Process Simulation of Power-to-X Systems—Modeling and Simulation of Biological Methanation
Abstract
:1. Introduction
1.1. Fundamentals of Biological Methanation
1.2. Technical Implementation of Biological Methanation
1.3. Challenges of Biological Methanation
2. Materials and Methods
2.1. Convection
2.2. Axial Mixing
2.3. Absorption and Desorption
2.4. Biological Reaction Kinetics
3. Results
3.1. Model Validation
3.1.1. Gas Inflow Rate
3.1.2. Liquid Flow Rate
3.1.3. Reactor Overhead Pressure
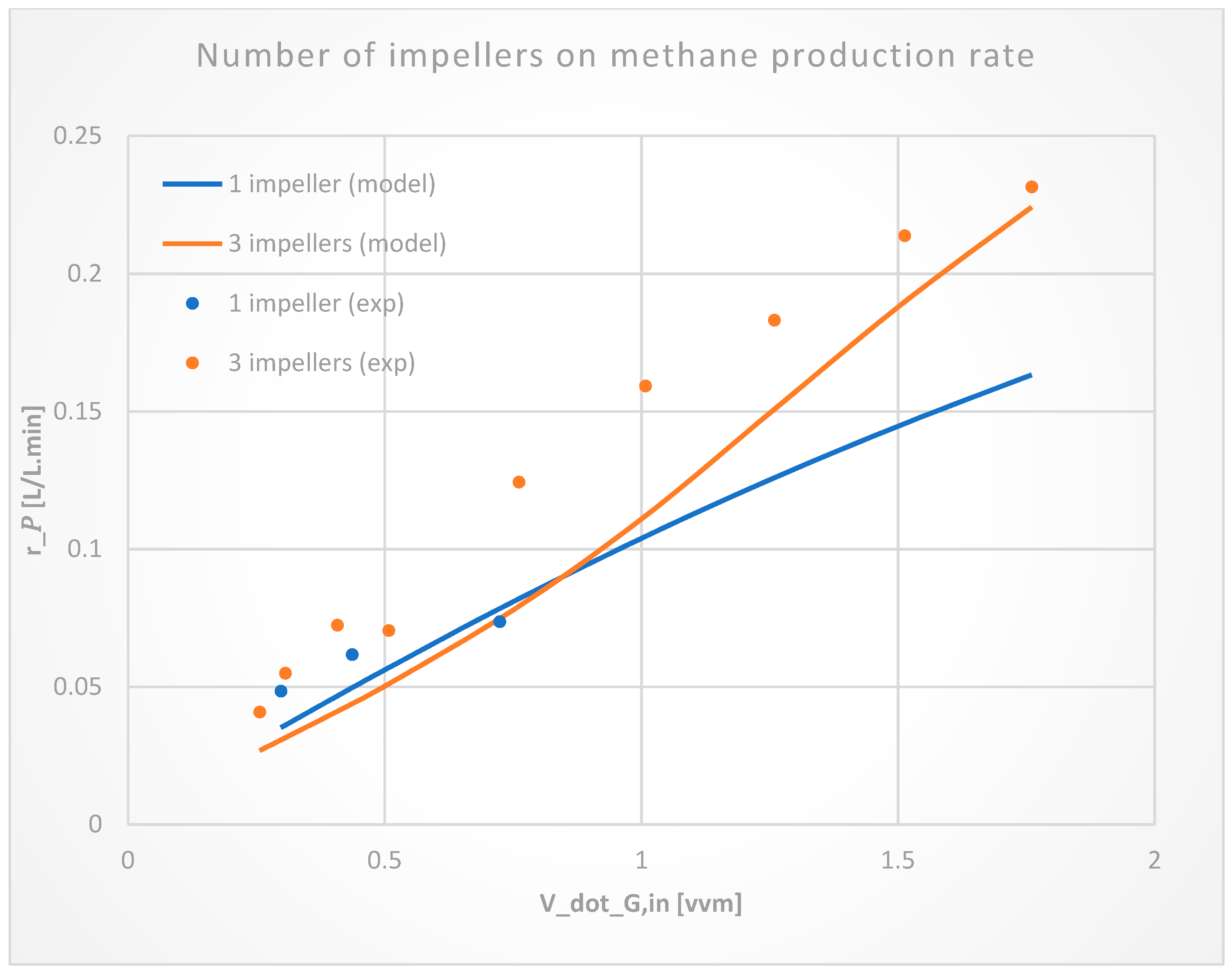
3.1.4. Number of Impellers
3.2. Sensitivity Analysis
3.2.1. Liquid Recycle Constant
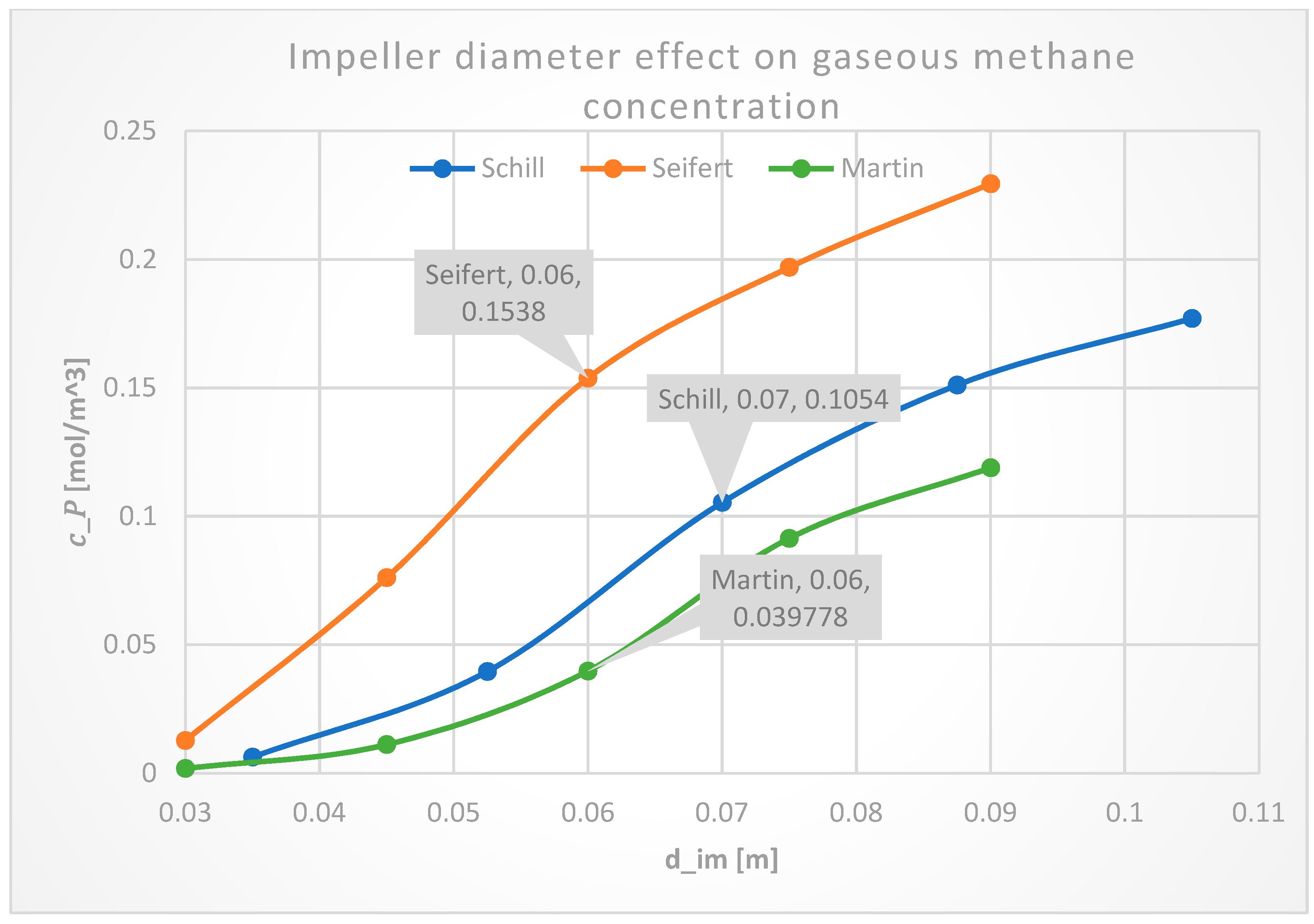
3.2.2. Impeller Diameter
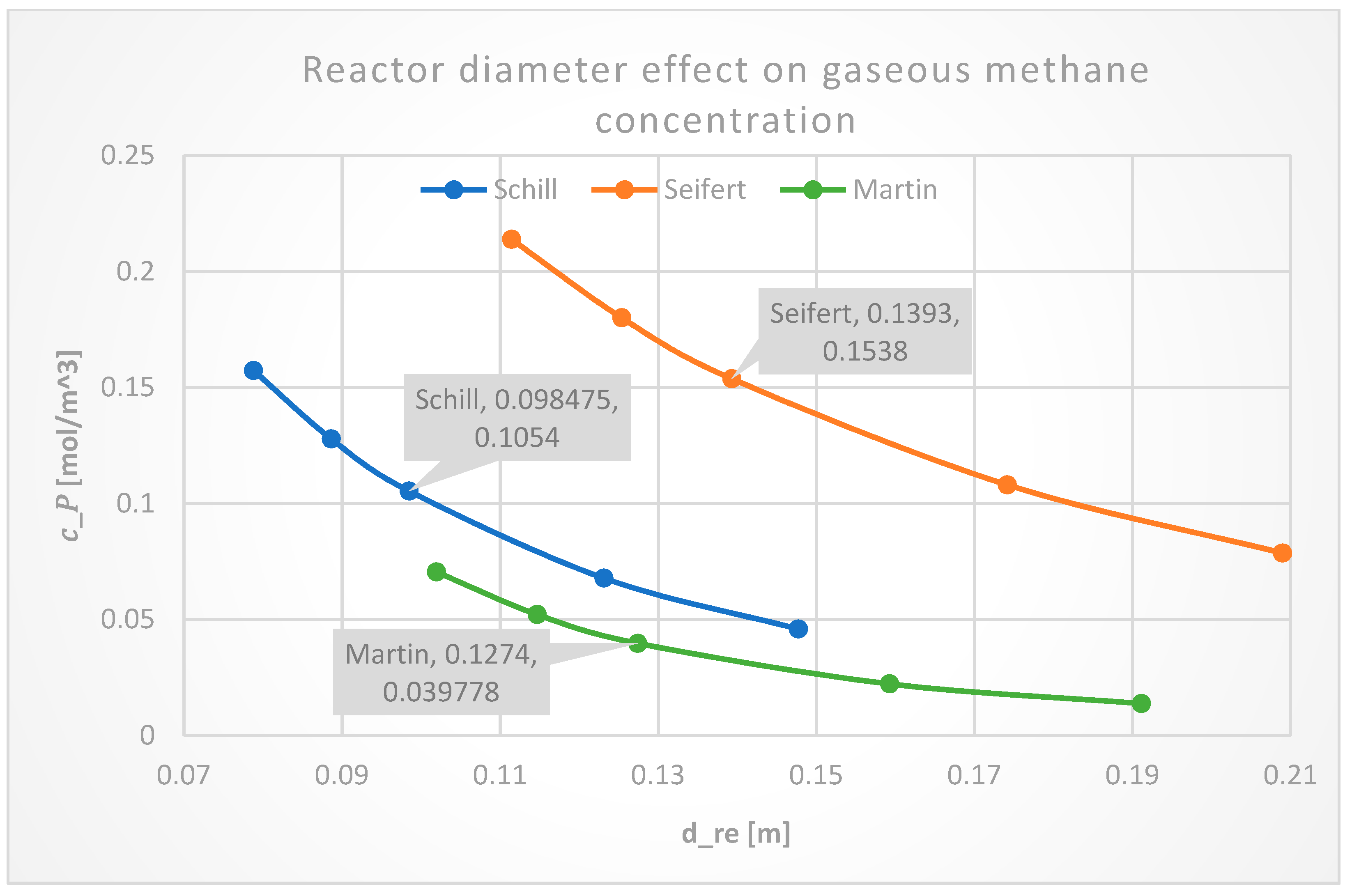
3.2.3. Reactor Diameter
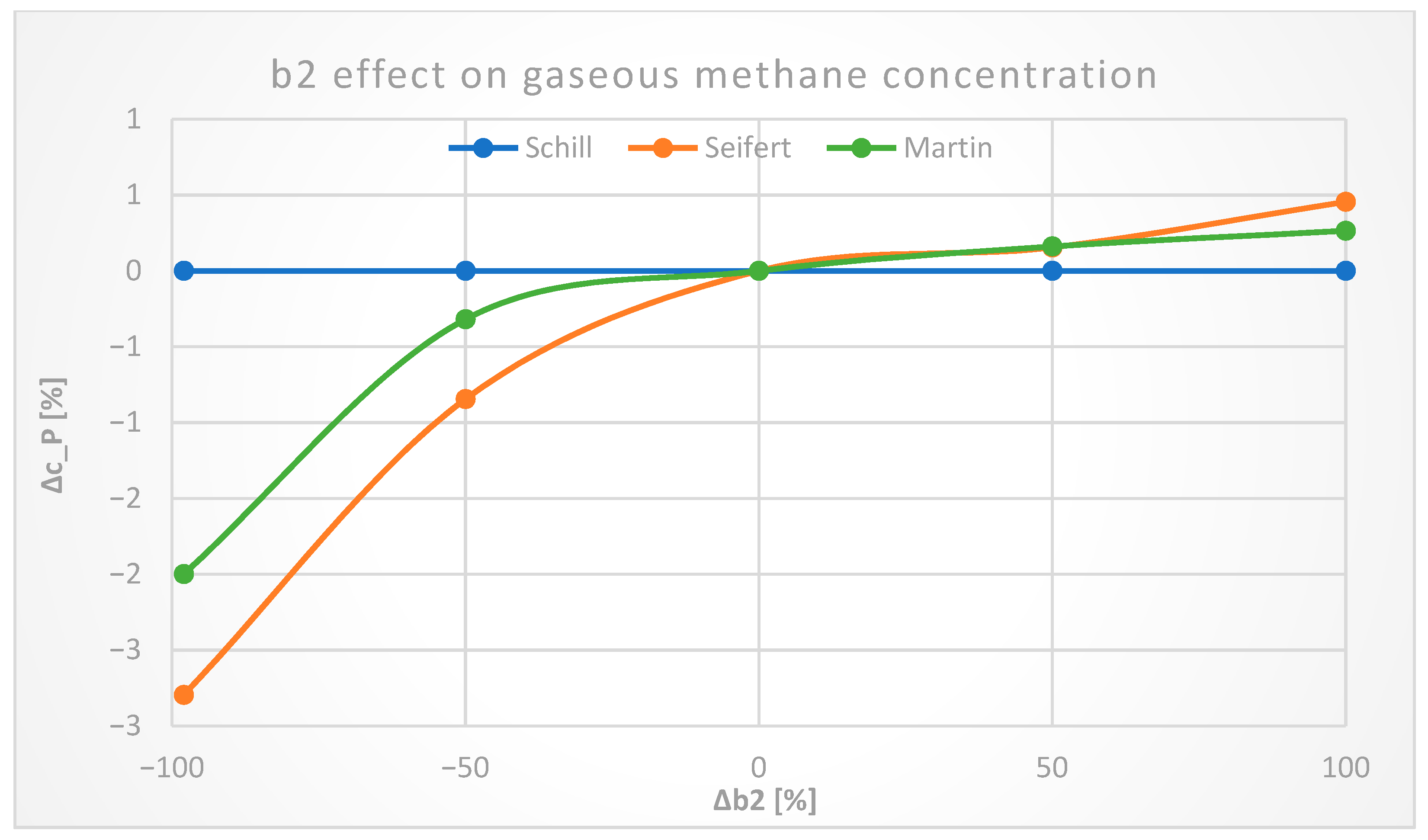
3.2.4. Axial Mixing Constants
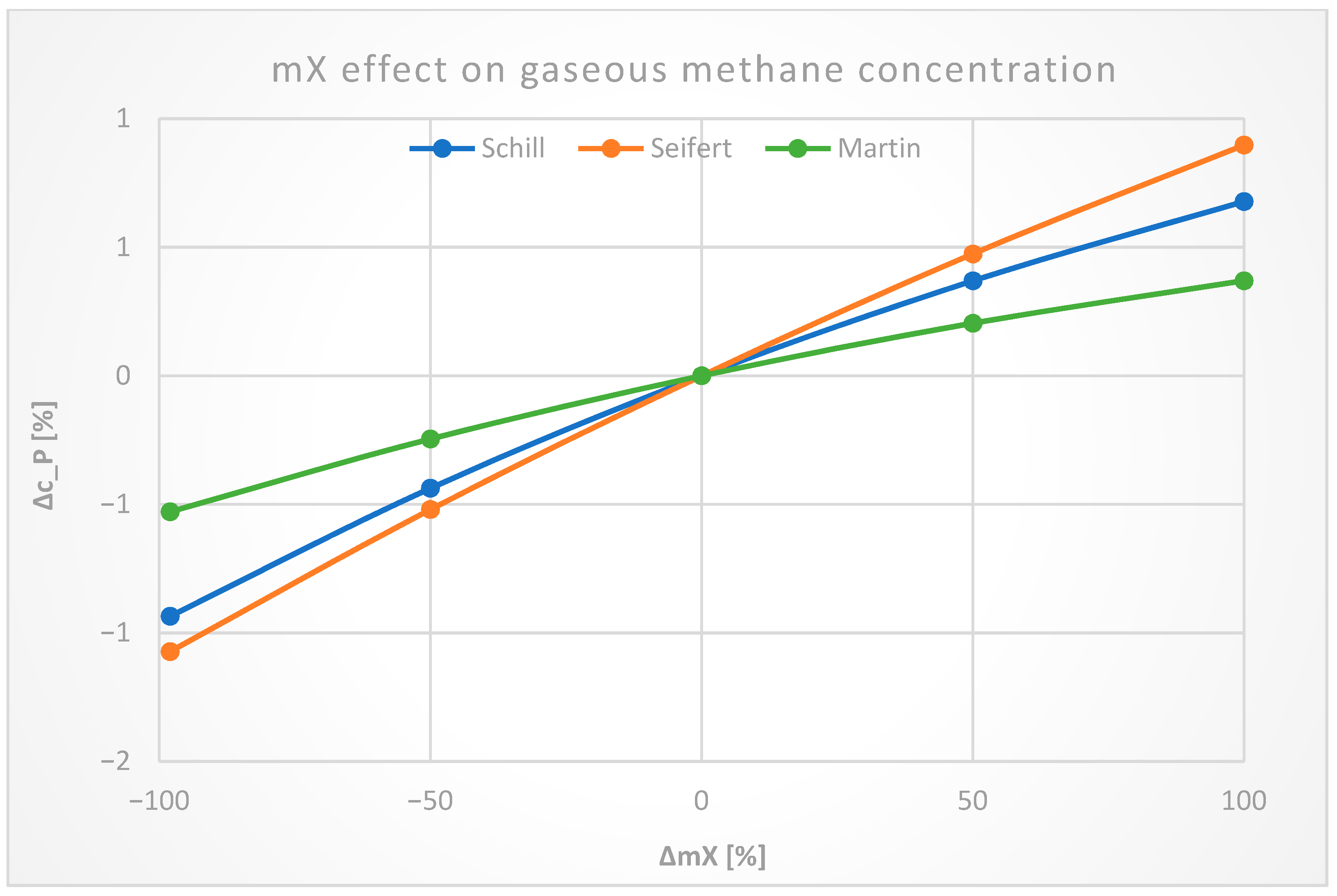
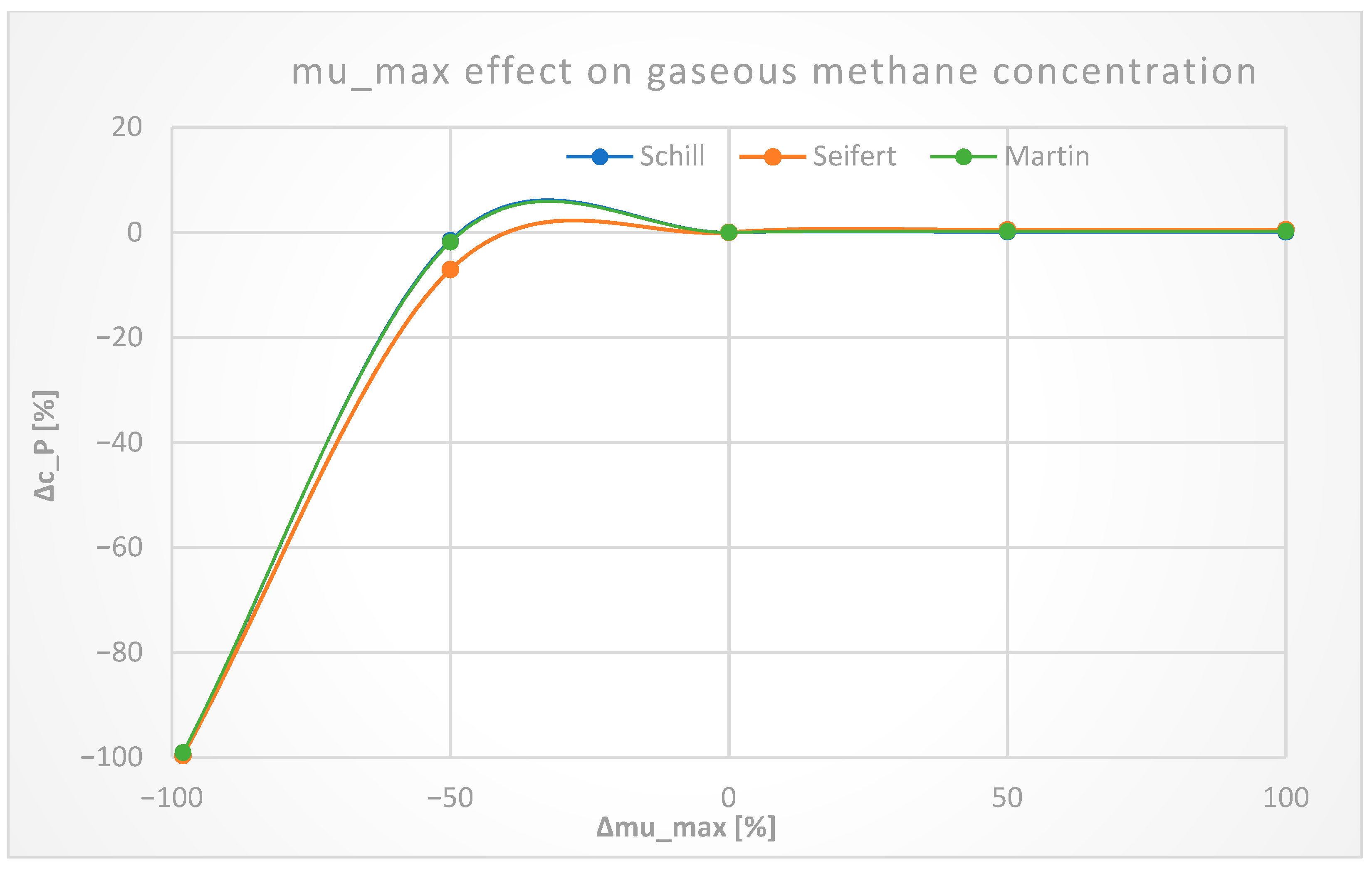
3.2.5. Biomass Kinetics Constants
4. Discussion
5. Conclusions
- Developing a model that considers the effects of parameters and operating conditions, such as pH, temperatures, and nutrients, simultaneously with reactor hydrodynamics, gas–liquid mass transfer, and microorganisms’ growth kinetics.
- Evaluating the possibilities of combining different reactor designs to achieve higher methane concentrations.
- Exploring novel technologies about more efficient ex situ bio-methanation, higher solubility, decreasing bubble diameter , and improving volumetric gas–liquid mass transfer coefficient .
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| BM | Biological methanation |
| CSTR | Continuously stirred tank reactor |
| GWP | Global warming potential |
| HFM | Hollow fiber membrane |
| HM | Hydrogenotrophic methanogenesis |
| LHV | Lower heating value |
| ODE | Ordinary differential equation |
| PtG | Power-to-gas |
| PtX | Power-to-x |
| TBR | Trickle-bed reactor |
| vvm | Gas volume flow per liquid volume in a minute |
Subscripts
| C | |
| ci | Circulation flow |
| D | |
| G | Gas phase |
| g | Gaseous component |
| in | Inlet |
| is | Inter-stage flow |
| L | Liquid phase |
| l | Absorbed gas component in liquid |
| im | Impeller |
| P | |
| re | Reactor |
| X | Biomass (archaea) |
| 0 | Ungassed condition |
Symbols
| Cross-sectional area of the reactor | |
| Interfacial area | |
| Constant | |
| concentration in liquid | |
| concentration in liquid | |
| Concentration in gaseous phase | |
| Concentration in liquid phase | |
| Biomass concentration in liquid phase | |
| Saturation solubility in liquid | |
| Average bubble diameter | |
| Impeller diameter | |
| Reactor diameter | |
| Gas diffusivity in liquid | |
| Liquid feed rate | |
| Gravitational constant | |
| Reactor height | |
| Henry’s solubility coefficient | |
| Inhibition factor due to the lack of substance [−] | |
| Saturation constant for | |
| Mass transfer coefficient | |
| Volumetric mass transfer coefficient | |
| Maintenance constant | |
| Mass flow rate | |
| Molar mass | |
| Stirring speed [rps] | |
| Number of cells in grid | |
| Number of impellers | |
| Power number | |
| Inter-stage number | |
| Circulation number | |
| Pressure | |
| Hydrostatic pressure | |
| Reactor overhead pressure | |
| Stirring power | |
| Maximum specific conversion rate | |
| Volumetric conversion rate | |
| Source term for gas phase | |
| Source term for liquid phase | |
| Time | |
| T | Temperature [°C] |
| Velocity | |
| Superficial velocity | |
| Active reactor volume | |
| Total reactor volume | |
| Volume flow rate | |
| Inter-stage liquid flow rate | |
| Circulation liquid flow rate | |
| Component molar fraction in gas phase | |
| Molar yield of substrate | |
| Constant | |
| Constant | |
| Axial mixing coefficient | |
| Energy dissipation | |
| Liquid recycle constant | |
| Dynamic viscosity | |
| Density | |
| Surface tension | |
| Gas hold-up without viscous effects | |
| Gas hold-up with viscous effects |
References
- Ellabban, O.; Abu-Rub, H.; Blaabjerg, F. Renewable Energy Resources: Current Status, Future Prospects and Their Enabling Technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Tan, C.H.; Nomanbhay, S.; Shamsuddin, A.H.; Park, Y.-K.; Hernández-Cocoletzi, H.; Show, P.L. Current Developments in Catalytic Methanation of Carbon Dioxide—A Review. Front. Energy Res. 2022, 9, 795423. [Google Scholar] [CrossRef]
- Guneratnam, A.J.; Ahern, E.; FitzGerald, J.A.; Jackson, S.A.; Xia, A.; Dobson, A.D.W.; Murphy, J.D. Study of the Performance of a Thermophilic Biological Methanation System. Bioresour. Technol. 2017, 225, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R. Archae—Timeline of the Third Domain. Nat. Rev. Microbiol. 2011, 9, 51–61. [Google Scholar] [CrossRef]
- Wagner, I.D.; Wiegel, J. Diversity of Thermophilic Anaerobes. Ann. N. Y. Acad. Sci. 2008, 1125, 1–43. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Purcell, R. CO2 Recycling by Reaction with Renewably-Generated Hydrogen. Int. J. Greenhouse Gas Control. 2010, 4, 44–50. [Google Scholar] [CrossRef]
- Burkhardt, M.; Koschack, T.; Busch, G. Biocatalytic Methanation of Hydrogen and Carbon Dioxide in an Anaerobic Three-Phase System. Bioresour. Technol. 2015, 178, 330–333. [Google Scholar] [CrossRef]
- Seifert, A.H.; Rittmann, S.; Herwig, C. Analysis of Process Related Factors to Increase Volumetric Productivity and Quality of Biomethane with Methanothermobacter Marburgensis. Appl. Energy 2014, 132, 155–162. [Google Scholar] [CrossRef]
- Shin, H.C.; Ju, D.-H.; Jeon, B.S.; Choi, O.; Kim, H.W.; Um, Y.; Lee, D.-H.; Sang, B.-I. Analysis of the Microbial Community in an Acidic Hollow-Fiber Membrane Biofilm Reactor (Hf-MBfR) Used for the Biological Conversion of Carbon Dioxide to Methane. PLoS ONE 2015, 10, e0144999. [Google Scholar] [CrossRef]
- Rittmann, S.; Seifert, A.; Herwig, C. Essential Prerequisites for Successful Bioprocess Development of Biological CH4 Production from CO2 and H2. Crit. Rev. Biotechnol. 2015, 35, 141–151. [Google Scholar] [CrossRef]
- Inkeri, E.; Tynjälä, T.; Laari, A.; Hyppänen, T. Dynamic One-Dimensional Model for Biological Methanation in a Stirred Tank Reactor. Appl. Energy 2018, 209, 95–107. [Google Scholar] [CrossRef]
- Schill, N.; van Gulik, W.M.; Voisard, D.; von Stockar, U. Continuous Cultures Limited by a Gaseous Substrate: Development of a Simple, Unstructured Mathematical Model and Experimental Verification with Methanobacterium Thermoautotrophicum. Biotechnol. Bioeng. 1996, 51, 645–658. [Google Scholar] [CrossRef]
- Díaz, I.; Pérez, C.; Alfaro, N.; Fdz-Polanco, F. A Feasibility Study on the Bioconversion of CO2 and H2 to Biomethane by Gas Sparging through Polymeric Membranes. Bioresour. Technol. 2015, 185, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.R.; Fornero, J.J.; Stark, R.; Mets, L.; Angenent, L.T. A Single-Culture Bioprocess of Methanothermobacter Thermautotrophicus to Upgrade Digester Biogas by CO2-to-CH4 Conversion with H2. Archaea 2013, 2013, e157529. [Google Scholar] [CrossRef]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Biological Methanation of CO2 in a Novel Biofilm Plug-Flow Reactor: A High Rate and Low Parasitic Energy Process. Appl. Energy 2017, 202, 238–247. [Google Scholar] [CrossRef]
- Rusmanis, D.; O’Shea, R.; Wall, D.M.; Murphy, J.D. Biological Hydrogen Methanation Systems—An Overview of Design and Efficiency. Bioengineered 2019, 10, 604–634. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Integrated Biogas Upgrading and Hydrogen Utilization in an Anaerobic Reactor Containing Enriched Hydrogenotrophic Methanogenic Culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef]
- Bernacchi, S.; Rittmann, S.; Seifert, A.H.; Krajete, A.; Herwig, C.; Bernacchi, S.; Rittmann, S.; Seifert, A.H.; Krajete, A.; Herwig, C. Experimental Methods for Screening Parameters Influencing the Growth to Product Yield (Y(x/CH4)) of a Biological Methane Production (BMP) Process Performed with Methanothermobacter Marburgensis. AIMS Bioeng. 2014, 1, 72–87. [Google Scholar] [CrossRef]
- Alitalo, A.; Niskanen, M.; Aura, E. Biocatalytic Methanation of Hydrogen and Carbon Dioxide in a Fixed Bed Bioreactor. Bioresour. Technol. 2015, 196, 600–605. [Google Scholar] [CrossRef]
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological Biogas Upgrading Capacity of a Hydrogenotrophic Community in a Trickle-Bed Reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Coker, A.K. Chapter 21—Industrial and Laboratory Reactors—Chemical Reaction Hazards and Process Integration of Reactors. In Ludwig’s Applied Process Design for Chemical and Petrochemical Plants (Fourth Edition); Coker, A.K., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2015; pp. 1095–1208. [Google Scholar] [CrossRef]
- Wilke, C.R. A Viscosity Equation for Gas Mixtures. J. Chem. Phys. 1950, 18, 517–519. [Google Scholar] [CrossRef]
- The Engineering Toolbox. Available online: https://www.engineeringtoolbox.com/gases-absolute-dynamic-viscosity-d_1888.html (accessed on 16 December 2022).
- Garcia-Ochoa, F.; Gomez, E. Theoretical Prediction of Gas–Liquid Mass Transfer Coefficient, Specific Area and Hold-up in Sparged Stirred Tanks. Chem. Eng. Sci. 2004, 59, 2489–2501. [Google Scholar] [CrossRef]
- Mazzeo, L.; Signorini, A.; Lembo, G.; Bavasso, I.; Di Palma, L.; Piemonte, V. In Situ Bio-Methanation Modelling of a Randomly Packed Gas Stirred Tank Reactor (GSTR). Processes 2021, 9, 846. [Google Scholar] [CrossRef]
- Vasconcelos, J.M.T.; Alves, S.S.; Barata, J.M. Mixing in Gas-Liquid Contactors Agitated by Multiple Turbines. Chem. Eng. Sci. 1995, 50, 2343–2354. [Google Scholar] [CrossRef]
- Schmitz, K.S. Chapter 5—Thermodynamics of the Liquid State. In Physical Chemistry; Schmitz, K.S., Ed.; Elsevier: Boston, MA, USA, 2017; pp. 203–260. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- The Engineering Toolbox. Available online: https://www.engineeringtoolbox.com/diffusion-coefficients-d_1404.html (accessed on 17 December 2022).
- Michel, B.J.; Miller, S.A. Power Requirements of Gas-Liquid Agitated Systems. AIChE J. 1962, 8, 262–266. [Google Scholar] [CrossRef]
- Nienow, A.W. Gas–liquid mixing studies: A comparison of Rushton turbines with some modern impellers. Chem. Eng. Res. Des. 1996, 74, 417–423. [Google Scholar]
- Bhavaraju, S.M.; Russell, T.W.F.; Blanch, H.W. The Design of Gas Sparged Devices for Viscous Liquid Systems. AIChE J. 1978, 24, 454–466. [Google Scholar] [CrossRef]
- Fardeau, M.-L.; Peillex, J.-P.; Belaïch, J.-P. Energetics of the Growth of Methanobacterium Thermoautotrophicum and Methanococcus Thermolithotrophicus on Ammonium Chloride and Dinitrogen. Arch. Microbiol. 1987, 148, 128–131. [Google Scholar] [CrossRef]
- Chezeau, B.; Vial, C. Chapter 19—Modeling and Simulation of the Biohydrogen Production Processes. In Biohydrogen, 2nd ed.; Biomass, Biofuels, Biochemicals; Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 445–483. [Google Scholar] [CrossRef]
- MathWorks. Available online: https://uk.mathworks.com/company/newsletters/articles/stiff-differential-equations.html (accessed on 17 December 2022).
- MathWorks. Available online: https://uk.mathworks.com/help/matlab/ref/ode15s.html (accessed on 17 December 2022).
- Eggers, N.; Böttger, J.N.J.; Kerpen, L.; Sankol, B.U.; Birth, T. Refining VDI Guideline 4663 to Evaluate the Efficiency of a Power-to-Gas Process by Employing Limit-Oriented Indicators. Energy Effic. 2021, 14, 73. [Google Scholar] [CrossRef]
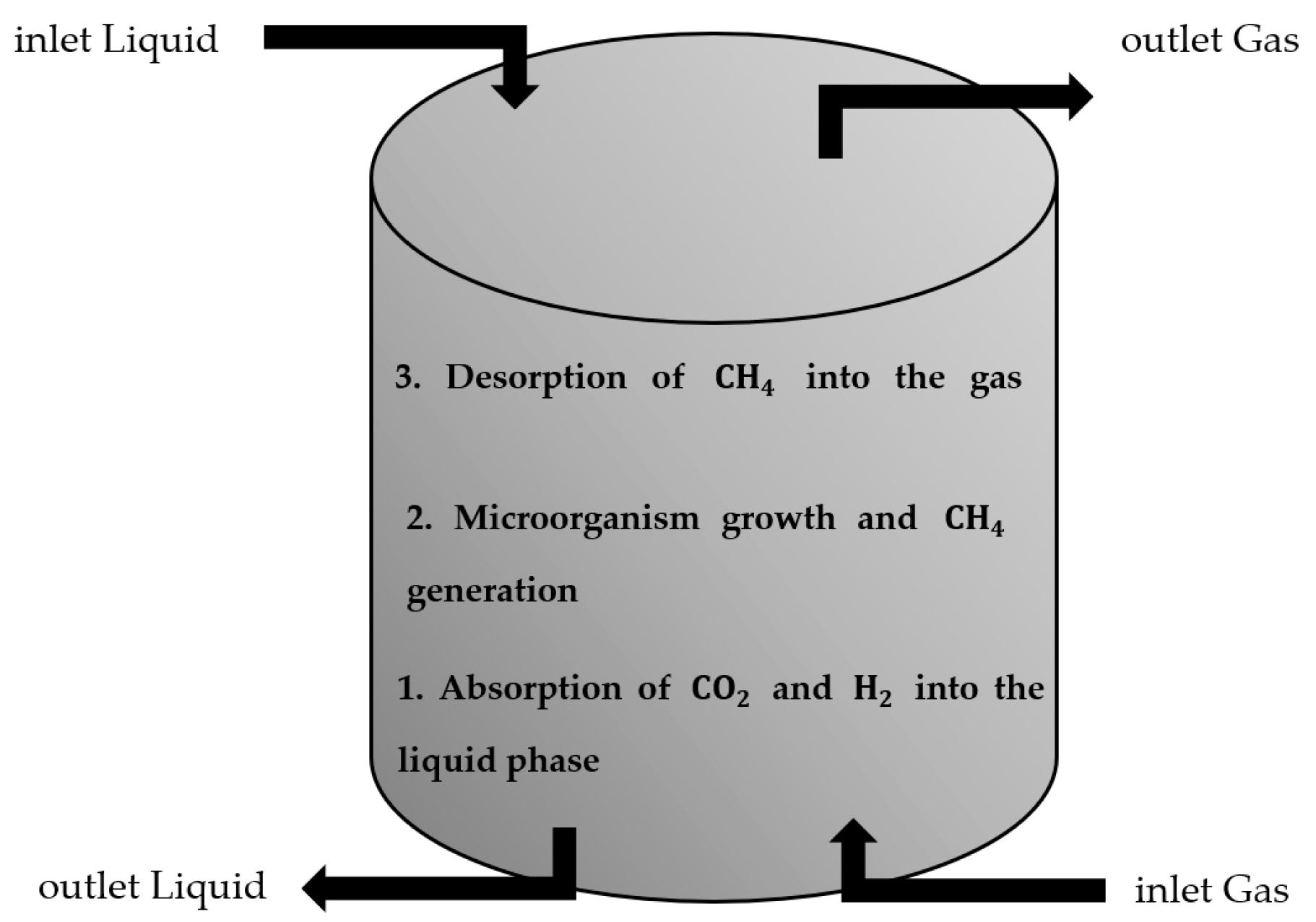
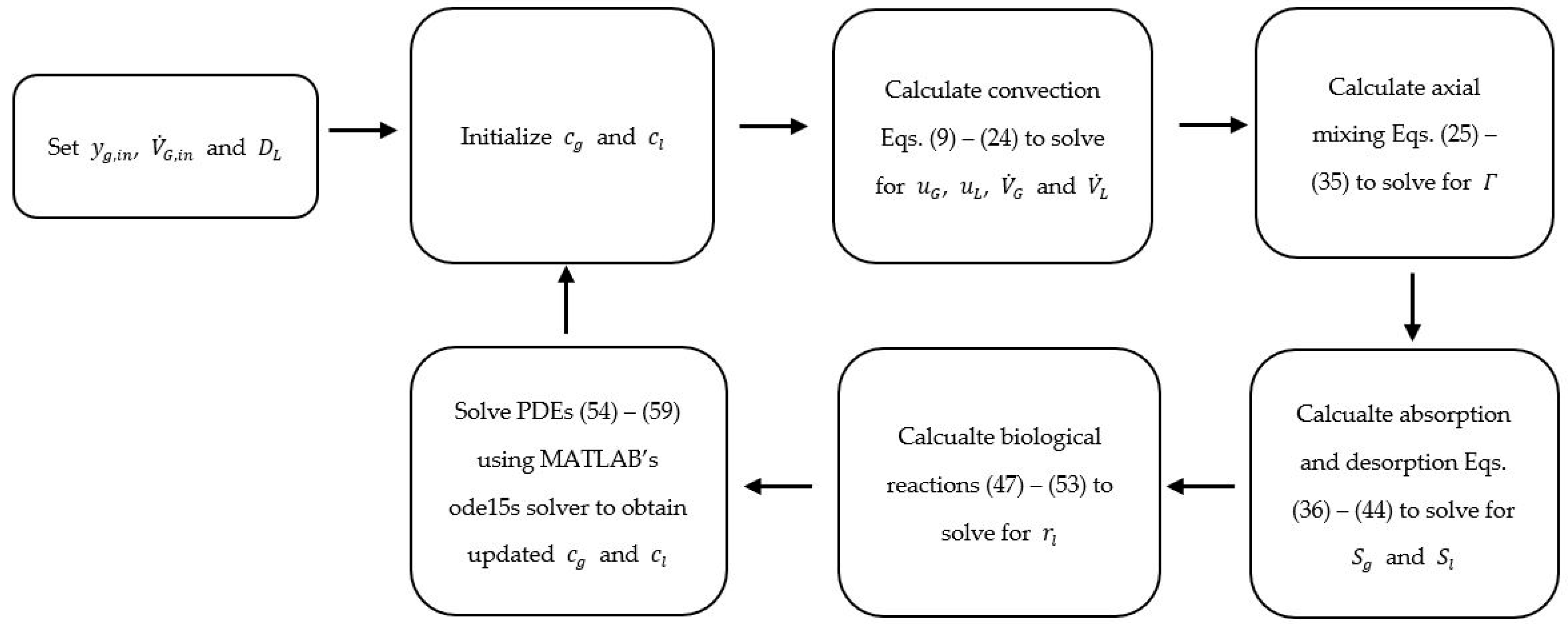
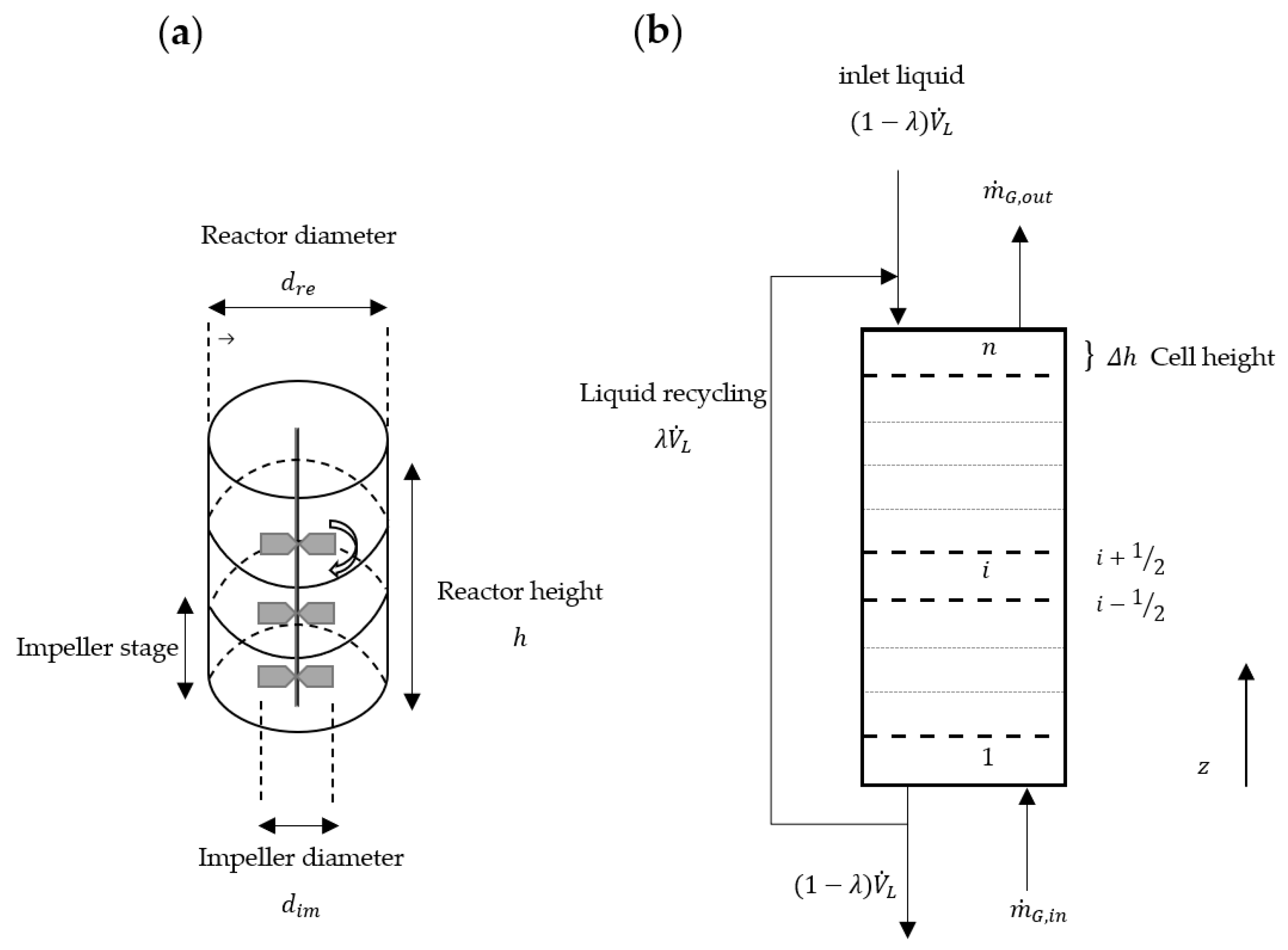
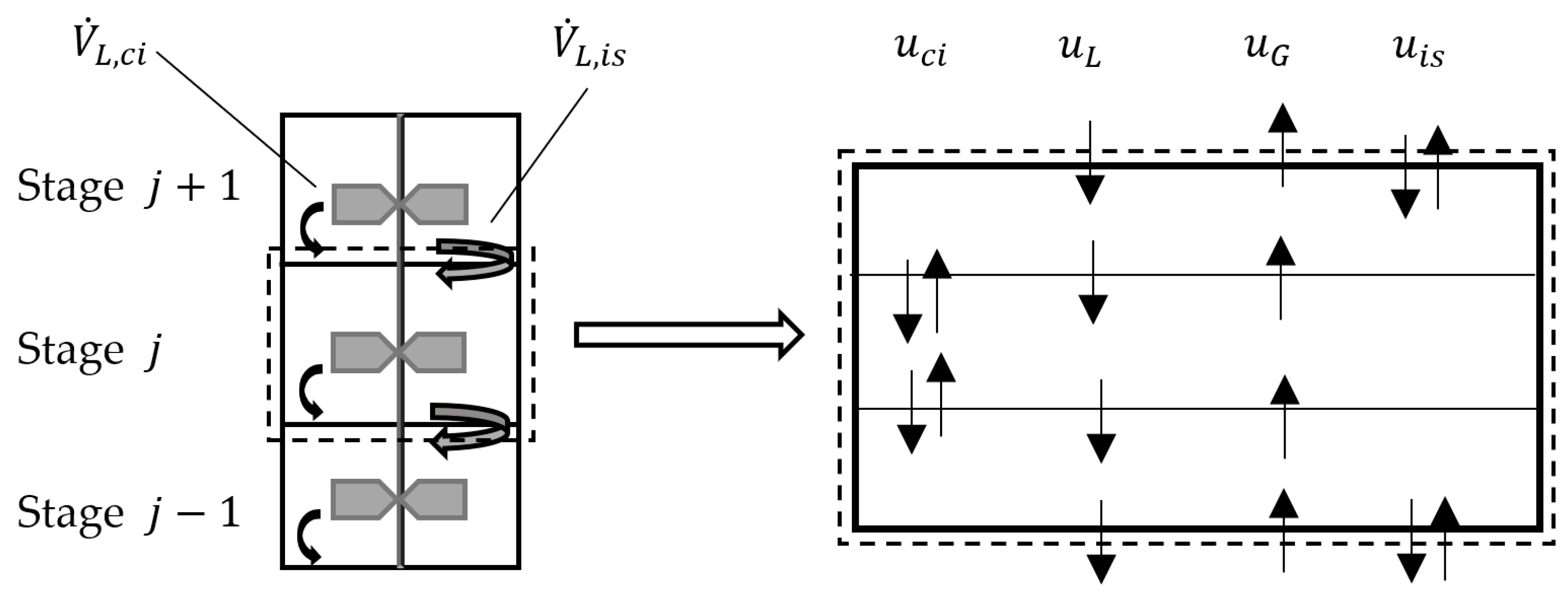
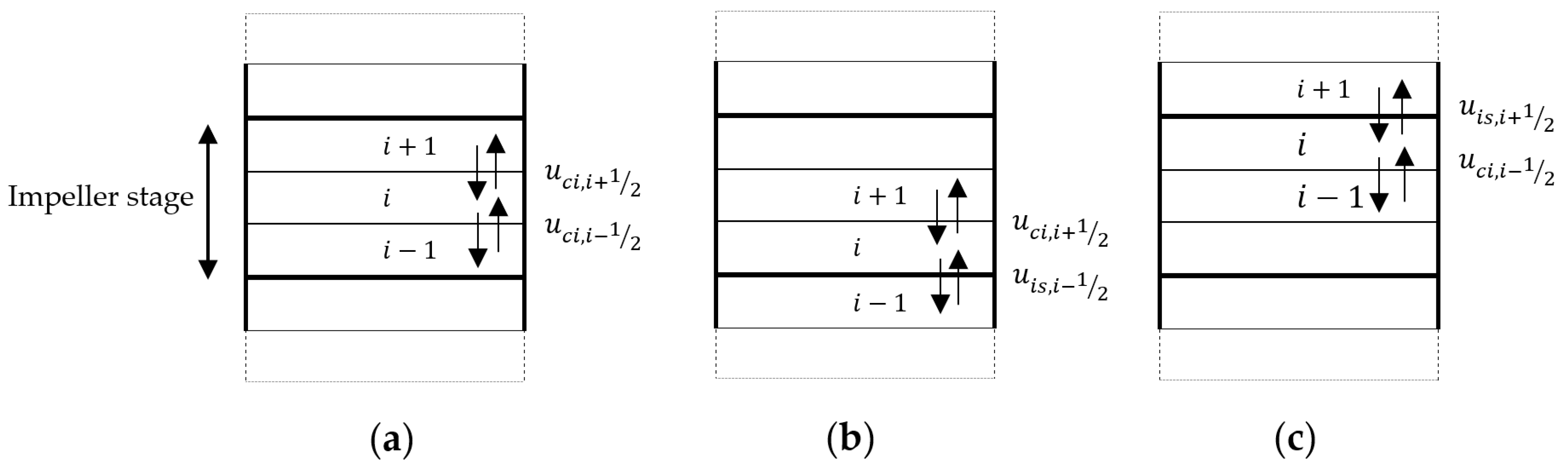
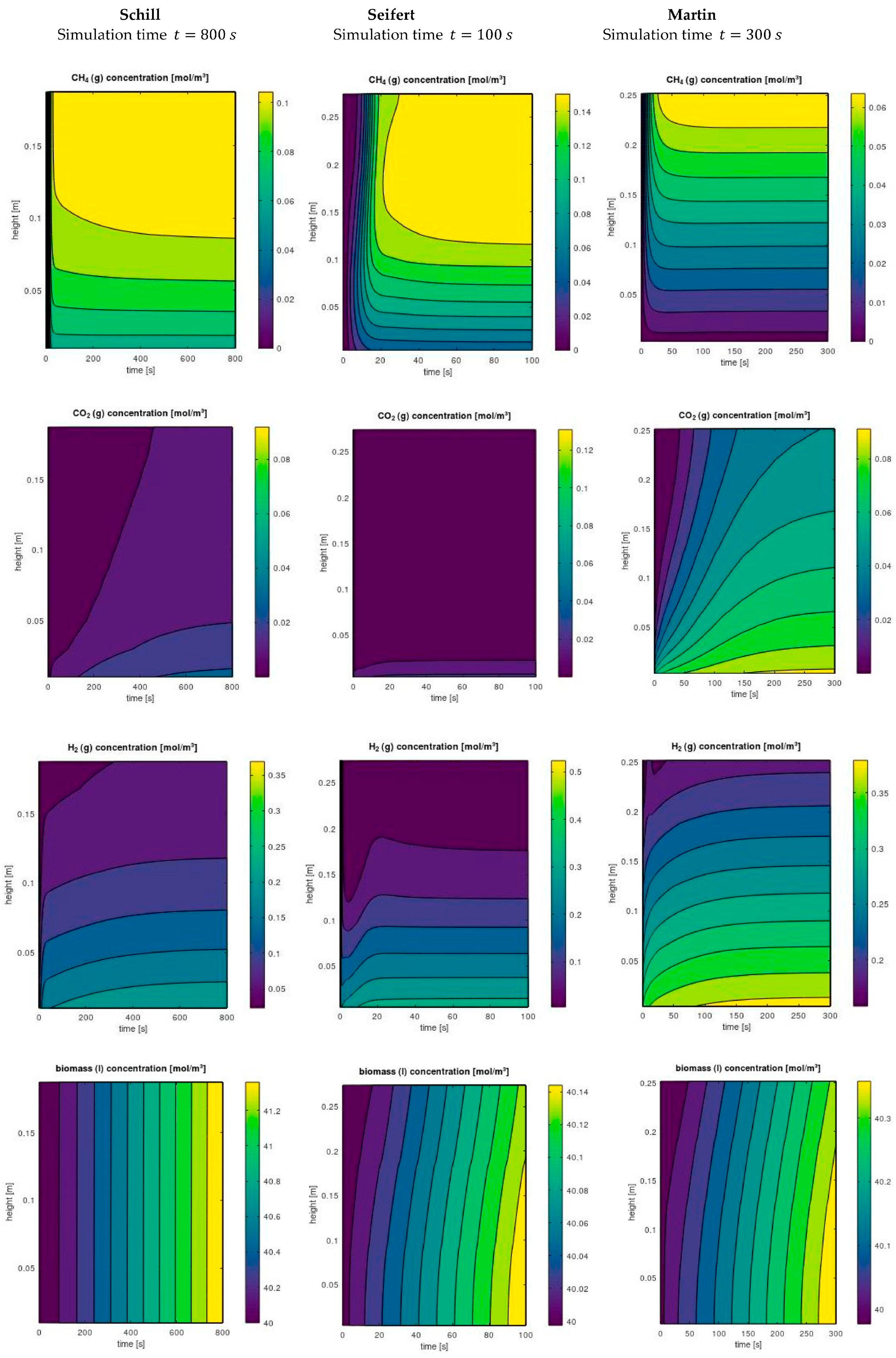

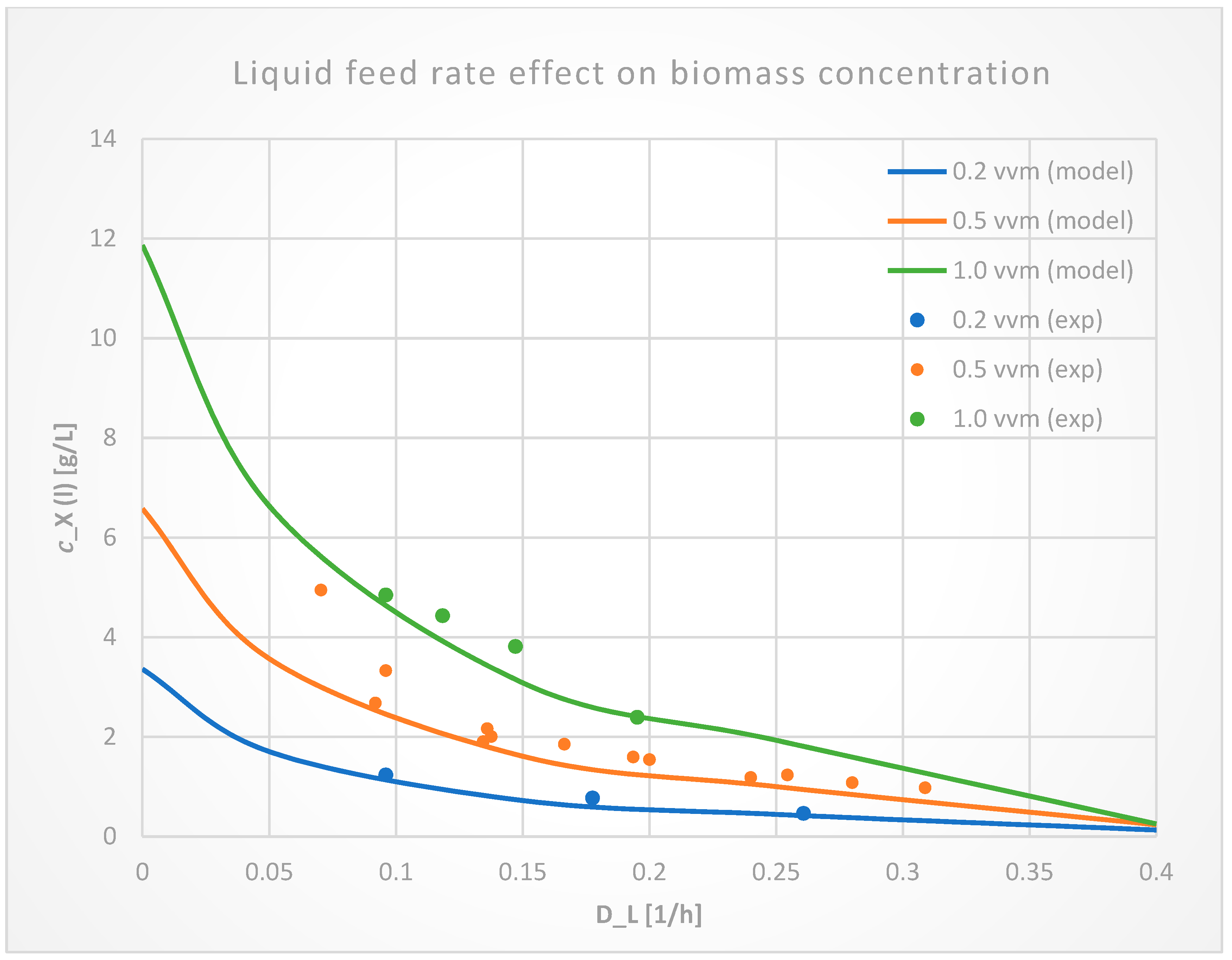
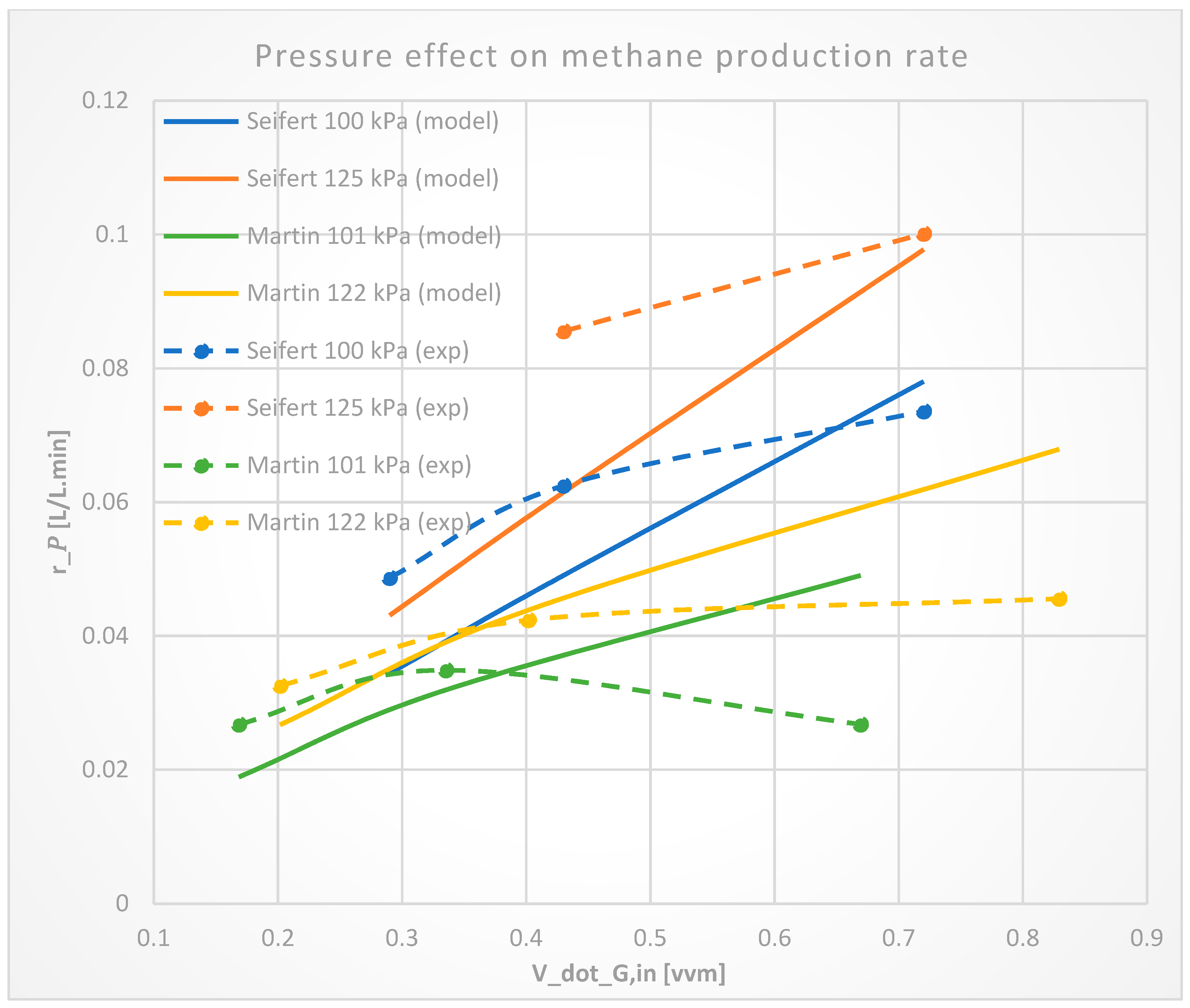



| Reference | pH | Temperature (°C) | Reactor Type |
|---|---|---|---|
| Jacob Guneratnam et al. [3] | 7.7–8.2 | 55 and 65 | Closed batch system |
| Bernacchi et al. [18] | 6–7.8 | 59–70 | CSTR 1 |
| Shin et al. [9] | 4.5–5.5 | 35–38 | Hf-MBfR 2 |
| Alitalo et al. [19] | 6.6–6.8 | 50 | Fixed bed reactor |
| Luo et al. [17] | 7.8 | 37 and 55 | CSTR |
| Díaz et al. [13] | 7.2 | 55 | Hollow-fiber MBR 3 |
| Martin et al. [14] | 6.85 and 7.35 | 60 | CSTR |
| Seifert et al. [8] | 6.85 | 65 | CSTR |
| Rachbauer et al. [20] | 7.4–7.7 | 37 | Trickle bed reactor |
| 11.993487 | |
| 16.330336 | |
| 9.747077 |
| 0.66 | |
| 13.25 | |
| 0.60 |
| 4.4597 | |
| CO2 | 4.8682 |
| 11.064 |
| Components | Subscripts |
|---|---|
| D | |
| C | |
| P | |
| N | |
| W | |
| Biomass | X |
| Subscripts | ||
|---|---|---|
| 0.00456 | ||
| 0.259 | ||
| 0.24 | ||
| 0.511 | ||
| 5.6 | ||
| 0.361 (± 0.011) | ||
| 1.67 (± 0.46) |
| Model | ||||||
|---|---|---|---|---|---|---|
| Schill | 1.5 | 2:1 * | 0.07 | 1000 | 1 | 60 |
| Seifert | 4.25 | 2:1 * | 0.06 | 1500 | 3 | 60 |
| Martin | 3.25 | 2:1 * | 0.06 | 700 | 4 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashkavand, M.; Heineken, W.; Birth, T. Process Simulation of Power-to-X Systems—Modeling and Simulation of Biological Methanation. Processes 2023, 11, 1510. https://doi.org/10.3390/pr11051510
Ashkavand M, Heineken W, Birth T. Process Simulation of Power-to-X Systems—Modeling and Simulation of Biological Methanation. Processes. 2023; 11(5):1510. https://doi.org/10.3390/pr11051510
Chicago/Turabian StyleAshkavand, Mostafa, Wolfram Heineken, and Torsten Birth. 2023. "Process Simulation of Power-to-X Systems—Modeling and Simulation of Biological Methanation" Processes 11, no. 5: 1510. https://doi.org/10.3390/pr11051510





