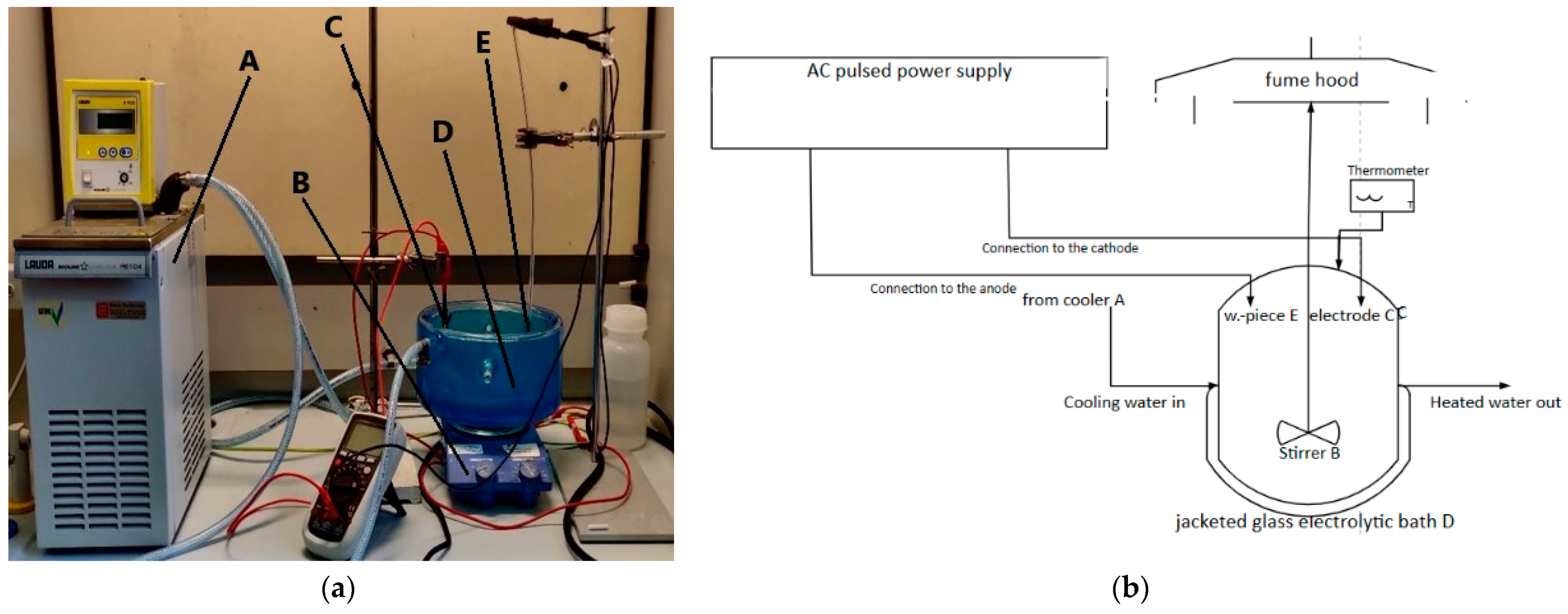
2.2.1. Experimental Setup
2.2.2. Design of Experiments
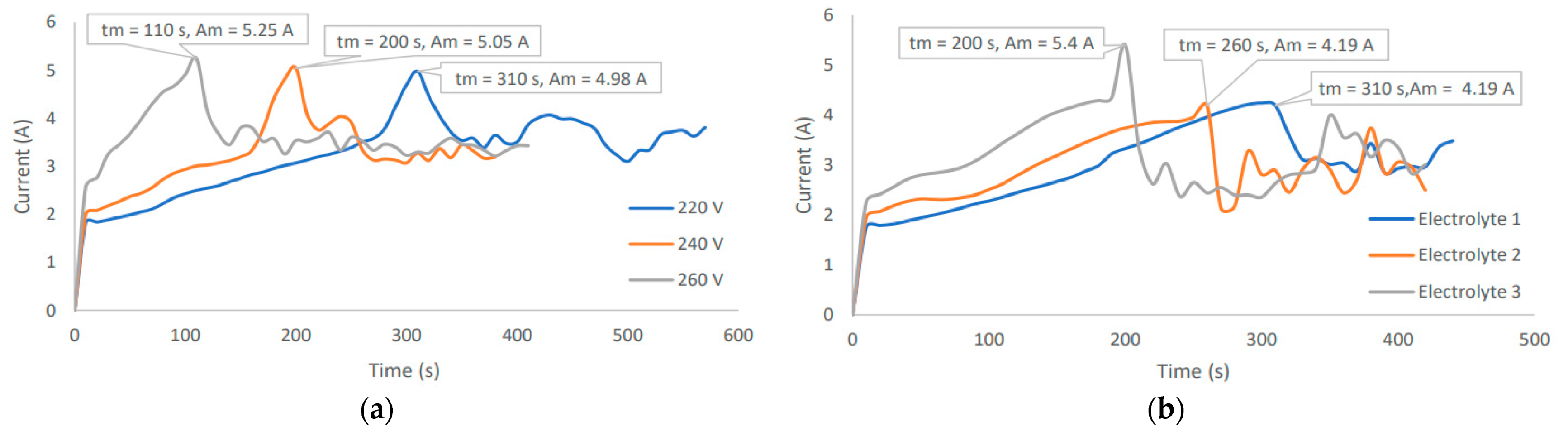
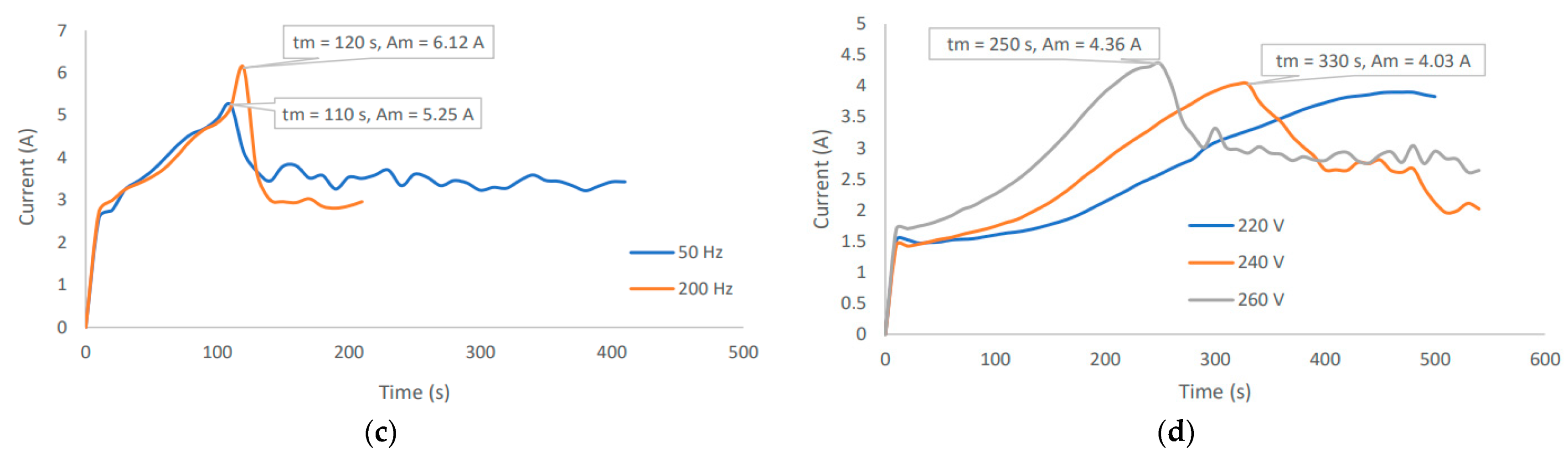
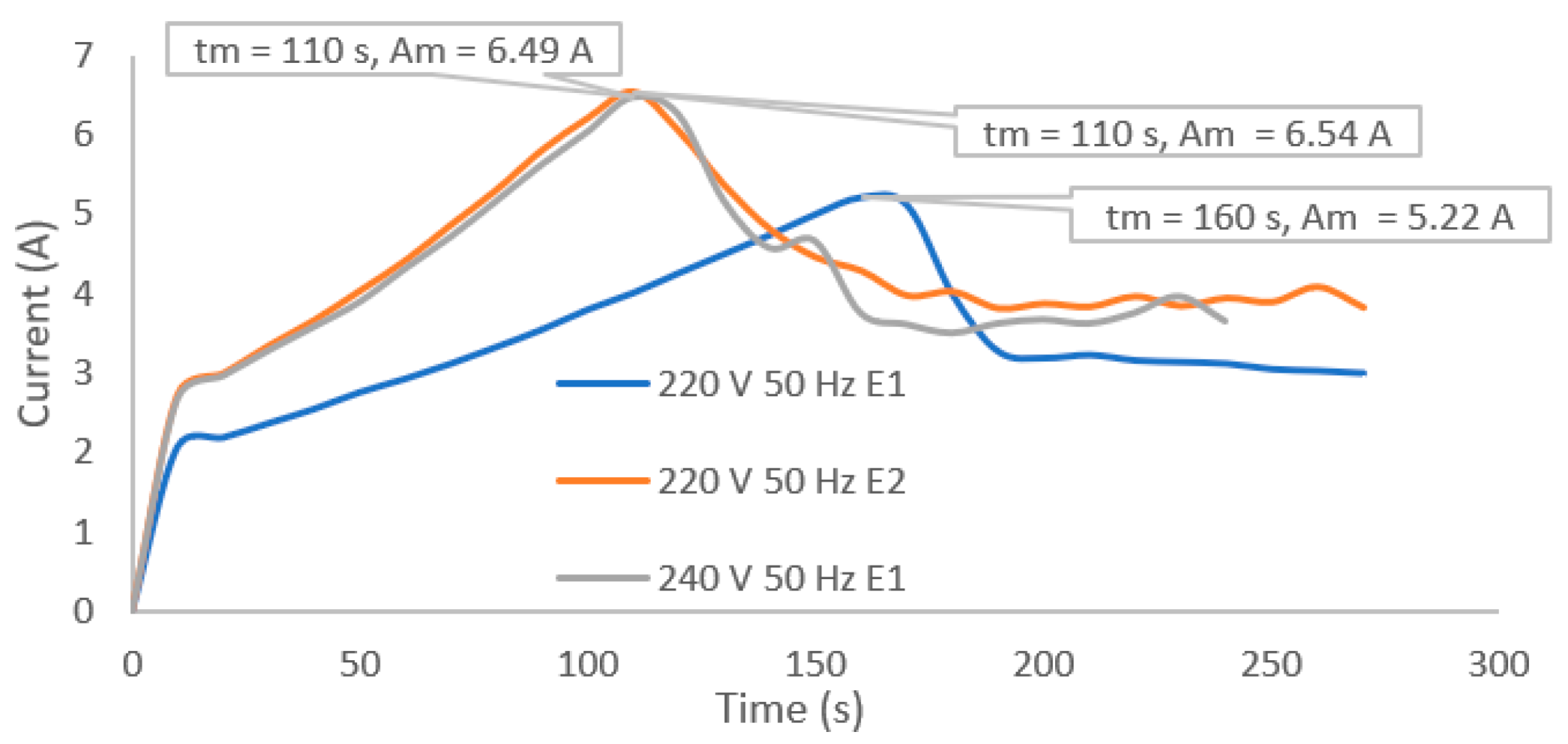
2.2.3. Current Profile Observation
2.3. Surface Characterization
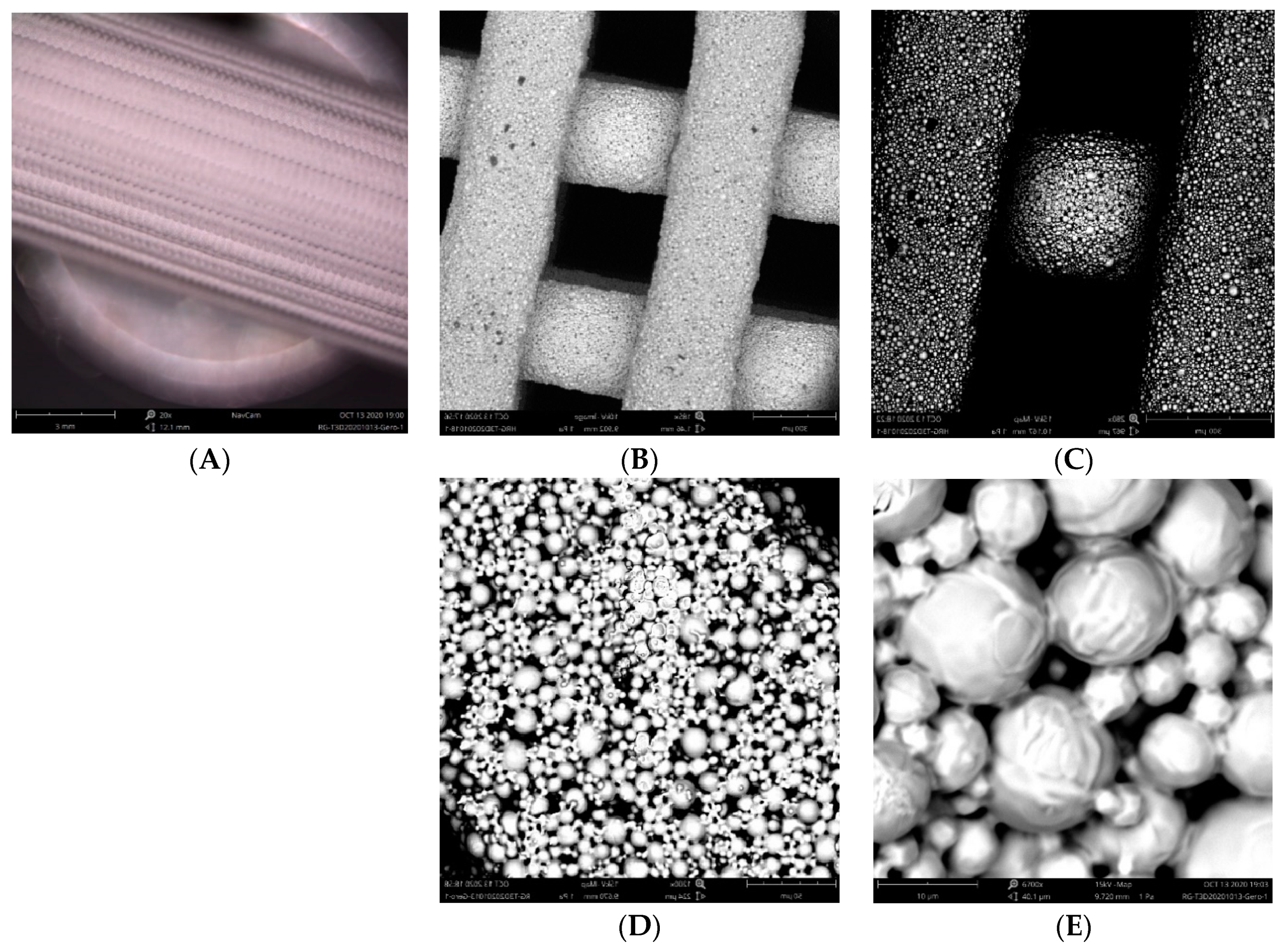
2.3.1. Surface Morphology
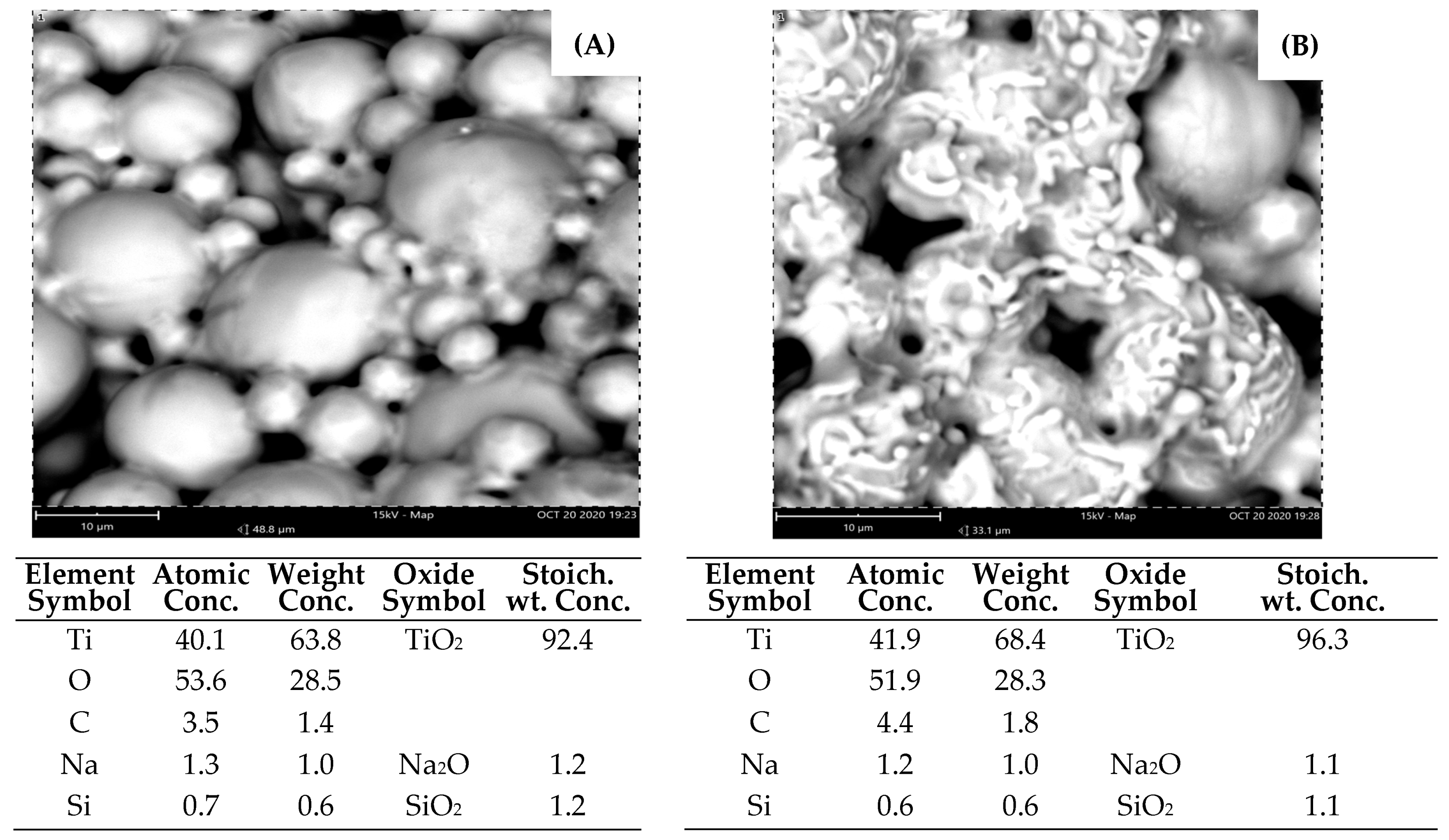
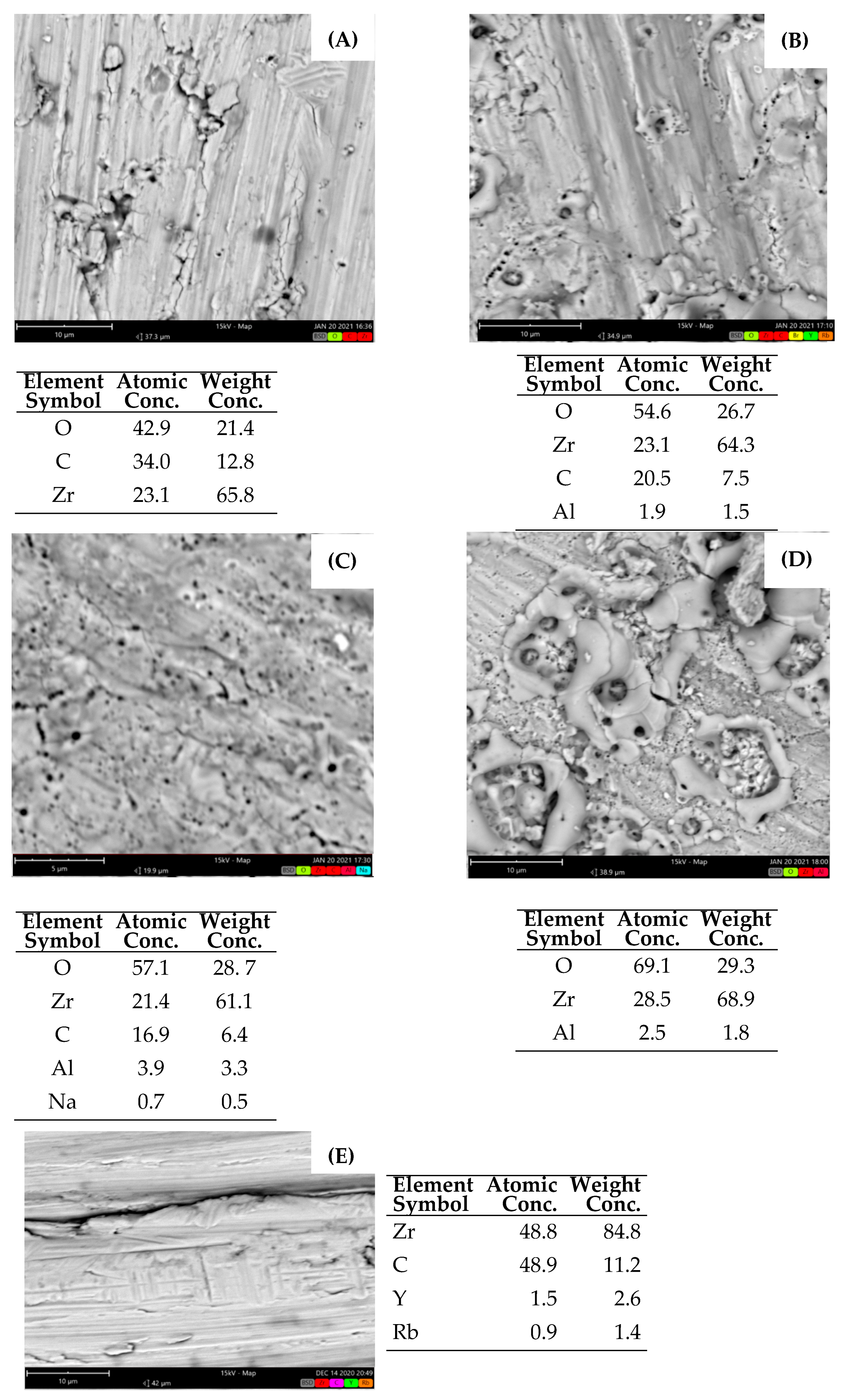
2.3.2. Element Identification and Phase Composition
3. Results and Discussion
3.1. SEM-EDX
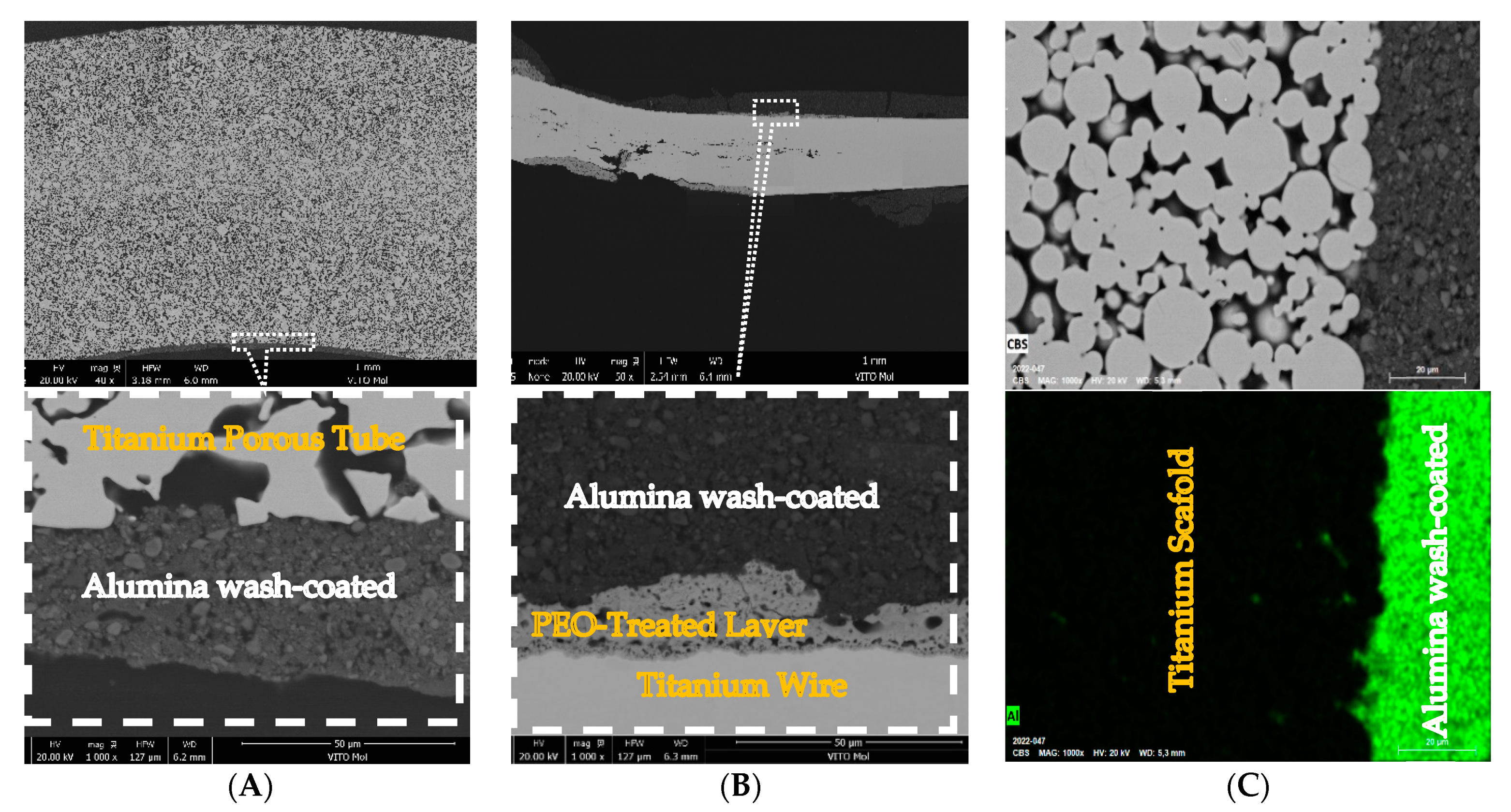
3.1.1. D-Printed Titanium Scaffold
3.1.2. Porous Titanium Tube Samples
3.1.3. Titanium Wire Samples
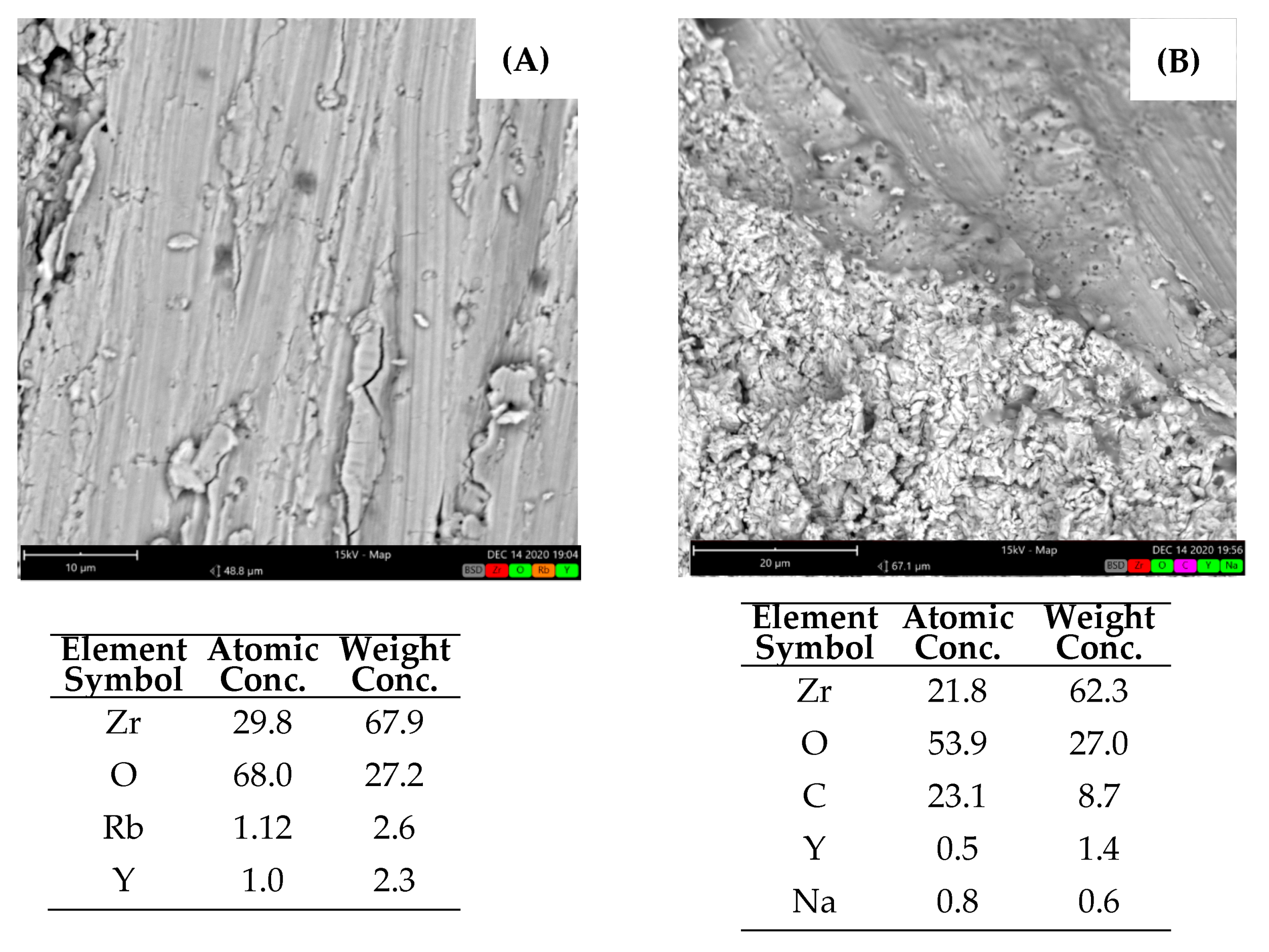
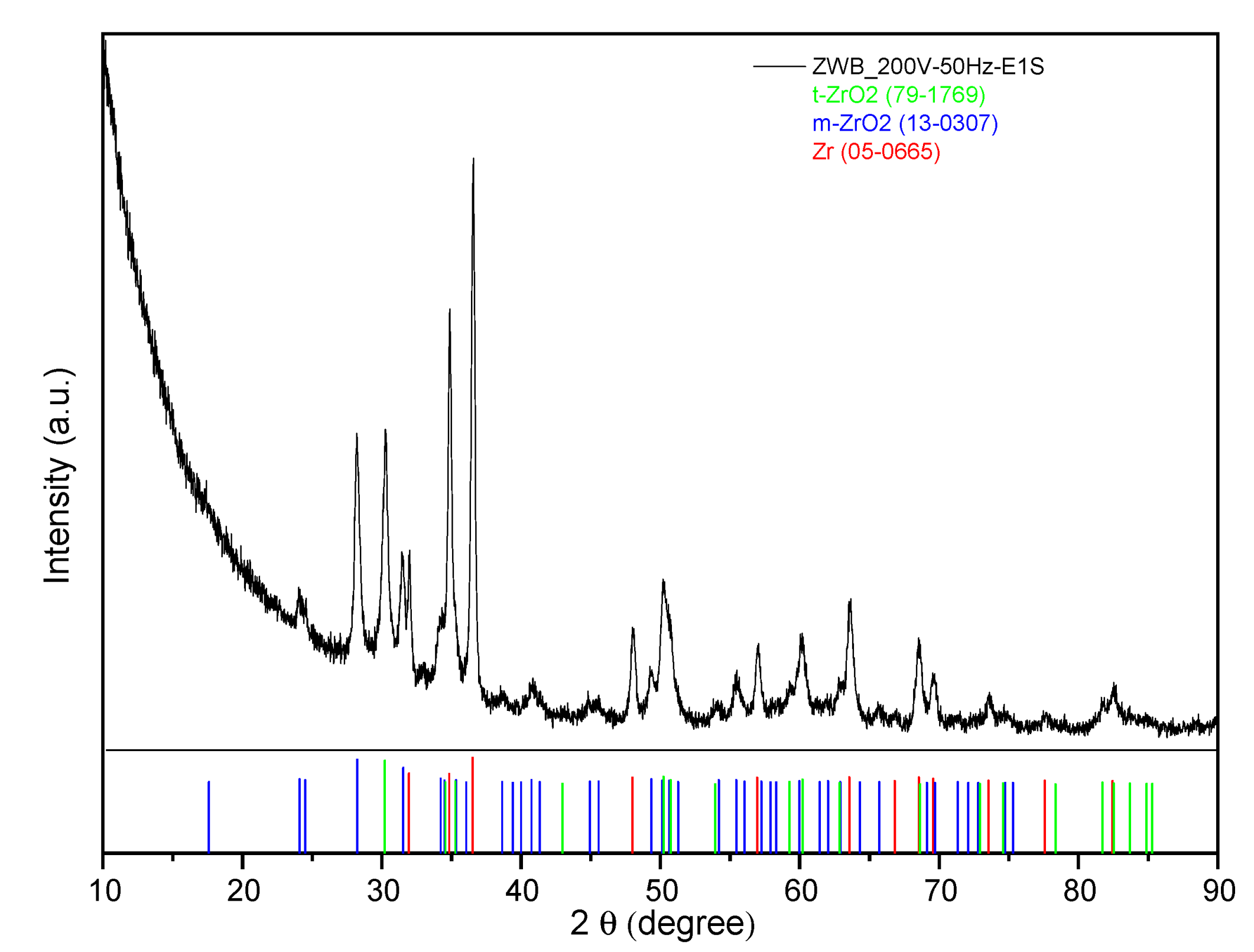
3.1.4. Zirconium Wire and Wire Bundle Samples
3.2. XRD
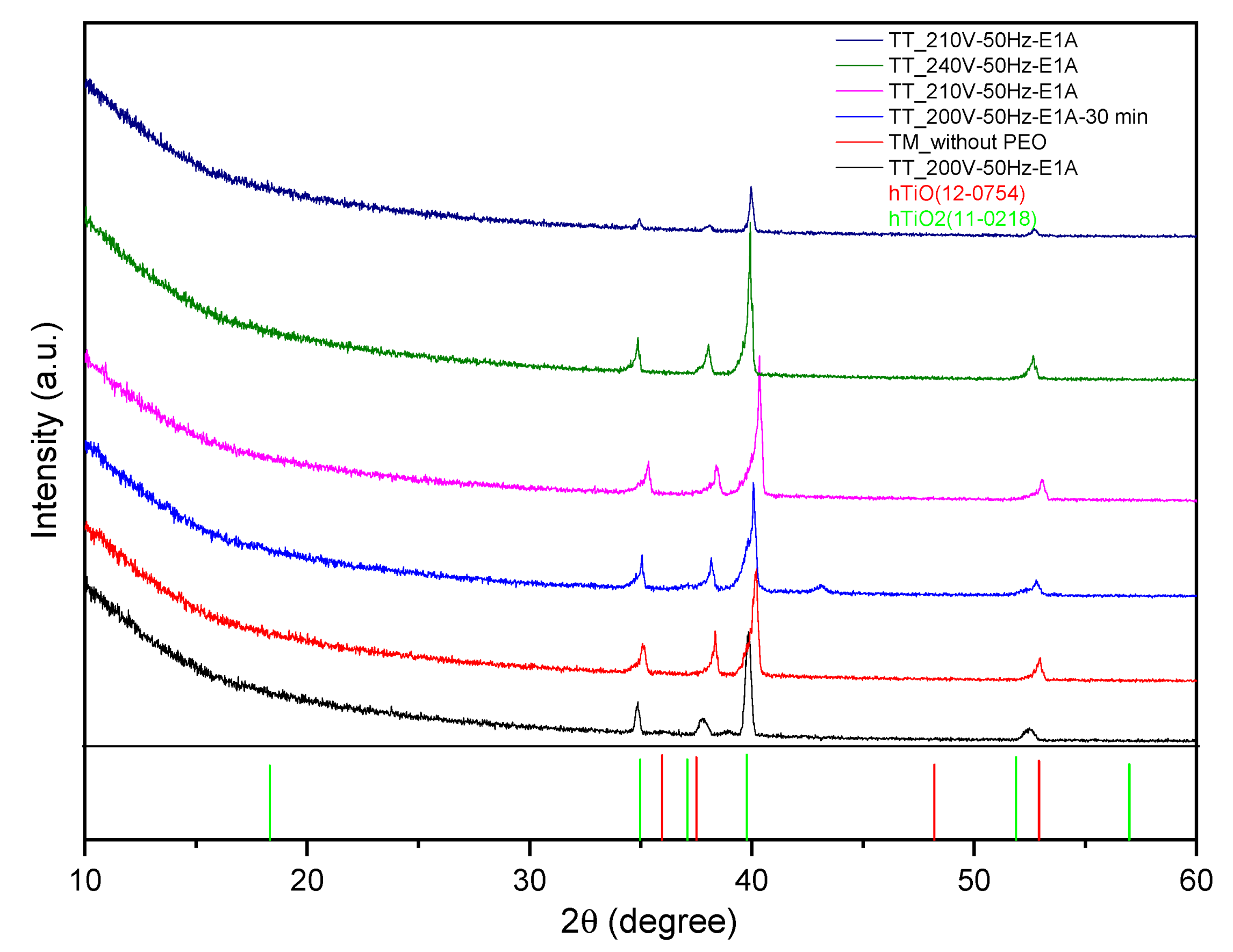
3.2.1. Porous Titanium Tube Samples
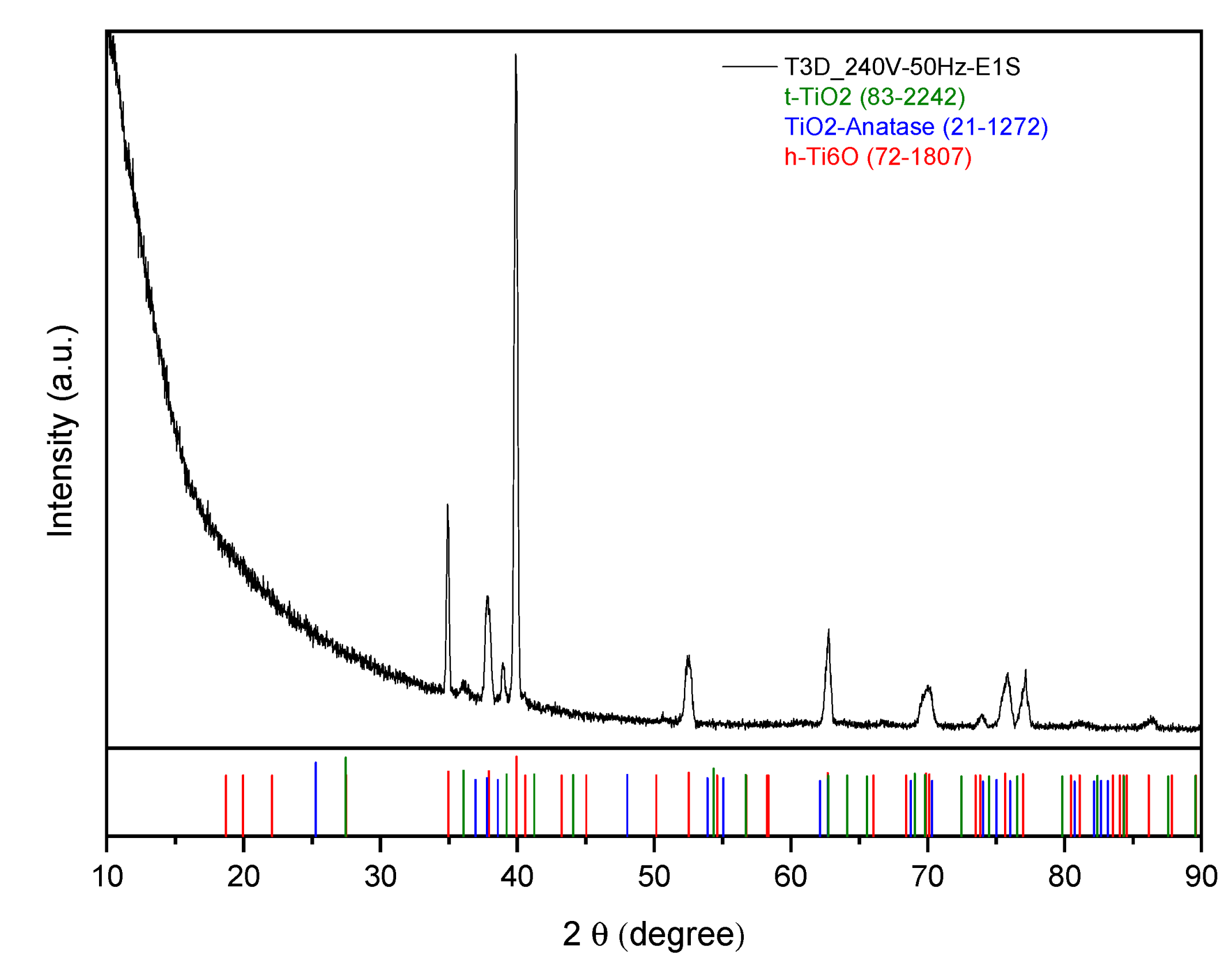
3.2.2. D-Printed Titanium Scaffold
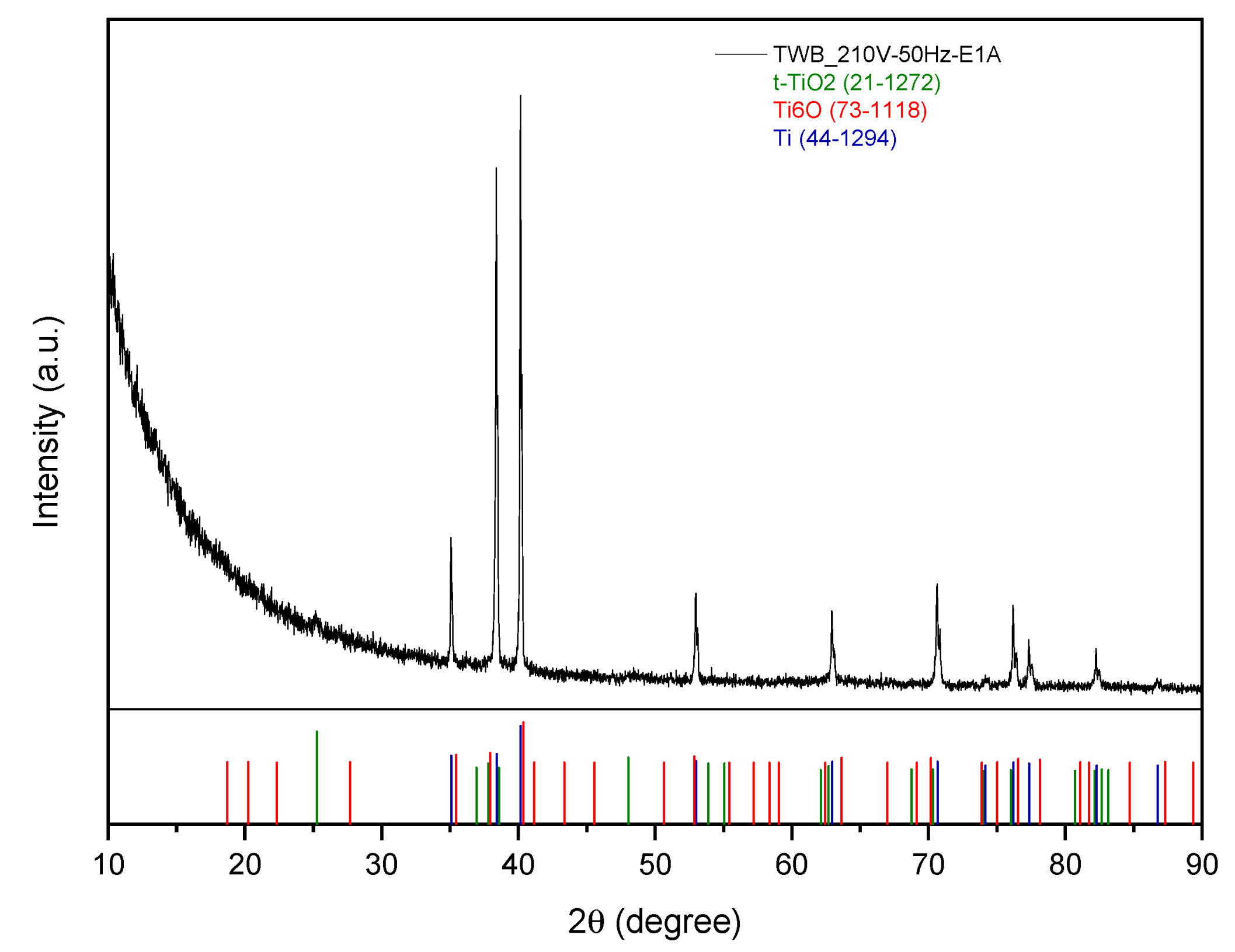
3.2.3. Titanium Wire Bundle
3.2.4. Zirconium Wire Bundles
3.3. Typical Coating Potential of the PEO Treated Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EDX | Energy-Dispersive X-Ray Spectroscopy |
| MAO | Micro Arc Oxidation |
| OCM | Oxidative Coupling of Methane |
| PEO | Plasma Electrolytic Oxidation |
| ROI | Region Of Interest |
| SEM | Scanning Electron Microscopy |
| TT | Titanium (Porous) Tube |
| TW/TWB | Titanium Wire/Titanium Wire Bundle Samples |
| T3D | Titanium 3D-Printed Scaffold |
| XRD | X-Ray Diffraction |
| ZW/ZWB | Zirconium Wire/Zirconium Wire Bundle Samples |
| Coating | refers to coating them via the alumina wash-coating method |
| Treatment | refers to PEO-treating the metal workpieces |
| Am | Sparking current | A |
| C | Current | A |
| D | Inter electrode distance | Cm |
| I | Current density | A.cm−2 |
| P | Potential | V |
| T | Oxidation time | S |
| tm | Sparking time | S |
Appendix A. Preliminary Experimentation and Observations
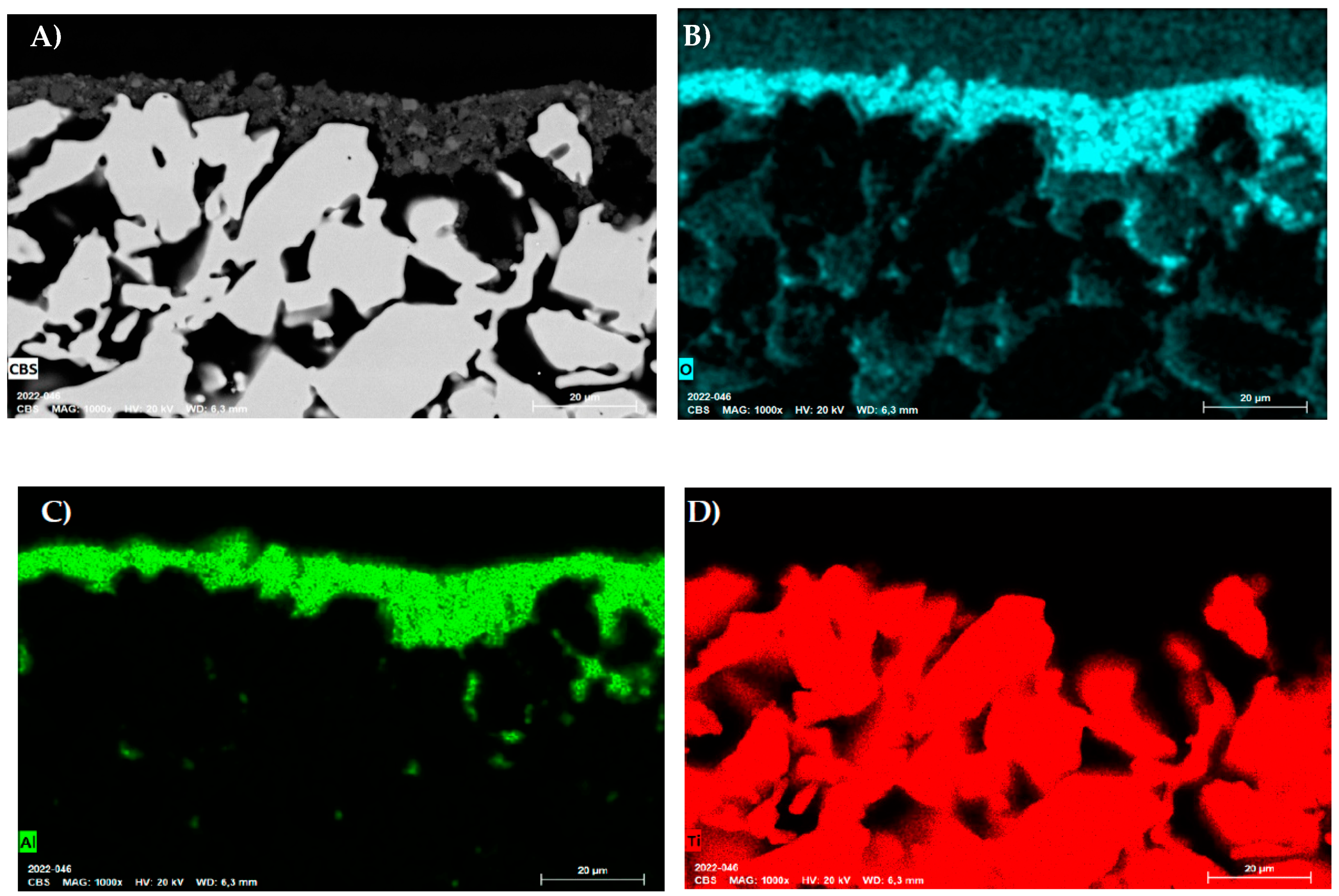
Appendix B. Additional Results for Characterization of Treated/Coated Samples
Appendix B.1. Porous Tube Samples
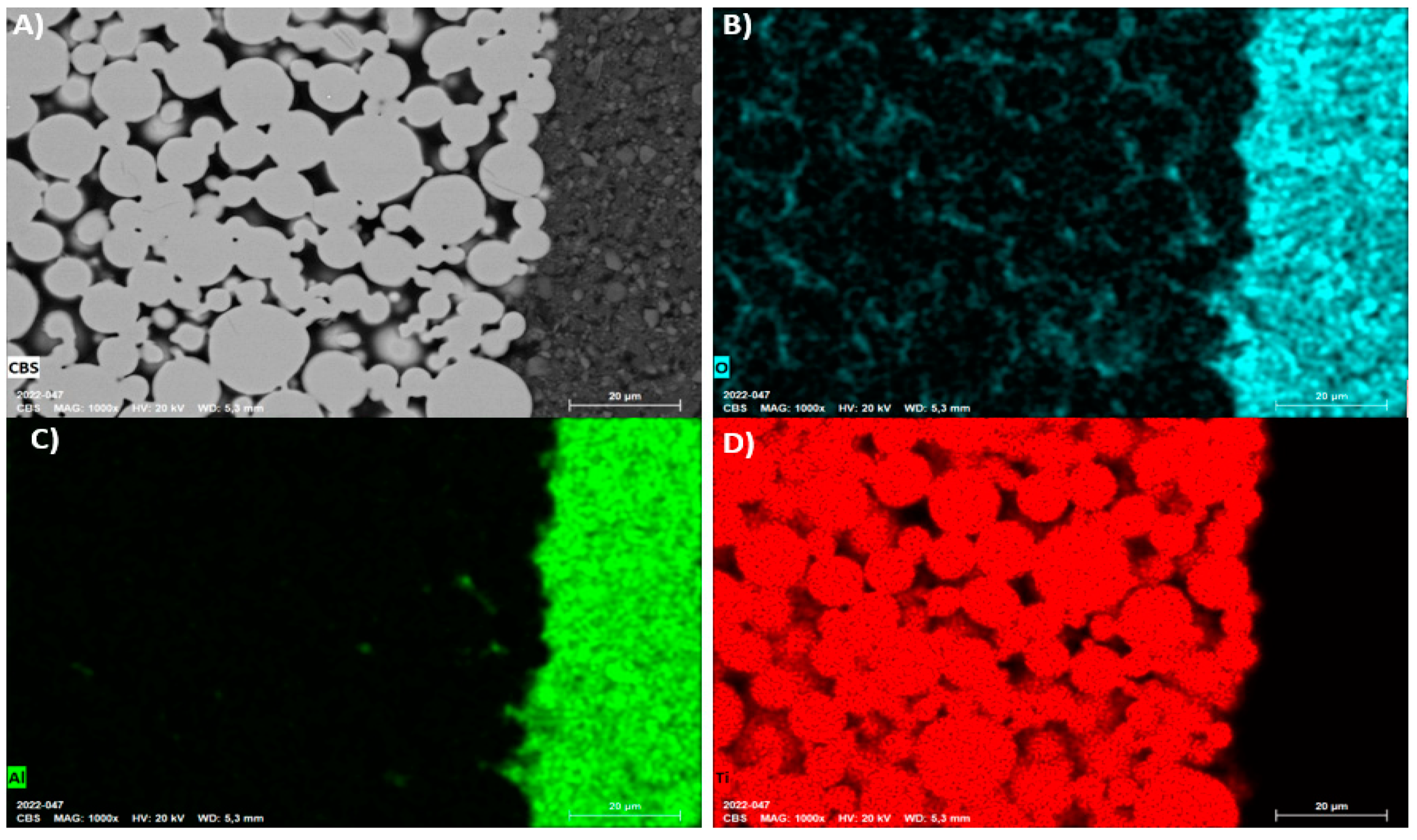
Appendix B.2. 3D-Printed Structures

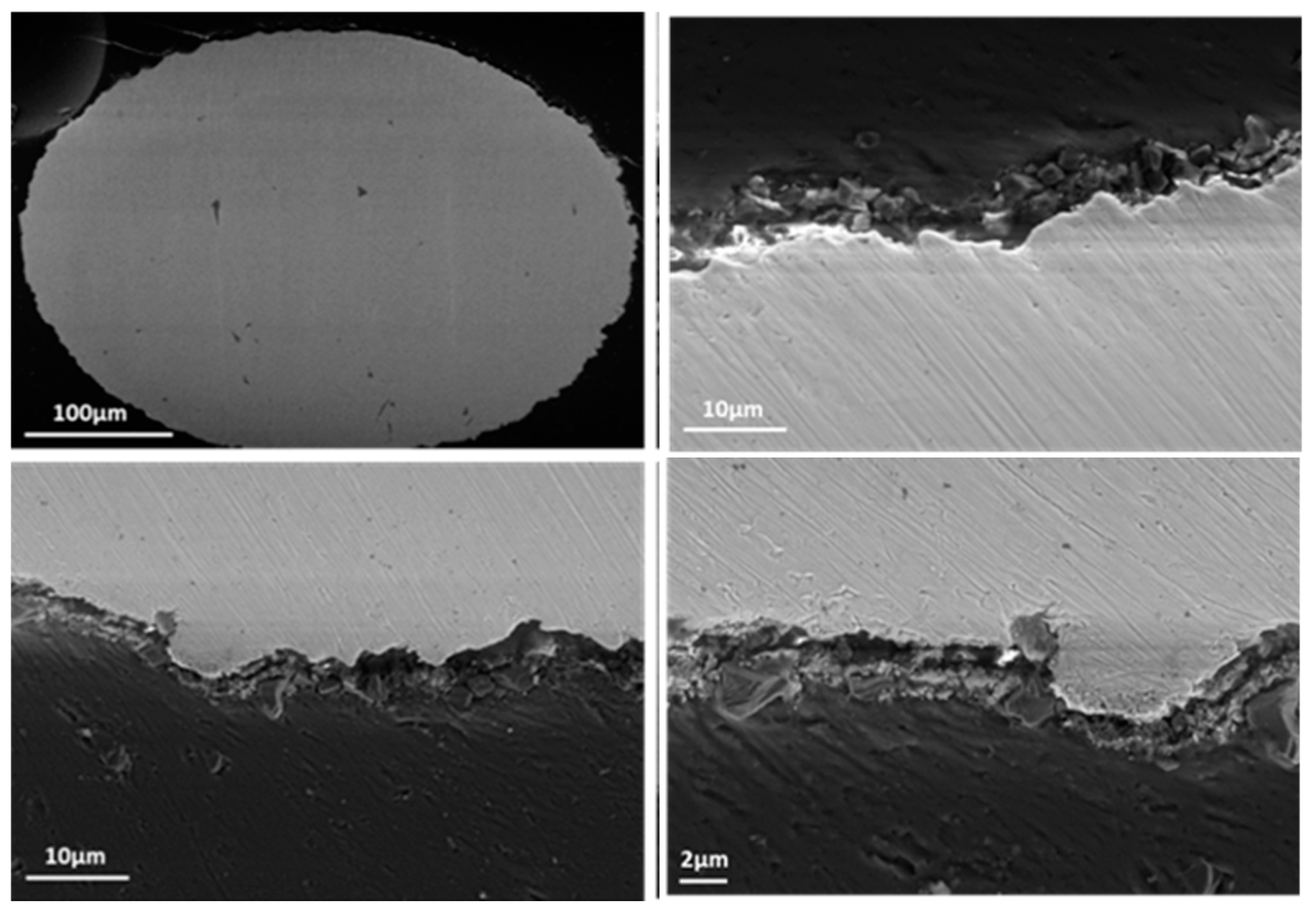
Appendix B.3. Wire Samples

Appendix C. Additional Results for Characterization of Treated/Coated Samples
References
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, mg, Ti) alloys. Trans. Inst. Met. Finish. 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 64, 127–162. [Google Scholar] [CrossRef]
- Rudnev, V.S.; Lukiyanchuk, I.V.; Kuryavyi, V.G. Electrolytic-plasma oxidation in borate electrolytes. Prot. Met. 2006, 42, 55–59. [Google Scholar] [CrossRef]
- Guan, Y.J.; Xia, Y. Correlation between discharging property and coatings microstructure during plasma electrolytic oxidation. Trans. Nonferrous Met. Soc. China 2006, 16, 1097–1102. [Google Scholar] [CrossRef]
- Khan, R.; Yerokhin, A.; Pilkington, T.; Leyland, A.; Matthews, A. Residual stresses in plasma electrolytic oxidation coatings on Al alloy produced by pulsed unipolar current. Surf. Coat. Technol. 2005, 200, 1580–1586. [Google Scholar] [CrossRef]
- Dehnavi, V.; Luan, B.L.; Shoesmith, D.W.; Liu, X.Y.; Rohani, S. Effect of duty cycle and applied current frequency on plasma electrolytic oxidation (PEO) coating growth behavior. Surf. Coat. Technol. 2013, 226, 100–107. [Google Scholar] [CrossRef]
- Sieber, M.; Simchen, F.; Morgenstern, R.; Scharf, I.; Lampke, T. Plasma Electrolytic Oxidation of High-Strength Aluminium Alloys—Substrate Effect on Wear and Corrosion Performance. Metals 2018, 8, 356. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Huang, Y.; Ovri, H.; Zheludkevich, M.L.; Kainer, K.U. Plasma electrolytic oxidation coatings on Mg alloy with addition of SiO2 particles. Electrochim. Acta 2016, 187, 20–33. [Google Scholar] [CrossRef]
- Lu, X.; Sah, S.P.; Scharnagl, N.; Störmer, M.; Starykevich, M.; Mohedano, M.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Degradation behavior of PEO coating on AM50 magnesium alloy produced from electrolytes with clay particle addition. Surf. Coat. Technol. 2015, 269, 155–169. [Google Scholar] [CrossRef]
- Hussein, R.; Northwood, D.; Nie, X. The effect of processing parameters and substrate composition on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloys. Surf. Coat. Technol. 2013, 237, 357–368. [Google Scholar] [CrossRef]
- Hussein, R.; Northwood, D.; Nie, X. The influence of pulse timing and current mode on the microstructure and corrosion behaviour of a plasma electrolytic oxidation (PEO) coated AM60B magnesium alloy. J. Alloys Compd. 2012, 541, 41–48. [Google Scholar] [CrossRef]
- Petković, M.; Stojadinović, S.; Vasilić, R.; Zeković, L. Characterization of oxide coatings formed on tantalum by plasma electrolytic oxidation in 12-tungstosilicic acid. Appl. Surf. Sci. 2011, 257, 10590–10594. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials 2018, 11, 545. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, J.; Peng, Z.; Wang, Q.; Matykina, E.; Skeldon, P.; Thompson, G. Wear-resistant coatings formed on Zircaloy-2 by plasma electrolytic oxidation in sodium aluminate electrolytes. Electrochim. Acta 2014, 116, 453–466. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.; Nie, X. Deposition and properties of zirconia coatings on a zirconium alloy produced by pulsed DC plasma electrolytic oxidation. Surf. Coat. Technol. 2013, 221, 150–157. [Google Scholar] [CrossRef]
- Sandhyarani, M.; Rao, T.P.; Rameshbabu, N. Role of electrolyte composition on structural, morphological and in-vitro biological properties of plasma electrolytic oxidation films formed on zirconium. Appl. Surf. Sci. 2014, 317, 198–209. [Google Scholar]
- Xue, W.; Zhu, Q.; Jin, Q.; Hua, M. Characterization of ceramic coatings fabricated on zirconium alloy by plasma electrolytic oxidation in silicate electrolyte. Mater. Chem. Phys. 2010, 120, 656–660. [Google Scholar] [CrossRef]
- Aktug, S.L.; Durdu, S.; Aktas, S.; Yalcin, E.; Usta, M. Characterization and investigation of in vitro properties of antibacterial copper deposited on bioactive ZrO2 coatings on zirconium. Thin Solid Films 2019, 681, 69–77. [Google Scholar] [CrossRef]
- Aliasghari, S.; Skeldon, P.; Thompson, G. Plasma electrolytic oxidation of titanium in a phosphate/silicate electrolyte and tribological performance of the coatings. Appl. Surf. Sci. 2014, 316, 463–476. [Google Scholar] [CrossRef]
- Habazaki, H.; Tsunekawa, S.; Tsuji, E.; Nakayama, T. Formation and characterization of wear-resistant PEO coatings formed on β-titanium alloy at different electrolyte temperatures. Appl. Surf. Sci. 2012, 259, 711–718. [Google Scholar] [CrossRef]
- Zehra, T.; Kaseem, M.; Hossain, S.; Ko, Y.-G. Fabrication of a Protective Hybrid Coating Composed of TiO2, MoO2, and SiO2 by Plasma Electrolytic Oxidation of Titanium. Metals 2021, 11, 1182. [Google Scholar] [CrossRef]
- Hao, J.; Jie, W.; Chen, H.; Ye, Y. The effect of SiO32− on micro-arc oxidization ceramic layer of TiAl alloy. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2005, 34, 1455–1459. [Google Scholar]
- Zhou, H.; Liu, Z.; Li, Z.; Du, J. Microarc oxidation coating and high-temperature oxidation resistant property on Ti alloy. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2005, 34, 1835–1838. [Google Scholar]
- Yao, Z.; Jiang, Z.; Wang, F.; Hao, G. Oxidation behavior of ceramic coatings on Ti-6Al-4V by micro-plasma oxidation. J. Mater. Process. Technol. 2007, 190, 117–122. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.; Jiang, Z.; Zhang, Y.; Liu, X. Investigation of the plasma electrolytic oxidation of Ti6Al4V under single-pulse power supply. Corros. Sci. 2011, 53, 2253–2262. [Google Scholar] [CrossRef]
- Quintero, D.; Galvis, O.; Calderón, J.; Castaño, J.; Echeverría, F. Effect of electrochemical parameters on the formation of anodic films on commercially pure titanium by plasma electrolytic oxidation. Surf. Coat. Technol. 2014, 258, 1223–1231. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.R.; Shamanian, M.; Apachitei, I.; Zadpoor, A.A. Influence of hydrothermal treatment on the surface characteristics and electrochemical behavior of Ti6Al4V bio-functionalized through plasma electrolytic oxidation. Surf. Coat. Technol. 2019, 374, 222–231. [Google Scholar] [CrossRef]
- Friedemann, A.E.R.; Gesing, T.M.; Plagemann, P. Electrochemical rutile and anatase formation on PEO surfaces. Surf. Coat. Technol. 2017, 315, 139–149. [Google Scholar] [CrossRef]
- Karbowniczek, J.; Muhaffel, F.; Cempura, G.; Cimenoglu, H.; Czyrska-Filemonowicz, A. Influence of electrolyte composition on microstructure, adhesion and bioactivity of micro-arc oxidation coatings produced on biomedical Ti6Al7Nb alloy. Surf. Coat. Technol. 2017, 321, 97–107. [Google Scholar] [CrossRef]
- Han, Y.; Hong, S.-H.; Xu, K. Porous nanocrystalline titania films by plasma electrolytic oxidation. Surf. Coat. Technol. 2002, 154, 314–318. [Google Scholar] [CrossRef]
- Stojadinović, S.; Vasilić, R.; Petković, M.; Kasalica, B.; Belča, I.; Žekić, A.; Zeković, L. Characterization of the plasma electrolytic oxidation of titanium in sodium metasilicate. Appl. Surf. Sci. 2013, 265, 226–233. [Google Scholar] [CrossRef]
- Tong, H.; Jin, F.; Shen, L. Fabrication of porous ceramic coatings embedded nanograins on titanium alloys. J. Korean Phys. Soc. 2005, 46, 134. [Google Scholar]
- Bakhtiari-Zamani, H.; Saebnoori, E.; Hassannejad, H.; Hassanzadeh-Tabrizi, A. Comparing Morphology and Corrosion Behavior of Nanostructured Coatings Obtained via Plasma Electrolytic Oxidation with Direct and Pulse Currents on Commercial Titanium Substrate. Surf. Eng. Appl. Electrochem. 2019, 55, 667–678. [Google Scholar] [CrossRef]
- Shin, K.R.; Ko, Y.G.; Shin, D.H. Effect of electrolyte on surface properties of pure titanium coated by plasma electrolytic oxidation. J. Alloys Compd. 2011, 509, S478–S481. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Dehghanian, C.; Montazeri, M.; Baradaran, A. Preparation of ceramic coating on Ti substrate by plasma electrolytic oxidation in different electrolytes and evaluation of its corrosion resistance: Part II. Appl. Surf. Sci. 2012, 258, 2416–2423. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Li, W. Antibacterial TiO2 coating incorporating silver nanoparticles by microarc oxidation and ion implantation. J. Nanomater. 2013, 2013, 2. [Google Scholar]
- Blackwood, D.J.; Chooi, S. Stability of protective oxide films formed on a porous titanium. Corros. Sci. 2002, 44, 395–405. [Google Scholar] [CrossRef]
- Wu, H.; Lu, X.; Long, B.; Wang, X.; Wang, J.; Jin, Z. The effects of cathodic and anodic voltages on the characteristics of porous nanocrystalline titania coatings fabricated by microarc oxidation. Mater. Lett. 2005, 59, 370–375. [Google Scholar] [CrossRef]
- Wang, L.; Shi, L.; Chen, J.; Shi, Z.; Ren, L.; Wang, Y. Biocompatibility of Si-incorporated TiO2 film prepared by micro-arc oxidation. Mater. Lett. 2014, 116, 35–38. [Google Scholar] [CrossRef]
- Di, S.; Guo, Y.; Lv, H.; Yu, J.; Li, Z. Microstructure and properties of rare earth CeO2-doped TiO2 nanostructured composite coatings through micro-arc oxidation. Ceram. Int. 2015, 41, 6178–6186. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J. Preparation of a Modified Micro-arc Oxidation Coating Using Al2O3 Particles on Ti6Al4V. J. Mater. Sci. Eng. 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Lukiyanchuk, I.V.; Rudnev, V.S.; Tyrina, L.M. Plasma electrolytic oxide layers as promising systems for catalysis. Surf. Coat. Technol. 2016, 307, 1183–1193. [Google Scholar] [CrossRef]
- Patcas, F.; Krysmann, W. Efficient catalysts with controlled porous structure obtained by anodic oxidation under spark-discharge. Appl. Catal. A Gen. 2007, 316, 240–249. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Qin, H.; Wu, M.; Yan, Z. Formation process of in situ oxide coatings with high porosity using one-step plasma electrolytic oxidation. Appl. Surf. Sci. 2016, 366, 432–438. [Google Scholar] [CrossRef]
- Mirelman, L.K.; Curran, J.A.; Clyne, T.W. The production of anatase-rich photoactive coatings by plasma electrolytic oxidation. Surf. Coat. Technol. 2012, 207, 66–71. [Google Scholar] [CrossRef]
- Rahimalimamaghani, A.; Godini, H.R.; Mboussi, M.; Tanaka, A.P.; Tenco, M.L.; Gallucci, F. Tailored Carbon Molecular Sieve Membranes for Selective CO2–Separation at Elevated Temperatures and Pressures. J. CO2 Util. 2023, 68, 102378. [Google Scholar] [CrossRef]
- Godini, H.R.; Gili, A.; Görke, O.; Simon, U.; Hou, K.; Wozny, G. Performance Analysis of a Porous Packed Bed Membrane Reactor for Oxidative Coupling of Methane: Structural and Operational Characteristics. Energy Fuels 2014, 28, 877–890. [Google Scholar] [CrossRef]
- Sadjadi, S.; Simon, U.; Godini, H.; Görke, O.; Schomäcker, R.; Wozny, G. Reactor material and gas dilution effects on the performance of miniplant-scale fluidized-bed reactors for oxidative coupling of methane. Chem. Eng. J. 2015, 281, 678–687. [Google Scholar] [CrossRef]
- Gallucci, F.; Medrano, J.A.; Roses, L.; Brunetti, A.; Barbieri, G.; Viviente, J.L. Process Intensification via Membrane Reactors, the DEMCAMER Project. Processes 2016, 4, 16. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Jacques, S.D.M.; Middelkoop, V.; Di Michiel, M.; Egan, C.K.; Ismagilov, I.Z.; Vaughan, G.B.M.; Gallucci, F.; van Sint Annaland, M.; Shearing, P.R.; et al. Real time chemical imaging of a working catalytic membrane reactor during oxidative coupling of methane. Chem. Commun. 2015, 51, 12752–12755. [Google Scholar] [CrossRef]
- Czuprat, O.; Schiestel, T.; Voss, H.; Caro, J. Oxidative Coupling of Methane in a BCFZ Perovskite Hollow Fiber Membrane Reactor. Ind. Eng. Chem. Res. 2010, 49, 10230–10236. [Google Scholar] [CrossRef]
- Song, J.; Hei, Y.; Li, C.; Yang, N.; Meng, B.; Tan, X.; Sunarso, J.; Liu, S. Dehydrogenation Coupling of Methane Using Catalyst-Loaded Proton-Conducting Perovskite Hollow Fiber Membranes. Membranes 2022, 12, 191. [Google Scholar] [CrossRef]
- Godini, H.R.; Kim, M.; Görke, O.; Khadivi, M.; Schomäcker, R.; Repke, J.-U. Membrane Engineering for the Treatment of Gases Volume 2: Gas-separation Issues Combined with Membrane Reactors. In Oxidative Coupling of Methane in Membrane Reactors; RSC (The Royal Society of Chemistry) Press: London, UK, 2018; ISBN 978-1-78801-219-5. [Google Scholar]
- Julbe, A.; Farrusseng, D.; Guizard, C. Limitations and potentials of oxygen transport dense and porous ceramic membranes for oxidation reactions. Catal. Today 2005, 104, 102–113. [Google Scholar] [CrossRef]
- Aseem, A.; Harold, M.P. C2 yield enhancement during oxidative coupling of methane in a nonpermselective porous membrane reactor. Chem. Eng. Sci. 2018, 175, 199–207. [Google Scholar] [CrossRef]
- Ramachandra, A.M.; Lu, Y.; Ma, Y.H.; Moser, W.R.; Dixon, A.G. Oxidative coupling of methane in porous Vycor membrane reactors. J. Memb. Sci. 1996, 116, 253–264. [Google Scholar] [CrossRef]
- Nozaki, T.; Hashimoto, S.; Omata, K.; Fujimoto, K. Oxidative coupling of methane with membrane reactors containing lead oxide. Ind. Eng. Chem. Res. 1993, 32, 1174–1179. [Google Scholar] [CrossRef]
- Salehi, M.-S.; Askarishahi, M.; Gallucci, F.; Godini, H.R. Selective CO2-Hydrogenation using a membrane reactor. Chem. Eng. Process. Process. Intensif. 2020, 160, 108264. [Google Scholar] [CrossRef]
- Julbe, A.; Farrusseng, D.; Guizard, C. Porous ceramic membranes for catalytic reactors—Overview and new ideas. J. Membr. Sci. 2001, 181, 3–20. [Google Scholar] [CrossRef]
- Hsieh, H.P. Inorganic membrane reactors—A review. AIChE Symp. Ser. 1989, 85, 53–67. [Google Scholar]
- Curran, J.; Clyne, T. Porosity in plasma electrolytic oxide coatings. Acta Mater. 2006, 54, 1985–1993. [Google Scholar] [CrossRef]
- Jayaraj, R.K.; Malarvizhi, S.; Balasubramanian, V. Optimizing the micro-arc oxidation (MAO) parameters to attain coatings with minimum porosity and maximum hardness on the friction stir welded AA6061 aluminium alloy welds. Def. Technol. 2017, 13, 111–117. [Google Scholar] [CrossRef]
- Karaji, Z.G.; Hedayati, R.; Pouran, B.; Apachitei, I.; Zadpoor, A.A. Effects of plasma electrolytic oxidation process on the mechanical properties of additively manufactured porous biomaterials. Mater. Sci. Eng. C 2017, 76, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wei, D.; Cheng, S.; Zhou, Y.; Jia, D.; Wang, Y.; Li, B. The effect of titanium bead diameter of porous titanium on the formation of micro-arc oxidized TiO2-based coatings containing Si and Ca. Ceram. Int. 2013, 39, 5725–5732. [Google Scholar] [CrossRef]
- Apachitei, I.; Leoni, A.; Riemslag, A.; Fratila-Apachitei, L.; Duszczyk, J. Enhanced fatigue performance of porous coated Ti6Al4V biomedical alloy. Appl. Surf. Sci. 2011, 257, 6941–6944. [Google Scholar] [CrossRef]
- Thukkaram, M.; Cools, P.; Nikiforov, A.; Rigole, P.; Coenye, T.; Van Der Voort, P.; Du Laing, G.; Vercruysse, C.; Declercq, H.; Morent, R.; et al. Antibacterial activity of a porous silver doped TiO2 coating on titanium substrates synthesized by plasma electrolytic oxidation. Appl. Surf. Sci. 2019, 500, 144235. [Google Scholar] [CrossRef]
- Gu, W.-C.; Lv, G.-H.; Chen, H.; Chen, G.-L.; Feng, W.-R.; Yang, S.-Z. PEO protective coatings on inner surface of tubes. Surf. Coat. Technol. 2007, 201, 6619–6622. [Google Scholar] [CrossRef]
- Gu, W.-C.; Lv, G.-H.; Chen, H.; Chen, G.-L.; Feng, W.; Zhang, G.-L.; Yang, S.-Z. Preparation of ceramic coatings on inner surface of steel tubes using a combined technique of hot-dipping and plasma electrolytic oxidation. J. Alloys Compd. 2007, 430, 308–312. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Q.; Yi, C.G.; Jiang, Z.H.; Wang, F.P. High emissivity coatings on titanium alloy prepared by micro-arc oxidation for high temperature application. J. Mater. Sci. 2012, 47, 2162–2168. [Google Scholar] [CrossRef]
- Sun, J.; Wu, J.S.; Zhao, B.; Wang, F. Microstructure, wear and high temperature oxidation resistance of nitrided TiA1 based alloys. Mater. Sci. Eng. A 2002, 329, 713–717. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, Z.; Jia, F.; Wang, Y.; Jiang, Z.; Bu, H. Preparation of PEO ceramic coating on Ti alloy and its high temperature oxidation resistance. Curr. Appl. Phys. 2010, 10, 698–702. [Google Scholar] [CrossRef]
- Rombouts, M.; Mullens, S.; Luyten, J.; Nuyts, P. The Production of Ti-6Al-4V Parts with Controlled Porous Architecture by Three-Dimensional Fiber Deposition in Innovative Developments in Design and Manufacturing (Book Chapter); Taylor Francis: Abingdon, UK, 2009. [Google Scholar]
- Jiang, B.L.; Wang, Y.M. Plasma electrolytic oxidation treatment of aluminium and titanium alloys. In Surface Engineering of Light Alloys; Elsevier: Amsterdam, The Netherlands, 2010; pp. 110–154. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Jin, F.; Chu, P.K.; Wang, K.; Zhao, J.; Huang, A.; Tong, H. Thermal stability of titania films prepared on titanium by micro-arc oxidation. Mater. Sci. Eng. A 2008, 476, 78–82. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Wang, L.; Fu, W.; Yi, G.; Chen, Z.; Gao, Z.; Pan, Q. Discharge Characteristics, Plasma Electrolytic Oxidation Mechanism and Properties of ZrO2 Membranes in K2ZrF6 Electrolyte. Membranes 2022, 12, 516. [Google Scholar] [CrossRef]
- Available online: https://www.aspire2050.eu/memere (accessed on 15 April 2023).
- Samadi, P.; Binczarski, M.J.; Pawlaczyk, A.; Rogowski, J.; Szynkowska-Jozwik, M.I.; Witonska, I.A. CO Oxidation over Pd Catalyst Supported on Porous TiO2 Prepared by Plasma Electrolytic Oxidation (PEO) of a Ti Metallic Carrier. Materials 2022, 15, 4301. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Cristiani, C.; Ferreira, C.; Kohler, S. Methods for the catalytic activation of metallic structured substrates. Catal. Sci. Technol. 2014, 4, 2846–2870. [Google Scholar] [CrossRef]
- Montebelli, A.; Visconti, C.G.; Groppi, G.; Tronconi, E.; Kohler, S. Optimization of compact multitubular fixed-bed reactors for the methanol synthesis loaded with highly conductive structured catalysts. Chem. Eng. J. 2014, 255, 257–265. [Google Scholar] [CrossRef]
- Visconti, C.G.; Montebelli, A.; Groppi, G.; Tronconi, E.; Kohler, S. Highly Conductive Structured Catalysts for the Intensification of Methanol Synthesis in Multitubular Reactors. In Methanol: Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 519–538. [Google Scholar] [CrossRef]
- Specchia, S.; Ercolino, G.; Karimi, S.; Italiano, C.; Vita, A.S. Solution combustion synthesis for preparation of structured catalysts: A mini-review on process intensification for energy applications and pollution control. Int. J. Self-Propagating High-Temp. Synth. 2017, 26, 166–186. [Google Scholar] [CrossRef]
- Pauletto, G.; Vaccari, A.; Groppi, G.; Bricaud, L.; Benito, P.; Boffito, D.C.; Lercher, J.A.; Patience, G.S. FeCrAl as a Catalyst Support. Chem. Rev. 2020, 120, 7516–7550. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Wybornov, S.; Staedler, T.; Hein, D.; Wiedenmann, F.; Krumm, W.; Rudnev, V.; Lukiyanchuk, I. Highly Efficient Nanoarchitectured Ni5TiO7 Catalyst for Biomass Gasification. ACS Appl. Mater. Interfaces 2012, 4, 4062–4066. [Google Scholar] [CrossRef] [PubMed]
- Kempler, P.A.; Nielander, A.C. Reliable reporting of Faradaic efficiencies for electrocatalysis research. Nat. Commun. 2023, 14, 1158. [Google Scholar] [CrossRef] [PubMed]



| Material | Length (cm) | Outer Diameter (mm) | Wall Thickness (mm) | Purity (%) | Filter Rating (µm) |
|---|---|---|---|---|---|
| Commercial Titanium | 10 | 13 | 2 | Min. 99.5% titanium | 0.45 |
| Sodium Metasilicate (Na2SiO3) | Sodium Aluminate (NaAlO2) | ||||||
|---|---|---|---|---|---|---|---|
| Classification | Composition | Classification | Composition | ||||
| Na2SiO3 (g/L) | Na2SiO3 (M) | KOH (g/L) | NaAlO2 (g/L) | NaAlO2 (M) | KOH (g/L) | ||
| Electrolyte1 (E1S) | 6.1 | 0.05 | 2 | Electrolyte1 (E1A) | 8.2 | 0.1 | 2 |
| Electrolyte2 (E2S) | 10 | 0.08 | 1 | Electrolyte2 (E2A) | 10 | 0.12 | 2 |
| Electrolyte3 (E3S) | 12.2 | 0.1 | 0.5 | Electrolyte3 (E3A) | 12.3 | 0.15 | 2 |
| Sample Type | Applied Potential Range (V) | Current (A) | Power Frequency (Hz) |
|---|---|---|---|
| Wires and wire bundles | 220–260 | 6.5 | 50 and 200 |
| Porous titanium tubes | 200 and 220 | 6.5 | 50 and 200 |
| 3D-printed structures | 200–240 | 6.5 | 50 and 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godini, H.R.; Prahlad, A.V.; Middelkoop, V.; Görke, O.; Li, S.; Gallucci, F. Electrochemical Surface Treatment for Tailored Porous Structures. Processes 2023, 11, 1260. https://doi.org/10.3390/pr11041260
Godini HR, Prahlad AV, Middelkoop V, Görke O, Li S, Gallucci F. Electrochemical Surface Treatment for Tailored Porous Structures. Processes. 2023; 11(4):1260. https://doi.org/10.3390/pr11041260
Chicago/Turabian StyleGodini, Hamid Reza, Anirudh Venkat Prahlad, Vesna Middelkoop, Oliver Görke, Sirui Li, and Fausto Gallucci. 2023. "Electrochemical Surface Treatment for Tailored Porous Structures" Processes 11, no. 4: 1260. https://doi.org/10.3390/pr11041260






