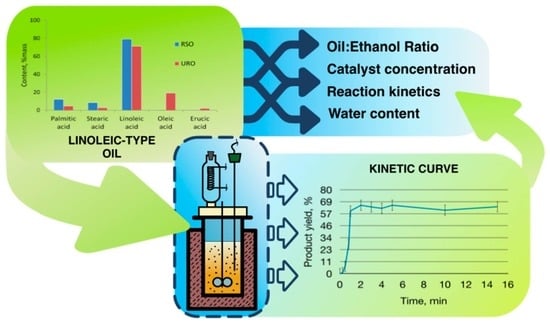
A New Approach for Synthesizing Fatty Acid Esters from Linoleic-Type Vegetable Oil
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Alcohol Drying
2.3. Synthesis of Fatty Acid Esters (FAEs)
2.3.1. Refined Sunflower Oil (RSO) Transesterification—Classical Method
2.3.2. Crude Rapeseed Oil (URO) Transesterification—Improved Method
2.4. Analysis of FAEs Composition
2.5. FTIR Analysis of FAEs
3. Results and Discussions
3.1. Influence of the Water Content
3.2. Influence of the Oil: Alcohol Ratio
3.3. Influence Catalyst Concentration
3.4. Process Kinetics
3.5. FAEs’ Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TFA | Triglycerides of Fatty Acids |
| FFA | Free Fatty Acids |
| FAE/FAEs | Fatty Acid Esters |
| RSO | Refined Sunflower Oil |
| URO | Unrefined Rapeseed Oil |
| FAE-RSO | Fatty Acid Esters of Refined Sunflower Oil |
| FAE-URO | Fatty Acid Esters of Unrefined Rapeseed Oil |
References
- Litvinenko, V.; Tsvetkov, P.; Dvoynikov, M.; Buslaev, G. Barriers to implementation of hydrogen initiatives in the context of global energy sustainable development. J. Min. Inst. 2020, 244, 421. [Google Scholar] [CrossRef]
- Pashkevich, M.; Bech, J.; Matveeva, V.; Alekseenko, A. Biogeochemical assessment of soils and plants in industrial, residential and recreational areas of Saint Petersburg. J. Min. Inst. 2020, 241, 125. [Google Scholar] [CrossRef]
- Litvinenko, V.; Bowbrick, I.; Naumov, I.; Zaitseva, Z. Global guidelines and requirements for professional competencies of natural resource extraction engineers: Implications for ESG principles and sustainable development goals. J. Clean. Prod. 2022, 338, 130530. [Google Scholar] [CrossRef]
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523. [Google Scholar] [CrossRef]
- Shishkova, I.; Stratiev, D.; Kolev, I.V.; Nenov, S.; Nedanovski, D.; Atanassov, K.; Ivanov, V.; Ribagin, S. Challenges in Petroleum Characterization—A Review. Energies 2022, 15, 7765. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Makhova, U.A.; Makhmudova, A.E.; Zuikov, A.V.; Kapustin, V.M.; Abdellatief, T.M.M.; Burov, N.O.; Geng, T.; Abdelkareem, M.A.; et al. Current Challenge and Innovative Progress for Producing HVO and FAME Biodiesel Fuels and Their Applications. Waste Biomass Valorization 2022, 14, 505–521. [Google Scholar] [CrossRef]
- Ershov, M.A.; Savelenko, V.D.; Shvedova, N.S.; Kapustin, V.M.; Abdellatief, T.M.M.; Karpov, N.V.; Dutlov, E.V.; Borisanov, D.V. An evolving research agenda of merit function calculations for new gasoline compositions. Fuel 2022, 322, 124209. [Google Scholar] [CrossRef]
- Tcvetkov, P. Climate Policy Imbalance in the Energy Sector: Time to Focus on the Value of CO2 Utilization. Energies 2021, 14, 411. [Google Scholar] [CrossRef]
- Sultanbekov, R.; Islamov, S.; Mardashov, D.; Beloglazov, I.; Hemmingsen, T. Research of the Influence of Marine Residual Fuel Composition on Sedimentation Due to Incompatibility. J. Mar. Sci. Eng. 2021, 9, 1067. [Google Scholar] [CrossRef]
- Litvinenko, V.; Petrov, E.; Vasilevskaya, D.; Yakovenko, A.; Naumov, I.; Ratnikov, M. Assessment of the role of the state in the management of mineral resources. J. Min. Inst. 2023, 259, 95–111. [Google Scholar] [CrossRef]
- Ershov, M.; Potanin, D.; Gueseva, A.; Abdellatief, T.M.M.; Kapustin, V. Novel strategy to develop the technology of high-octane alternative fuel based on low-octane gasoline Fischer-Tropsch process. Fuel 2020, 261, 116330. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Politaeva, N.; Smyatskaya, Y.; Ivanova, A. Lemna Minor Cultivation for Biofuel Production. IOP Conf. Ser. Earth Environ. Sci. 2019, 272, 022058. [Google Scholar] [CrossRef]
- Sverguzova, S.; Sapronova, Z.; Zubkova, O.; Svyatchenko, A.; Shaikhieva, K.; Voronina, Y. Electric steelmaking dust as a raw material for coagulant production. J. Min. Inst. 2023, 260, 279–288. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Kononchuk, O.; Alekseev, A.; Zubkova, O.; Udovitsky, V. Scientific Background for Processing of Aluminum Waste. E3S Web Conf. 2017, 21, 02003. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Ivanov, M.; Dinkov, R.; Argirov, G.; Vasilev, S.; Yordanov, D. Validation of Diesel Fraction Content in Heavy Oils Measured by High Temperature Simulated Distillation and Physical Vacuum Distillation by Performance of Commercial Distillation Test and Process Simulation. Appl. Sci. 2022, 12, 11824. [Google Scholar] [CrossRef]
- Ershov, M.A.; Grigorieva, E.V.; Abdellatief, T.M.M.; Chernysheva, E.A.; Makhin, D.Y.; Kapustin, V.M. A new approach for producing mid-ethanol fuels E30 based on low-octane hydrocarbon surrogate blends. Fuel Process. Technol. 2021, 213, 106688. [Google Scholar] [CrossRef]
- Boutesteijn, C.; Drabik, D.; Venus, T.J. The interaction between EU biofuel policy and first- and second-generation biodiesel production. Ind. Crops Prod. 2017, 106, 124–129. [Google Scholar] [CrossRef]
- Janda, K.; Stankus, E. Munich Personal RePEc Archive Biofuels Markets and Policies in Russia; University Library of Munich: Munich, Germany, 2017. [Google Scholar]
- Balajii, M.; Niju, S. Biochar-derived heterogeneous catalysts for biodiesel production. Environ. Chem. Lett. 2019, 17, 1447–1469. [Google Scholar] [CrossRef]
- Pashkevich, M.A.; Kharko, P.A. The use of a composite mix to remove metals from acidic drainage waters at tailings facilities. Obogashchenie Rud 2022, 4, 40–47. [Google Scholar] [CrossRef]
- Cheremisina, E.; Cheremisina, O.; Ponomareva, M.; Bolotov, V.; Fedorov, A. Kinetic features of the hydrogen sulfide sorption on the ferro-manganese material. Metals 2021, 11, 90. [Google Scholar] [CrossRef]
- Murad, P.C.; Hamerski, F.; Corazza, M.L.; Luz, L.F.L.; Voll, F.A.P. Acid-catalyzed esterification of free fatty acids with ethanol: An assessment of acid oil pretreatment, kinetic modeling and simulation. React. Kinet. Mech. Catal. 2018, 123, 505–515. [Google Scholar] [CrossRef]
- Lucena, I.L.; Saboya, R.M.A.; Oliveira, J.F.G.; Rodrigues, M.L.; Torres, A.E.B.; Cavalcante, C.L.; Parente, E.J.S.; Silva, G.F.; Fernandes, F.A.N. Oleic acid esterification with ethanol under continuous water removal conditions. Fuel 2011, 90, 902–904. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Dinkov, R.; Nenov, S.; Sotirov, S.; Sotirova, E.; Kolev, I.; Ivanov, V.; Ribagin, S.; Atanassov, K.; et al. Prediction of petroleum viscosity from molecular weight and density. Fuel 2023, 331, 125679. [Google Scholar] [CrossRef]
- Chew, S.C.; Ali, M.A. Recent advances in ultrasound technology applications of vegetable oil refining. Trends Food Sci. Technol. 2021, 116, 468–479. [Google Scholar] [CrossRef]
- Gea, S.; Irvan, I.; Wijaya, K.; Nadia, A.; Pulungan, A.N.; Sihombing, J.L.; Rahayu, R. Bio-oil hydrodeoxygenation over acid activated-zeolite with different Si/Al ratio. Biofuel Res. J. 2022, 9, 1630–1639. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Atabani, A.E.; Chong, W.T. A global comparative review of biodiesel production from jatropha curcas using different homogeneous acid and alkaline catalysts: Study of physical and chemical properties. Renew. Sustain. Energy Rev. 2013, 24, 514–533. [Google Scholar] [CrossRef]
- Raita, M.; Champreda, V.; Laosiripojana, N. Biocatalytic ethanolysis of palm oil for biodiesel production using microcrystalline lipase in tert-butanol system. Process Biochem. 2010, 45, 829–834. [Google Scholar] [CrossRef]
- Salisbury, C.; O’Cathain, A.; Edwards, L.; Thomas, C.; Gaunt, D.; Hollinghurst, S.; Nicholl, J.; Large, S.; Yardley, L.; Lewis, G.; et al. Effectiveness of an integrated telehealth service for patients with depression: A pragmatic randomised controlled trial of a complex intervention. Lancet Psychiatry 2016, 3, 515–525. [Google Scholar] [CrossRef]
- Eremeeva, A.M.; Kondrasheva, N.K.; Khasanov, A.F.; Oleynik, I.L. Environmentally Friendly Diesel Fuel Obtained from Vegetable Raw Materials and Hydrocarbon Crude. Energies 2023, 16, 2121. [Google Scholar] [CrossRef]
- de Oliveira, V.F.; Parente, E.J.S.; Manrique-Rueda, E.D.; Cavalcante, C.L.; Luna, F.M.T. Fatty acid alkyl esters obtained from babassu oil using C1–C8 alcohols and process integration into a typical biodiesel plant. Chem. Eng. Res. Des. 2020, 160, 224–232. [Google Scholar] [CrossRef]
- Oloyede, C.T.; Jekayinfa, S.O.; Alade, A.O.; Ogunkunle, O.; Laseinde, O.T.; Adebayo, A.O.; Abdulkareem, A.I.; Smaisim, G.F.; Fattah, I.M.R. Synthesis of Biobased Composite Heterogeneous Catalyst for Biodiesel Production Using Simplex Lattice Design Mixture: Optimization Process by Taguchi Method. Energies 2023, 16, 2197. [Google Scholar] [CrossRef]
- Kondrasheva, N.; Eremeeva, A. Production of biodiesel fuel from vegetable raw materials. J. Min. Inst. 2023, 260, 248–256. [Google Scholar] [CrossRef]
- Lugani, Y.; Rai, R.; Prabhu, A.A.; Maan, P.; Hans, M.; Kumar, V.; Kumar, S.; Chandel, A.K.; Sengar, R.S. Recent advances in bioethanol production from lignocelluloses: A comprehensive review with a focus on enzyme engineering and designer biocatalysts. Biofuel Res. J. 2020, 7, 1267–1295. [Google Scholar] [CrossRef]
- Belozertseva, N.E.; Bogdanov, I.A.; Altynov, A.A.; Balzhanova, A.T.; Belinskaya, N.S.; Kirgina, M.V. Selection of the most beneficial raw materials for the synthesis of biodiesel from a standpoint of its yield and physicochemical properties. Proc. Univ. Appl. Chem. Biotechnol. 2020, 10, 114–123. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Abdulahad, W.S. Transesterification of mustard (Brassica nigra) seed oil with ethanol: Purification of the crude ethyl ester with activated carbon produced from de-oiled cake. Energy Convers. Manag. 2014, 77, 495–503. [Google Scholar] [CrossRef]
- Pinnarat, T.; Savage, P.E. Noncatalytic esterification of oleic acid in ethanol. J. Supercrit. Fluids 2010, 53, 53–59. [Google Scholar] [CrossRef]
- Santana, H.S.; Tortola, D.S.; Reis, É.M.; Silva, J.L.; Taranto, O.P. Transesterification reaction of sunflower oil and ethanol for biodiesel synthesis in microchannel reactor: Experimental and simulation studies. Chem. Eng. J. 2016, 302, 752–762. [Google Scholar] [CrossRef]
- Veljković, V.B.; Biberdžić, M.O.; Banković-Ilić, I.B.; Djalović, I.G.; Tasić, M.B.; Nježić, Z.B.; Stamenković, O.S. Biodiesel production from corn oil: A review. Renew. Sustain. Energy Rev. 2018, 91, 531–548. [Google Scholar] [CrossRef]
- Natarajan, Y.; Nabera, A.; Salike, S.; Dhanalakshmi Tamilkkuricil, V.; Pandian, S.; Karuppan, M.; Appusamy, A. An overview on the process intensification of microchannel reactors for biodiesel production. Chem. Eng. Process.-Process Intensif. 2019, 136, 163–176. [Google Scholar] [CrossRef]
- Permyakova, I.A.; Vol’khin, V.V.; Kazakov, D.A.; Kaczmarski, K.; Kudryashova, O.S.; Sukhoplecheva, E.A. Phase Equilibria in Triacylglycerols–Ethanol–Oleic Acid–Athyl Oleate Quasi-Quaternary System. Eurasian Chem. J. 2014, 16, 257–264. [Google Scholar] [CrossRef]
- Golubev, V.; Litvinova, T. Dynamic simulation of industrial-scale gibbsite crystallization circuit. J. Min. Inst. 2021, 247, 88–101. [Google Scholar] [CrossRef]
- Raupov, I.; Burkhanov, R.; Lutfullin, A.; Maksyutin, A.; Lebedev, A.; Safiullina, E. Experience in the Application of Hydrocarbon Optical Studies in Oil Field Development. Energies 2022, 15, 3626. [Google Scholar] [CrossRef]
- Cheremisina, O.; Litvinova, T.; Sergeev, V.; Ponomareva, M.; Mashukova, J. Application of the Organic Waste-Based Sorbent for the Purification of Aqueous Solutions. Water 2021, 13, 3101. [Google Scholar] [CrossRef]
- Povarov, V.; Efimov, I. Use of the UNIFAC model in the calculation of physicochemical properties of ecotoxicants for technological and ecoanalytical purposes. J. Min. Inst. 2023, 260, 238–247. [Google Scholar] [CrossRef]
- Gerasimov, A.; Ustinov, I.; Zyryanova, O. Use of clay-containing waste as pozzolanic additives. J. Min. Inst. 2023, 260, 313–320. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Uemura, Y. Isopropanolysis of Cottonseed Oil to Biodiesel via Potassium Hydroxide Catalyst. Procedia Eng. 2016, 148, 473–478. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Rodríguez, J.J.; Tejedor, A. Biodiesel fuels from vegetable oils: Transesterification of Cynara cardunculus L. Oils with ethanol. Energy Fuels 2002, 16, 443–450. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J. Optimization of Brassica carinata oil methanolysis for biodiesel production. J. Am. Oil Chem. Soc. 2005, 82, 899–904. [Google Scholar] [CrossRef]
- Crapiste, G.H.; Brevedan, M.I.V.; Carelli, A.A. Oxidation of sunflower oil during storage. J. Am. Oil Chem. Soc. 1999, 76, 1437–1443. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Barrios, C.C.; Alés-Álvarez, F.J.; Dominguez-Sáez, A.; Alvarez-Mateos, P. Biodiesel production from waste cooking oil in an oscillatory flow reactor. Performance as a fuel on a TDI diesel engine. Renew. Energy 2018, 125, 546–556. [Google Scholar] [CrossRef]
- Knerelman, E.I.; Yarullin, R.S.; Davydova, G.I.; Startseva, G.P.; Churkina, V.Y.; Matkovsky, P.E.; Aldoshin, S.M. Comparative features of the infrared spectra C18-carboxic acids, their methyl esters (biodiesel) and triglycerides (vegetable oils). Vestn. Kazan. Tekhnol. Univ. 2008, 6, 68–78. [Google Scholar]
- Sultanova, G.I.; Sayfetdinova, G.A.; Rakhmatullina, A.P.; Akhmedyanova, R.A.; Liakumovich, A.G. Effect of potassium salts of stearic and oleic acids on the emulsion copolymerization of styrene and alpha-methylstyrene. Bull. Kazan Technol. Univ. 2006, 2, 67–71. [Google Scholar]
- Gomes, M.G.; Santos, D.Q.; De Morais, L.C.; Pasquini, D. Purification of biodiesel by dry washing, employing starch and cellulose as natural adsorbents. Fuel 2015, 155, 1–6. [Google Scholar] [CrossRef]


















| Alcohol | Molecular Formula | MPC of the Working Area, mg/m3 |
|---|---|---|
| Methanol | CH3OH | 5 |
| Ethanol | C2H5OH | 1000–2000 |
| Propanol | C3H7OH | 10 |
| Butanol | C4H9OH | 10 |
| Oil Type | Acid Value, mg KOH/g Oil | % Acid | Moisture, %Mass |
|---|---|---|---|
| Refined Sunflower Oil | 3.49 | 1.76 | 0.076 |
| Unrefined Rapeseed Oil | 32.36 | 16.27 | 0.063 |
| No. Test Series | 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|---|
| Temperature, °C | 70 | 70 | 70 | 70 | ||
| Stirring speed, rpm | 200 ÷ 250 | 200 ÷ 250 | 200 ÷ 250 | 200 ÷ 250 | ||
| Catalyst | KOH | NaOH | KOH | NaOH | KOH | KOH |
| Catalyst quantity, mass% (by weight of alcohol) | 1 | 1 | 0.5 ÷ 2.5 | 1 | ||
| Ethanol drying degree, %vol. | 95 | 95 | 95 | 91 ÷ 99 | ||
| Synthesis time, hour | 2.5 | 0 ÷ 2.5 | 2.5 | 2.5 | ||
| Val/Voil | 1÷5 | 3 | 3 | 3 | ||
| No. Test Series | 0 | 1 | 2 | 3 | 4 | ||
|---|---|---|---|---|---|---|---|
| Temperature, °C | 70 | 70 | 70 | 70 | 70 | ||
| Stirring speed, rpm | 200 ÷ 250 | 200 ÷ 250 | 200 ÷ 250 | 200 ÷ 250 | 200 ÷ 250 | ||
| Catalyst | KOH | KOH | KOH | KOH | KOH | ||
| Catalyst quantity, mass% (by weight of alcohol) | 1 | 1 | 0.25 ÷ 2 | 0.5 | 1 | 0.5 | |
| Ethanol drying degree, % vol. | 93 | 91 ÷ 99 | 95 | 99 | 99 | 99 | |
| Synthesis time, hour | 2.5 | 2.5 | 2.5 | 2.5 | 0 ÷ 2.5 | ||
| Val/Voil | 3 | 3 | 3 | 1 ÷ 5 | 3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosolapova, S.M.; Smal, M.S.; Rudko, V.A.; Pyagay, I.N. A New Approach for Synthesizing Fatty Acid Esters from Linoleic-Type Vegetable Oil. Processes 2023, 11, 1534. https://doi.org/10.3390/pr11051534
Kosolapova SM, Smal MS, Rudko VA, Pyagay IN. A New Approach for Synthesizing Fatty Acid Esters from Linoleic-Type Vegetable Oil. Processes. 2023; 11(5):1534. https://doi.org/10.3390/pr11051534
Chicago/Turabian StyleKosolapova, Sofia M., Makar S. Smal, Viacheslav A. Rudko, and Igor N. Pyagay. 2023. "A New Approach for Synthesizing Fatty Acid Esters from Linoleic-Type Vegetable Oil" Processes 11, no. 5: 1534. https://doi.org/10.3390/pr11051534
APA StyleKosolapova, S. M., Smal, M. S., Rudko, V. A., & Pyagay, I. N. (2023). A New Approach for Synthesizing Fatty Acid Esters from Linoleic-Type Vegetable Oil. Processes, 11(5), 1534. https://doi.org/10.3390/pr11051534