Graphene Oxide Membranes for Ionic/Molecular Filtration: From Self-Assembly to Design
Abstract
:1. Introduction
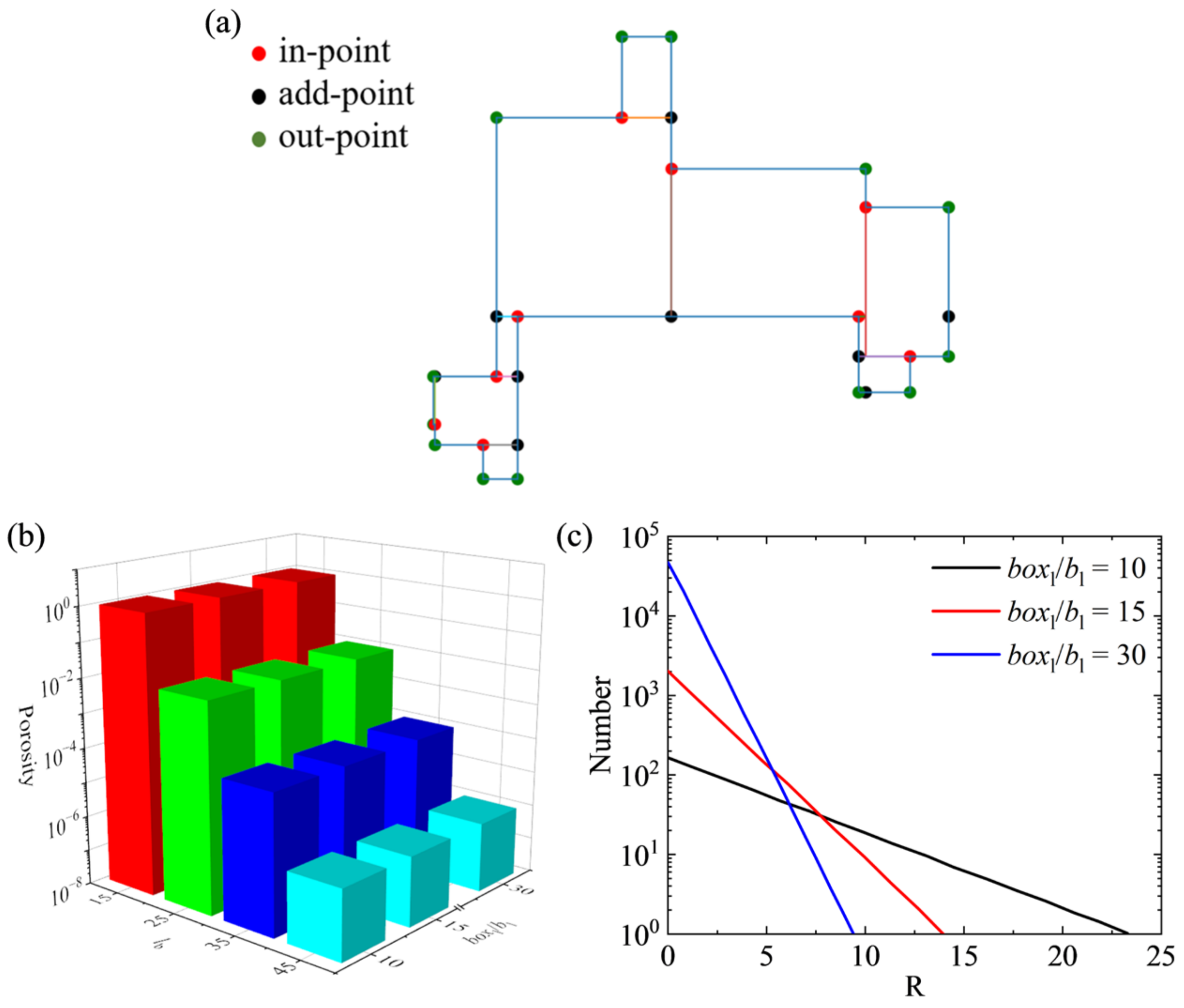
2. Materials and Methods
- If a block is in contact with the base, we set lbl to 1;
- If a newly generated block overlaps with an existing one, we mark the block layer where the overlapping block is present as lbl − 1.
3. Results and Discussion
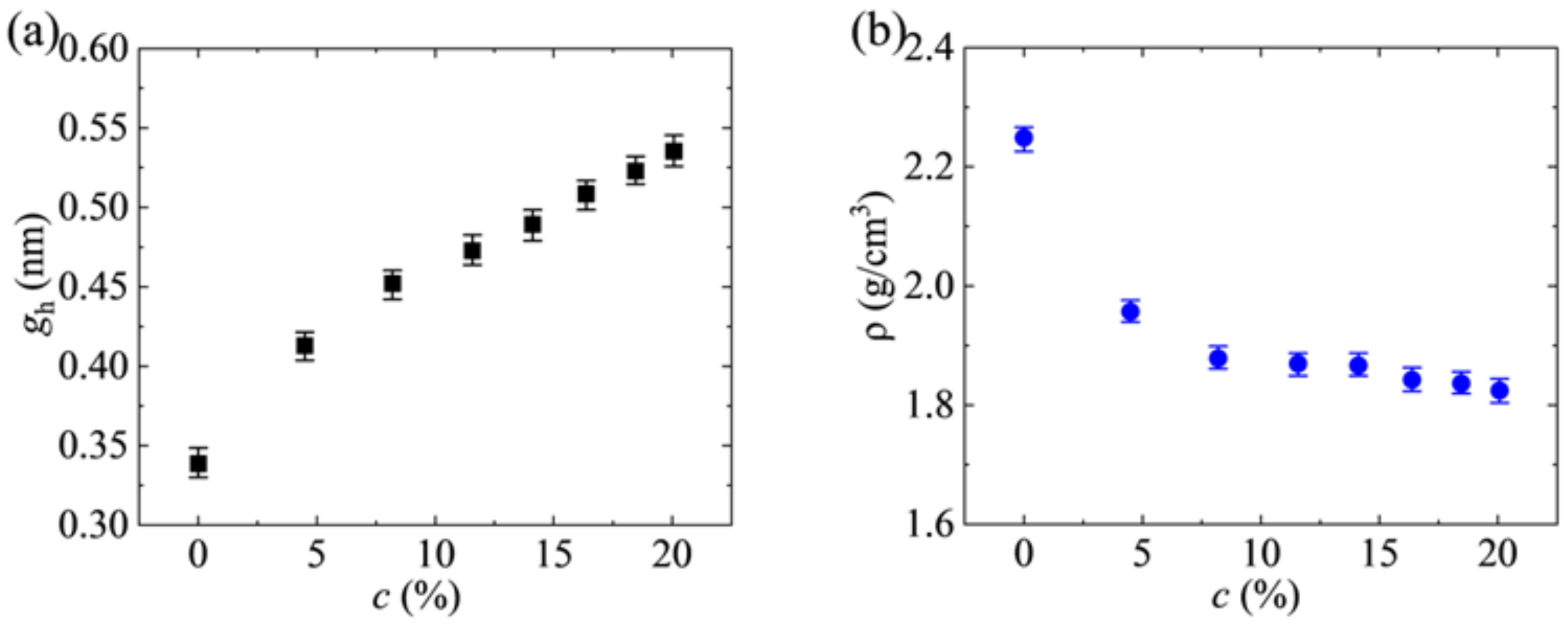
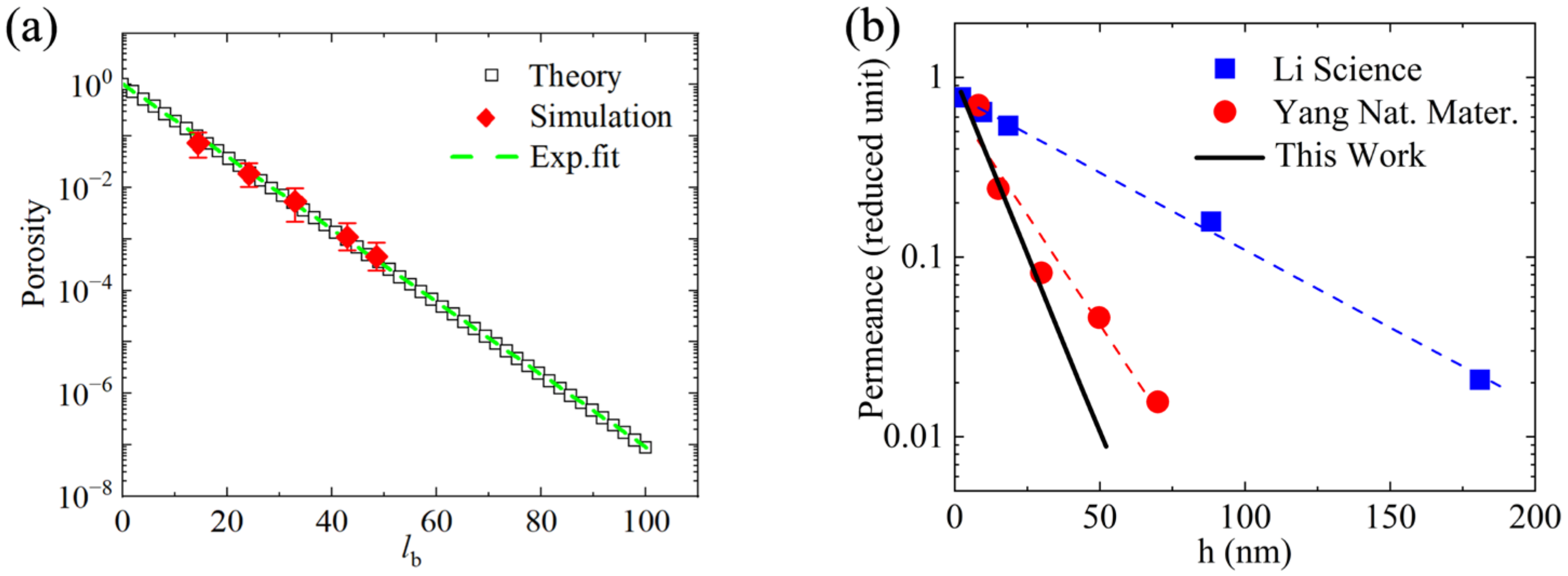
3.1. Porosity Calculate
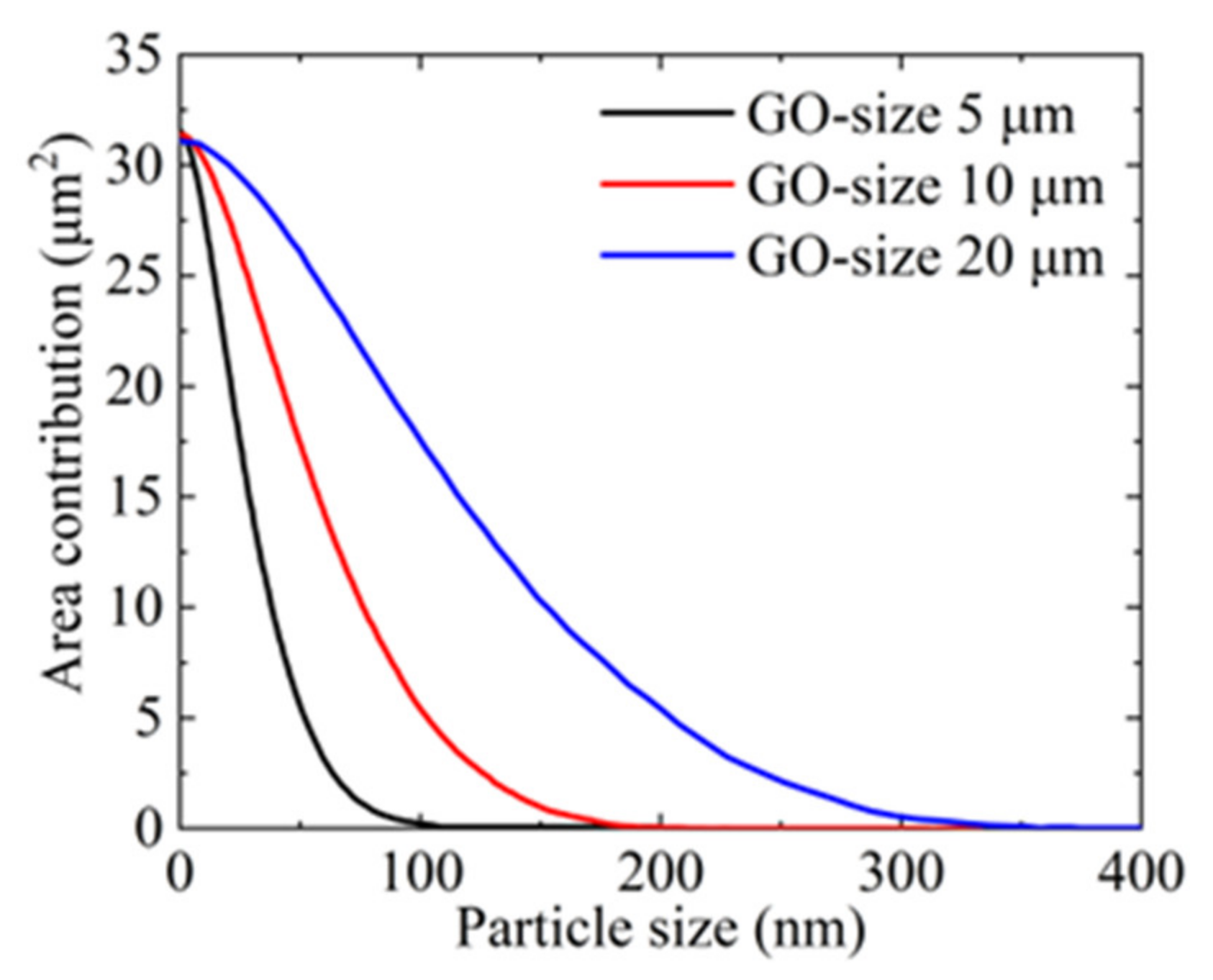
3.2. Effect of GO Flakes on the Formation of Holes
3.3. Promotional Applications
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kwon, O.; Choi, Y.; Choi, E.; Kim, M.; Woo, Y.C.; Kim, D.W. Fabrication Techniques for Graphene Oxide-Based Molecular Separation Membranes: Towards Industrial Application. Nanomaterials 2021, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Noble, R.D.; Falconer, J.L. Zeolite Membranes: Microstructure Characterization and Permeation Mechanisms. Accounts Chem. Res. 2011, 44, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.M.; Verweij, H. High-Selectivity, High-Flux Silica Membranes for Gas Separation. Science 1998, 279, 1710–1711. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Foley, H.C. Ultrasonic Deposition of High-Selectivity Nanoporous Carbon Membranes. Science 1999, 285, 1902–1905. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Ahmad, A.; Vijaya Bhaskar Reddy, A. Toxicology and Environmental Application of Carbon Nanocomposite. In Environmental Remediation Through Carbon Based Nano Composites; Springer: Singapore, 2021; pp. 1–18. [Google Scholar]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with Cavities Tuned for Fast Selective Transport of Small Molecules and Ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef]
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M.; Parveen, T.; Safian, M.T.-u. Environmental Applications of Smart Polymer Composites. In Smart Polymer Nanocomposites; Woodhead Publishing: Sawston, UK, 2021; pp. 295–312. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Li, D.; Xiong, Q.; Santoso, I.; Yu, X.; Chen, W.; Rusydi, A.; Wee, A.T.S. Anomalous Photoresponse in the Deep-Ultraviolet Due to Resonant Excitonic Effects in Oxygen Plasma Treated Few-Layer Graphene. Carbon 2016, 106, 330–335. [Google Scholar] [CrossRef]
- Qiu, R.; Xiao, J.; Chen, X.D.; Selomulya, C.; Zhang, X.; Woo, M.W. Relationship between Desalination Performance of Graphene Oxide Membranes and Edge Functional Groups. ACS Appl. Mater. Inter. 2020, 12, 4769–4776. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water through Helium-Leak-Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yoon, H.W.; Yoon, S.-M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective Gas Transport Through Few-Layered Graphene and Graphene Oxide Membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Su, Y.; Chi, C.; Cherian, C.T.; Huang, K.; Kravets, V.G.; Wang, F.C.; Zhang, J.C.; Pratt, A.; Grigorenko, A.N.; et al. Ultrathin Graphene-Based Membrane with Precise Molecular Sieving and Ultrafast Solvent Permeation. Nat. Mater. 2017, 16, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable Sieving of Ions Using Graphene Oxide Membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Liu, H.; Wang, K.; Zhong, M.; Wu, D.; Zhu, H. Selective Ion Transport Through Functionalized Graphene Membranes Based on Delicate Ion–Graphene Interactions. J. Phys. Chem. C 2014, 118, 19396–19401. [Google Scholar] [CrossRef]
- Sun, P.; Zheng, F.; Zhu, M.; Song, Z.; Wang, K.; Zhong, M.; Wu, D.; Little, R.B.; Xu, Z.; Zhu, H. Selective Trans-Membrane Transport of Alkali and Alkaline Earth Cations Through Graphene Oxide Membranes Based on Cation-π Interactions. ACS Nano 2014, 8, 850–859. [Google Scholar] [CrossRef]
- Morelos-Gomez, A.; Terashima, S.; Yamanaka, A.; Cruz-Silva, R.; Ortiz-Medina, J.; Sánchez-Salas, R.; Fajardo-Díaz, J.L.; Muñoz-Sandoval, E.; López-Urías, F.; Takeuchi, K.; et al. Graphene Oxide Membranes for Lactose-Free Milk. Carbon 2021, 181, 118–129. [Google Scholar] [CrossRef]
- Su, Y.; Kravets, V.G.; Wong, S.L.; Waters, J.; Geim, A.K.; Nair, R.R. Impermeable Barrier Films and Protective Coatings Based on Reduced Graphene Oxide. Nat. Commun. 2014, 5, 4843–4847. [Google Scholar] [CrossRef]
- Kanwal, A.; Yaqoob, A.A.; Siddique, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Umar, K. Hybrid Nanocomposites Based on Graphene and its Derivatives: From Preparation to Applications. In Graphene and Nanoparticles Hybrid Nanocomposites: From Preparation to Applications; Springer: Singapore, 2021; pp. 261–281. [Google Scholar]
- Huang, H.; Song, Z.; Wei, N.; Shi, L.; Mao, Y.; Ying, Y.; Sun, L.; Xu, Z.; Peng, X. Ultrafast Viscous Water Flow Through Nanostrand-Channelled Graphene Oxide Membranes. Nat. Commun. 2013, 4, 2979–2987. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nanofiltration Membrane Intercalated by Carbon Nanotubes. ACS Appl. Mater. Inter. 2015, 7, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Khansanami, M.; Esfandiar, A. High Flux and Complete Dyes Removal from Water by Reduced Graphene Oxide Laminate on Poly Vinylidene Fluoride/Graphene Oxide Membranes. Environ. Res. 2021, 201, 111576. [Google Scholar] [CrossRef]
- Beluci, N.d.C.L.; Homem, N.C.; Amorim, M.T.S.P.; Bergamasco, R.; Vieira, A.M.S. Biopolymer Extracted from Moringa Oleifera Lam. in Conjunction with Graphene Oxide to Modify Membrane Surfaces. Environ. Technol. 2020, 41, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.D.; Woellner, C.F.; Autreto, P.A.; Galvao, D.S. Water/Alcohol Separation in Graphene Oxide Membranes: Insights from Molecular Dynamics and Monte Carlo Simulations. Mrs Adv. 2018, 3, 109–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wang, C.; Zhang, Y.; Zhao, J.; Wei, N. Lamellar Water Induced Quantized Interlayer Spacing of Nanochannels Walls. Green Energy Environ. 2022. [Google Scholar] [CrossRef]
- Huang, P.Y.; Ruiz-Vargas, C.S.; Van Der Zande, A.M.; Whitney, W.S.; Levendorf, M.P.; Kevek, J.W.; Garg, S.; Alden, J.S.; Hustedt, C.J.; Zhu, Y. Grains and Grain Boundaries in Single-Layer Graphene Atomic Patchwork Quilts. Nature 2011, 469, 389–392. [Google Scholar] [CrossRef]
- Duong, D.L.; Han, G.H.; Lee, S.M.; Gunes, F.; Kim, E.S.; Kim, S.T.; Kim, H.; Ta, Q.H.; So, K.P.; Yoon, S.J.; et al. Probing Graphene Grain Boundaries with Optical Microscopy. Nature 2012, 490, 235–239. [Google Scholar] [CrossRef]
- Madauß, L.; Foller, T.; Plass, J.; Kumar, P.V.; Musso, T.; Dunkhorst, K.; Joshi, R.; Schleberger, M. Selective Proton Transport for Hydrogen Production Using Graphene Oxide Membranes. J. Phys. Chem. Lett. 2020, 11, 9415–9420. [Google Scholar] [CrossRef]
- Valencia, L.; Monti, S.; Kumar, S.; Zhu, C.; Liu, P.; Yu, S.; Mathew, A.P. Nanocellulose/Graphene Oxide Layered Membranes: Elucidating Their Behaviour During Filtration of Water and Metal Ions in Real Time. Nanoscale 2019, 11, 22413–22422. [Google Scholar] [CrossRef]
- Giri, A.K.; Teixeira, F.; Cordeiro, M.N.D. Salt Separation from Water Using Graphene Oxide Nanochannels: A Molecular Dynamics Simulation Study. Desalination 2019, 460, 1–14. [Google Scholar] [CrossRef]
- Saidi, P.; Béland, L.K.; Daymond, M.R. Graphene Oxide Membranes for Water Isotope Filtration: Insight at the Nano-and Microscale. J. Phys. Chem. C 2020, 124, 26864–26873. [Google Scholar] [CrossRef]
- Shih, C.-J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-Dependent Behavior of Graphene Oxide Aqueous Solutions: A Comparative Experimental and Molecular Dynamics Simulation Study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z. Multimodal and Self-Healable Interfaces Enable Strong and Tough Graphene-Derived Materials. J. Mech. Phys. Solids. 2014, 70, 30–41. [Google Scholar] [CrossRef]




| Abbreviation of the Variable | Means |
|---|---|
| boxl | Box length |
| boxw | Box width |
| bl | GO block length |
| bw | GO block width |
| bh | GO block height |
| nb | Number of GO block |
| ns | Number of the flake in the GO block |
| lb | Layer number of GO block |
| lg | The number of layers in the GO block |
| lbl | The number of layer where the GO block is located |
| gh | Interlayer spacing of GO flakes |
| α | The average coverage of GO blocks in each layer |
| c | Oxidation concentration of GO flakes |
| Particle | Particle Size (μm) |
|---|---|
| Viruses | 0.005–0.2 |
| Tobacco smoke | 0.01–1 |
| Dust | 0.01–100 |
| Bacteria | 0.05–40 |
| Fungus debris and spores | 0.7–80 |
| Human skin cells | 1–25 |
| Pollens | 10–100 |
| Hair | 17–100 |
| Sand | 80–750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, N.; Xu, K.; Guo, J.; Wei, N.; Yan, J. Graphene Oxide Membranes for Ionic/Molecular Filtration: From Self-Assembly to Design. Processes 2023, 11, 1520. https://doi.org/10.3390/pr11051520
Ma N, Xu K, Guo J, Wei N, Yan J. Graphene Oxide Membranes for Ionic/Molecular Filtration: From Self-Assembly to Design. Processes. 2023; 11(5):1520. https://doi.org/10.3390/pr11051520
Chicago/Turabian StyleMa, Niping, Ke Xu, Jianhua Guo, Ning Wei, and Junxia Yan. 2023. "Graphene Oxide Membranes for Ionic/Molecular Filtration: From Self-Assembly to Design" Processes 11, no. 5: 1520. https://doi.org/10.3390/pr11051520





