Dissolving Activated Carbon Pellets for Ibuprofen Removal at Point-of-Entry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbates
2.2. Activated Carbon Pelletization
2.3. Sample Characterization
2.3.1. BET Surface Area
2.3.2. Ash Content
2.3.3. Pellet Dissolvability
2.3.4. Sample Density
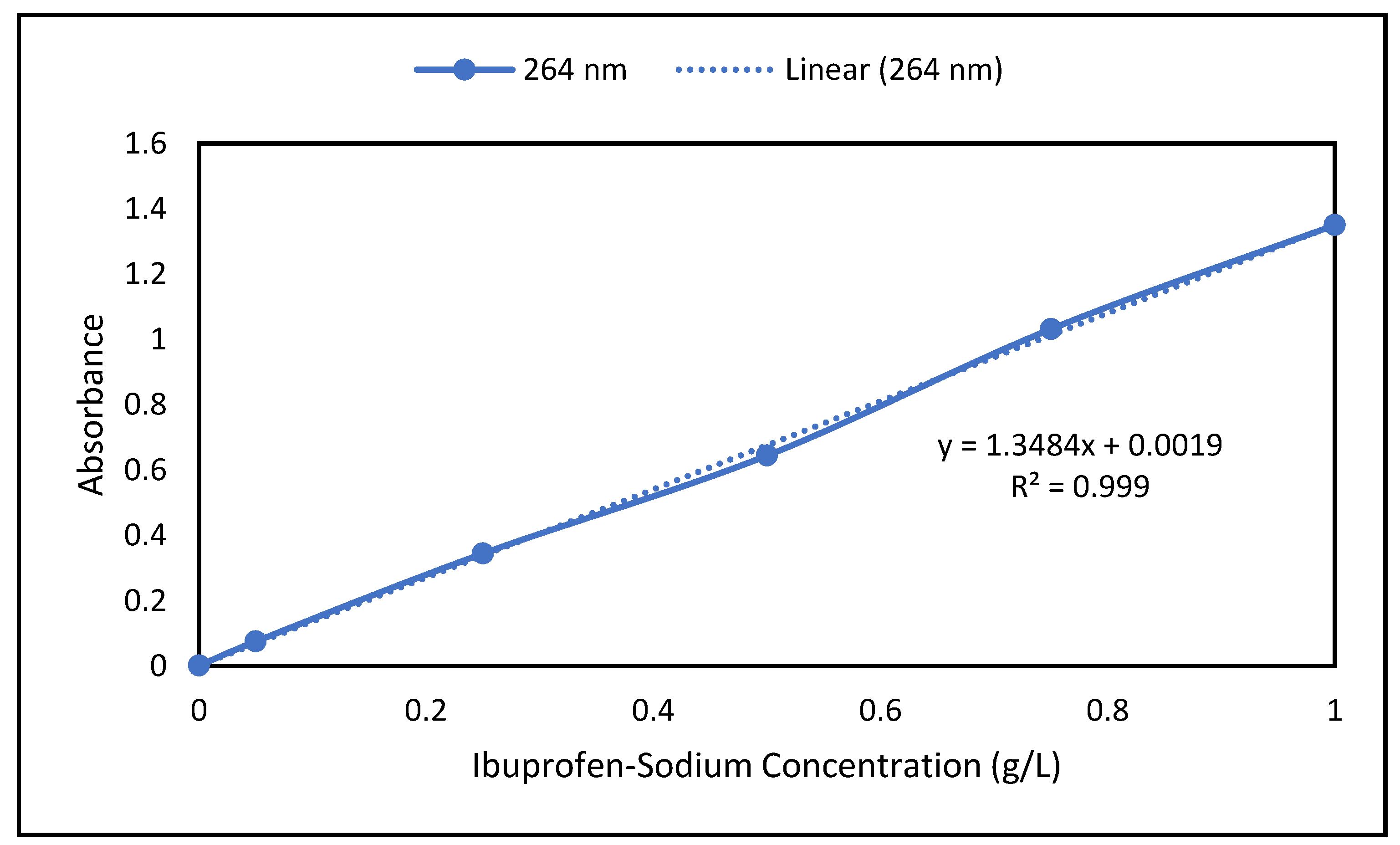
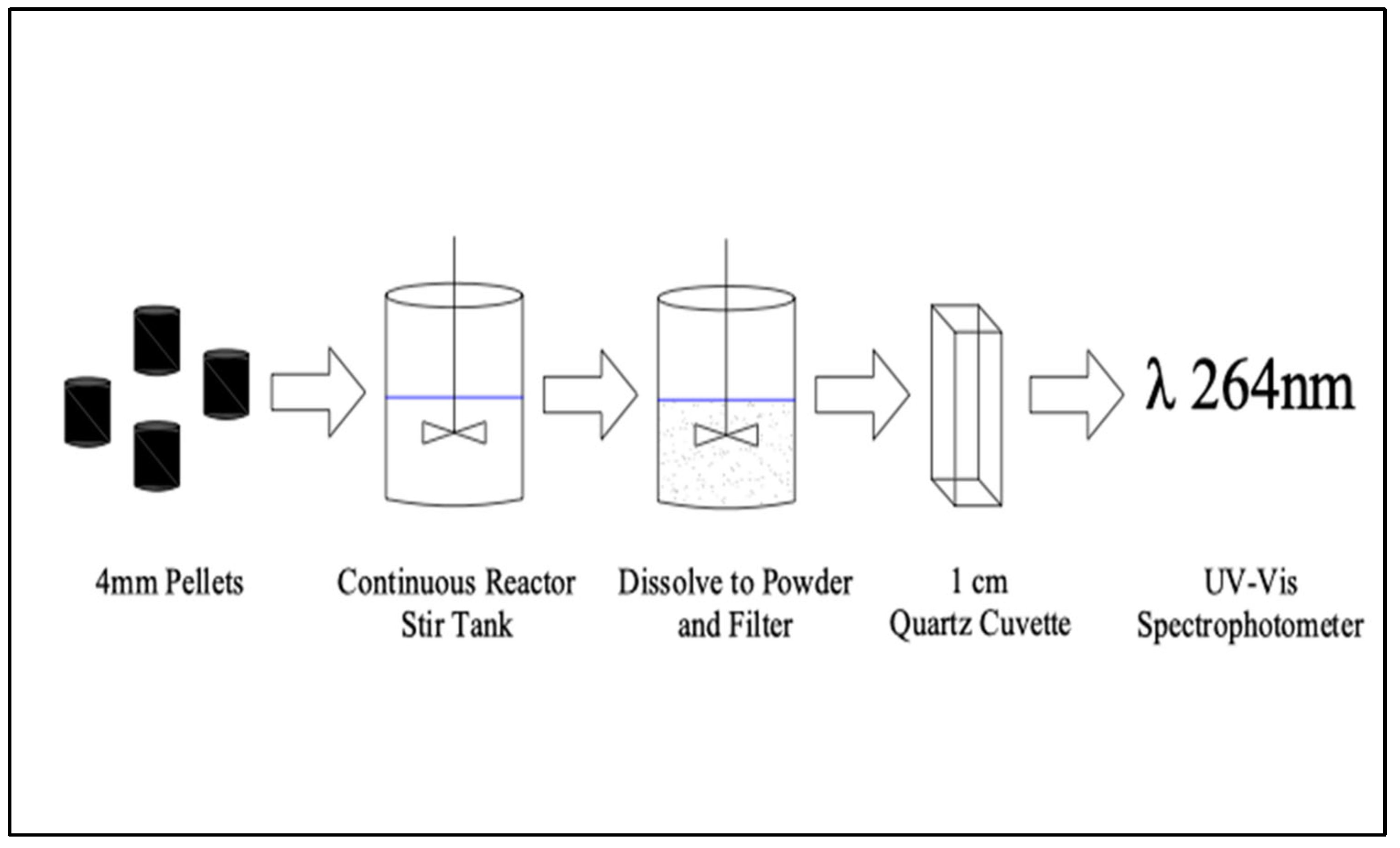
2.4. Analytical Method and Calibration
2.5. Batch Adsorption
3. Results and Discussion
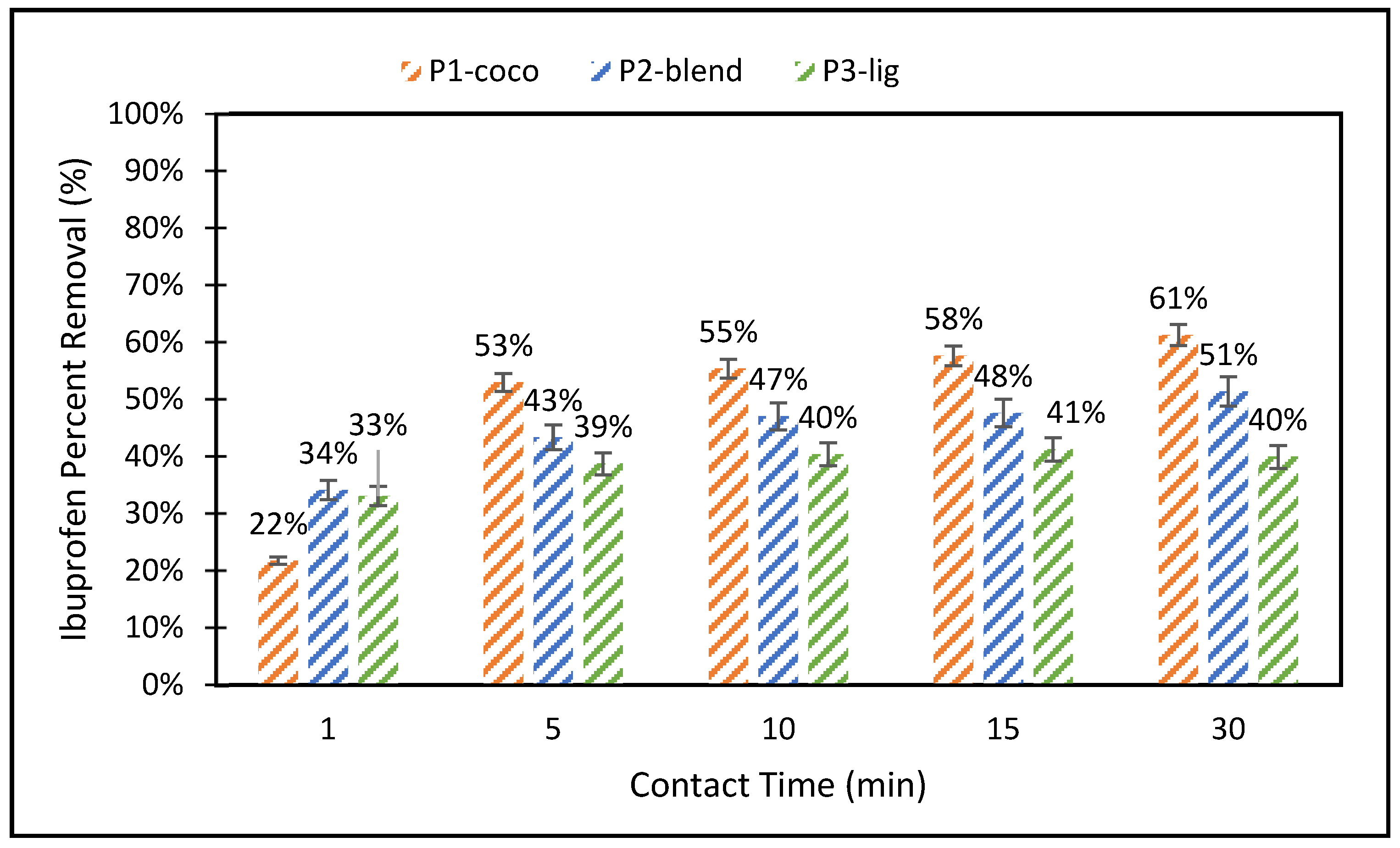
3.1. Pellet Dissolvability-Basket Test
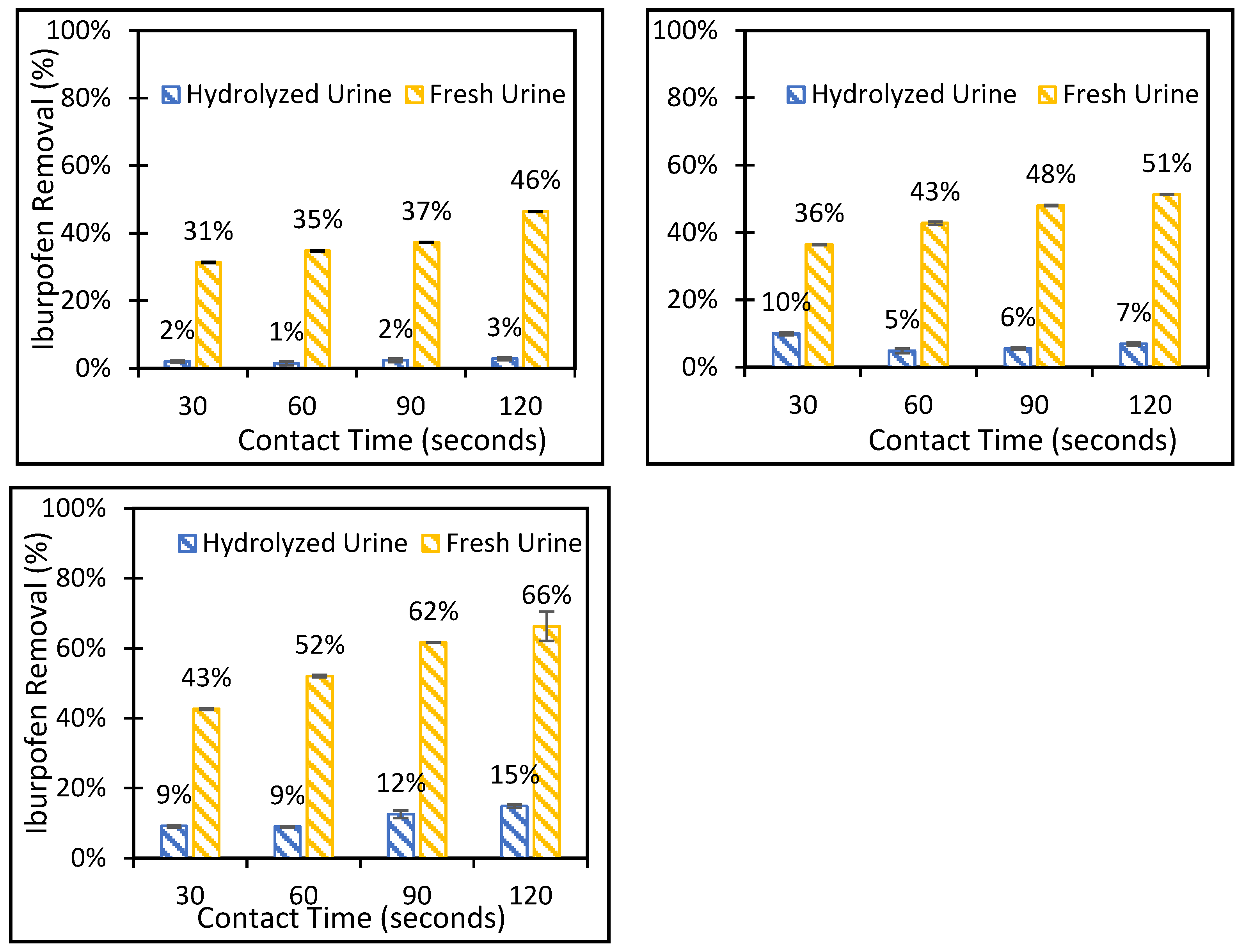
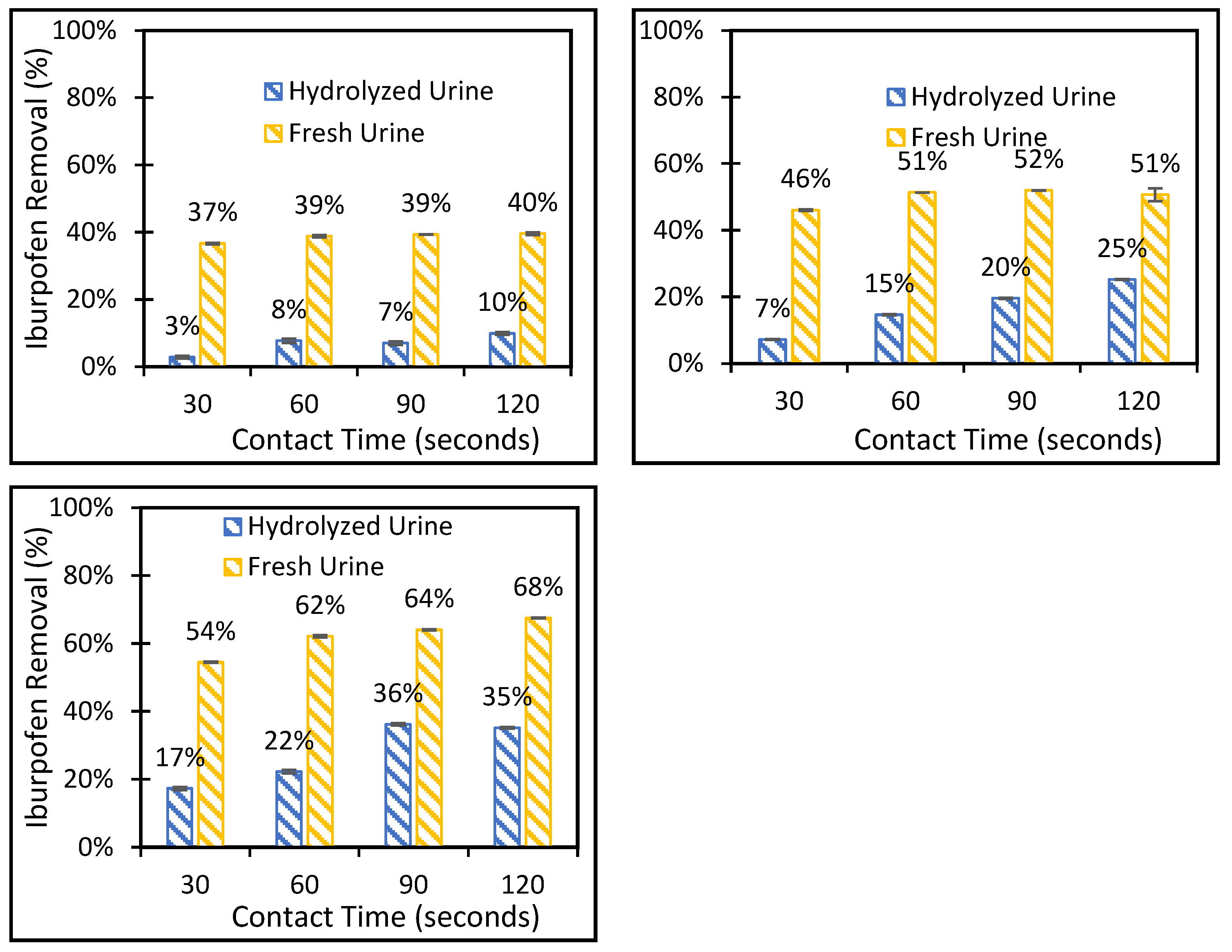
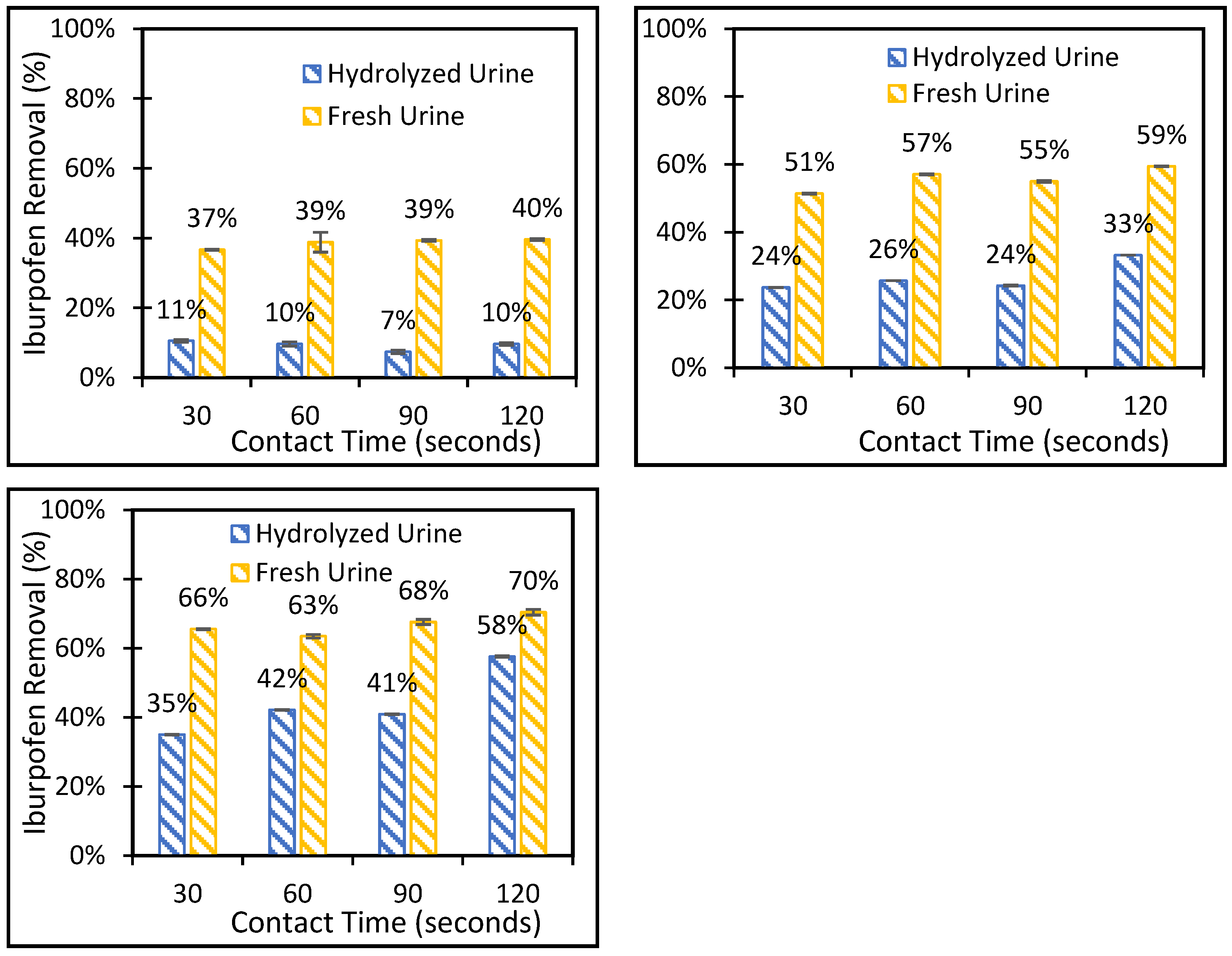
3.2. Batch Adsorption-Deionized Water and Synthetic Urine Solutions
3.3. Cost-Benefit Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Lienert, J.; Bürki, T.; Escher, B. Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine. Water Sci. Technol. 2007, 56, 87–96. [Google Scholar] [CrossRef]
- Hernando, M.; Mezcua, M.; Fernández-Alba, A.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Dos Santos, C.; Nardocci, A. Prioritization of pharmaceuticals in drinking water exposure based on toxicity and environmental fate assessment by in silico tools: An integrated and transparent ranking. Comput. Toxicol. 2019, 9, 12–21. [Google Scholar] [CrossRef]
- Webb, S.; Ternes, T.; Gibert, M.; Olejniczak, K. Indirect human exposure to pharmaceuticals via drinking water. Toxicol. Lett. 2003, 142, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Möller, T.; Sylvester, P.; Shepard, D.; Morassi, E. Arsenic in groundwater in New England—Point-of-entry and point-of-use treatment of private wells. Desalination 2009, 243, 293–304. [Google Scholar] [CrossRef]
- Ayotte, J.D.; Montgomery, D.L.; Flanagan, S.M.; Robinson, K.W. Arsenic in Groundwater in Eastern New England: Occurrence, Controls, and Human Health Implications. Environ. Sci. Technol. 2003, 37, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Mukoko, T.; Mupa, M.; Guyo, U.; Dziike, F. Preparation of rice hull activated carbon for the removal of selected pharmaceutical waste compounds in hospital effluent. J. Environ. Anal. Toxicol. 2015, S7, 2161-0525. [Google Scholar] [CrossRef]
- Inomata, K.; Kanazawa, K.; Urabe, Y.; Hosono, H.; Araki, T. Natural gas storage in activated carbon pellets without a binder. Carbon 2002, 40, 87–93. [Google Scholar] [CrossRef]
- Finn, M.; Giampietro, G.; Mazyck, D.; Rodriguez, R. Activated Carbon for Pharmaceutical Removal at Point-of-Entry. Processes 2021, 9, 1091. [Google Scholar] [CrossRef]
- Matsui, Y.; Nakao, S.; Sakamoto, A.; Taniguchi, T.; Pan, L.; Matsushita, T.; Shirasaki, N. Adsorption capacities of activated carbons for geosmin and 2-methylisoborneol vary with activated carbon particle size: Effects of adsorbent and adsorbate characteristics. Water Res. 2015, 85, 95–102. [Google Scholar] [CrossRef]
- Finn, M.; Rodriguez, R.; Contrino, D.; Swenson, J.; Mazyck, D.W.; Suau, S. Impact of Inherent Magnesium in Biochar for Phosphate Removal from Reclaimed Water Streams. J. Environ. Eng. 2022, 148, 04021085. [Google Scholar] [CrossRef]
- Valcarce, C.O.; Gonzaga, E.W.; Mazyck, D.W. Evaluating the Efficacy of PAC and Water Parameters to Remove Trace Organics. J. Am. Water Work. Assoc. 2017, 109, E50–E60. [Google Scholar] [CrossRef]
- Skoczko, I.; Guminski, R. Research on the Development of Technologies for the Production of Granulated Activated Carbons Using Various Binders. Materials 2020, 13, 5180. [Google Scholar] [CrossRef]
- Briens, L.; Bowden-Green, B. A comparison of liquid binders for drum granulation of biochar powder. Powder Technol. 2020, 367, 487–496. [Google Scholar] [CrossRef]
- Amaya, A.; Medero, N.; Tancredi, N.; Silva, H.; Deiana, C. Activated carbon briquettes from biomass materials. Bioresour. Technol. 2007, 98, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.; Parra, J.B.; Carvalho, A.P.; Ania, C.O. Waste-derived activated carbons for removal of ibuprofen from solution: Role of surface chemistry and pore structure. Bioresour. Technol. 2009, 100, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Guedidi, H.; Reinert, L.; Lévêque, J.-M.; Soneda, Y.; Bellakhal, N.; Duclaux, L. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon N. Y. 2013, 54, 432–443. [Google Scholar] [CrossRef]
- Johns, M.M.; Marshall, W.E.; Toles, C.A. Agricultural by-products as granular activated carbons for adsorbing dissolved metals and organics. J. Chem. Technol. Biotechnol. 1998, 71, 131–140. [Google Scholar] [CrossRef]
- Ding, L.; Snoeyink, V.L.; Mariñas, B.J.; Yue, Z.; Economy, J. Effects of Powdered Activated Carbon Pore Size Distribution on the Competitive Adsorption of Aqueous Atrazine and Natural Organic Matter. Environ. Sci. Technol. 2008, 42, 1227–1231. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Q.; Wang, Z.; Liu, S.; Zou, C.; Guo, J.; Guo, X. Competitive adsorption of benzene and water vapor on lignite-based activated carbon: Experiment and molecular simulation study. Chem. Eng. J. 2020, 398, 125557. [Google Scholar] [CrossRef]
- Yi, P.; Zuo, X.; Lang, D.; Wu, M.; Dong, W.; Chen, Q.; Zhang, L. Competitive adsorption of methanol co-solvent and dioctyl phthalate on functionalized graphene sheet: Integrated investigation by molecular dynamics simulations and quantum chemical calculations. J. Colloid Interface Sci. 2022, 605, 354–363. [Google Scholar] [CrossRef]
- Solanki, A.; Boyer, T.H. Physical-chemical interactions between pharmaceuticals and biochar in synthetic and real urine. Chemosphere 2019, 218, 818–826. [Google Scholar] [CrossRef]
- Solanki, A.; Boyer, T.H. Pharmaceutical removal in synthetic human urine using biochar. Environ. Sci. Water Res. Technol. 2017, 3, 553–565. [Google Scholar] [CrossRef]
- Landry, K.A.; Sun, P.; Huang, C.-H.; Boyer, T.H. Ion-exchange selectivity of diclofenac, ibuprofen, ketoprofen, and naproxen in ureolyzed human urine. Water Res. 2015, 68, 510–521. [Google Scholar] [CrossRef]
- ASTM D2866-11. Standard Test Method for Total Ash Content of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2019; Volume 15.01.
- ASTM D2854-09; Standard Test Method for Apparent Density of Activated Carbon. ASTM International: West Conshohocken, PA, USA, 2019; Volume 15.01.
- ASTM D8176-18; Standard Test Method for Mechanically Tapped Density of Activated Carbon (Powdered and Fine Mesh). ASTM International: West Conshohocken, PA, USA, 2019; Volume 15.01.
- Vassilev, S.V.; Kitano, K.; Vassileva, C.G. Some relationships between coal rank and chemical and mineral composition. Fuel 1996, 75, 1537–1542. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Xing, L.; Han, G.; Liu, J.; Fan, G. Exploring on aqueous chemistry of micron-sized lignite particles in lignite–water slurry: Effects of pH on humics dissolution. Fuel 2016, 181, 94–101. [Google Scholar] [CrossRef]
- Daifullah, A.; Girgis, B. Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 181–193. [Google Scholar] [CrossRef]
- Moghadamnia, Y.; Kazemi, S.; Rezaee, B.; Rafati-Rahimzadeh, M.; Ebrahimpour, S.; Aghapour, F. New formulation of ibuprofen on absorption-rate: A comparative bioavailability study in healthy volunteers. Casp. J. Intern. Med. 2019, 10, 150–155. [Google Scholar] [CrossRef]
- Old Toilets, vs. New|Home Guides|SF Gate. Available online: https://homeguides.sfgate.com/old-toilets-vs-new-88835.html (accessed on 4 March 2022).
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- López-Ramón, V.; Moreno-Castilla, C.; Rivera-Utrilla, J.; Radovic, L.R. Ionic strength effects in aqueous phase adsorption of metal ions on activated carbons. Carbon 2003, 41, 2020–2022. [Google Scholar] [CrossRef]
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption of Selected Pharmaceuticals and an Endocrine Disrupting Compound by Granular Activated Carbon. 1. Adsorption Capacity and Kinetics. Environ. Sci. Technol. 2009, 43, 1467–1473. [Google Scholar] [CrossRef]
- Belo, C.R.; Cansado, I.P.D.P.; Mourão, P.A.M. Synthetic polymers blend used in the production of high activated carbon for pesticides removals from liquid phase. Environ. Technol. 2017, 38, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- McDougall, G.J. The physical nature and manufacture of activated carbon. J. S. Afr. Inst. Min. Metall. 1991, 91, 109–120. [Google Scholar]
- Lignite Activated Carbon-Lignite Activated Carbon Manufacturers, Suppliers and Exporters on Alibaba.com. Chemical Auxiliary Agent. Available online: https://www.alibaba.com/trade/search?fsb=y&IndexArea=product_en&CatId=&SearchText=lignite+activated+carbon&viewtype=&tab= (accessed on 12 April 2022).
- Coconut Activated Carbon-Coconut Activated Carbon Manufacturers, Suppliers and Exporters on Alibaba.com. Chemical Auxiliary Agent. Available online: https://www.alibaba.com/trade/search?fsb=y&IndexArea=product_en&CatId=&SearchText=coconut+activated+carbon (accessed on 12 April 2022).
- Milici, R.C. Depletion of Appalachian coal reserves—How soon? Int. J. Coal Geol. 2000, 44, 251–266. [Google Scholar] [CrossRef]
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of activated carbon production from coconut shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef]


| Chemical Name | Supplier | Fresh Urine (mmol L−1) | Fresh Urine (mg mL−1) | Hydrolyzed Urine (mmol L−1) | Hydrolyzed Urine (mg mL−1) |
|---|---|---|---|---|---|
| Sodium Chloride | Fisher Scientific, Waltham, MA, USA | 44 | 2.57 | 60 | 3.51 |
| Sodium Sulfate | Fisher Scientific, Waltham, MA, USA | 15 | 2.13 | 15 | 2.13 |
| Potassium Chloride | Fisher Scientific, Waltham, MA, USA | 40 | 2.98 | 40 | 2.98 |
| Magnesium Chloride | Fisher Scientific, Waltham, MA, USA | 4 | 0.81 | - | - |
| Sodium Phosphate Dibasic Anhydrous | Acros Organics, Waltham, MA, USA | 20 | 0.62 | 5 | 0.16 |
| Calcium Chloride Dihydrate | Acros Organics, Waltham, MA, USA | 4 | 0.59 | - | - |
| Ibuprofen Sodium Salt | Sigma Aldrich, St. Louis, MO, USA | 2 | 0.50 | 2 | 0.50 |
| Sodium Citrate | Sigma Aldrich, St. Louis, MO, USA | 0.07 | 0.02 | 0.07 | 0.02 |
| Urea-N | Acros Organics, Waltham, MA, USA | 500 | 7.00 | - | - |
| Ammonium Hydroxide, ACS grade | JT Baker Avantor, Allentown, PA, USA | - | - | 233 | 15.70 mL |
| Ammonium Bicarbonate | Fisher Scientic, Waltham, MA, USA | - | - | 267 | 21.11 |
| Pellet Sample ID | Pellet Formulation |
|---|---|
| P1-coco | 1.2 lb Coconut PAC and 0.3 lb inorganic binder |
| P2-blend | 0.6 lb Coconut PAC, 0.6 lb Lignite PAC, 0.3 lb inorganic binder |
| P3-lig | 1.2 lb Lignite PAC and 0.3 lb inorganic binder |
| Powdered Sample ID | Tapped Density (g/mL) | Ash Content (%) | Contact pH | BET (m2/g) | Average Pore Size (Å) | Cumulative Pore Volume (cc/g) | Meso-Pore Volume (cc/g) | Micro-Pore Volume (cc/g) |
|---|---|---|---|---|---|---|---|---|
| Lignite PAC | 0.51 | 28.5 | 10.5 | 509 | 37.1 | 0.472 | 0.248 | 0.152 |
| Coconut PAC | 0.93 | 1.8 | 9.7 | 1088 | 17.8 | 0.483 | 0.036 | 0.388 |
| Pellet Sample ID | Apparent Density (g/mL) | Ash Content (%) | Contact pH | BET (m2/g) | Average Pore Size (Å) | Cumulative Pore Volume (cc/g) | Meso-Pore Volume (cc/g) | Micro-Pore Volume (cc/g) |
|---|---|---|---|---|---|---|---|---|
| P1-coco | 0.45 | 19.4 | 8.6 | 933 | 19.1 | 0.445 | 0.060 | 0.332 |
| P2-blend | 0.50 | 39.8 | 7.4 | 606 | 25.7 | 0.390 | 0.131 | 0.204 |
| P3-lig | 0.48 | 42.4 | 8.9 | 456 | 35.9 | 0.409 | 0.205 | 0.136 |
| Pellet ID | Raw Material Cost ($/lb) | Total Pellet Cost ($/lb) | Q1 min-fresh urine (g Ibuprofen/g Pellet) | Q1 min-deionized water (g Ibuprofen/g Pellet) |
|---|---|---|---|---|
| P1-coco | 0.90 | 0.74 | 0.070 | 0.042 |
| P2-blend | 0.78 | 0.64 | 0.088 | 0.066 |
| P3-lig | 0.65 | 0.54 | 0.089 | 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finn, M.; Yackel, N.; Giampietro, G.; Mazyck, D. Dissolving Activated Carbon Pellets for Ibuprofen Removal at Point-of-Entry. Processes 2023, 11, 1470. https://doi.org/10.3390/pr11051470
Finn M, Yackel N, Giampietro G, Mazyck D. Dissolving Activated Carbon Pellets for Ibuprofen Removal at Point-of-Entry. Processes. 2023; 11(5):1470. https://doi.org/10.3390/pr11051470
Chicago/Turabian StyleFinn, Michelle, Noelle Yackel, Gabrielle Giampietro, and David Mazyck. 2023. "Dissolving Activated Carbon Pellets for Ibuprofen Removal at Point-of-Entry" Processes 11, no. 5: 1470. https://doi.org/10.3390/pr11051470





