Development of Geopolymer Mortars Using Air-Cooled Blast Furnace Slag and Biomass Bottom Ashes as Fine Aggregates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Geopolymeric Mortar Preparation
2.2.2. Mortar Characterization
2.2.3. Leaching Study
3. Results and Discussion
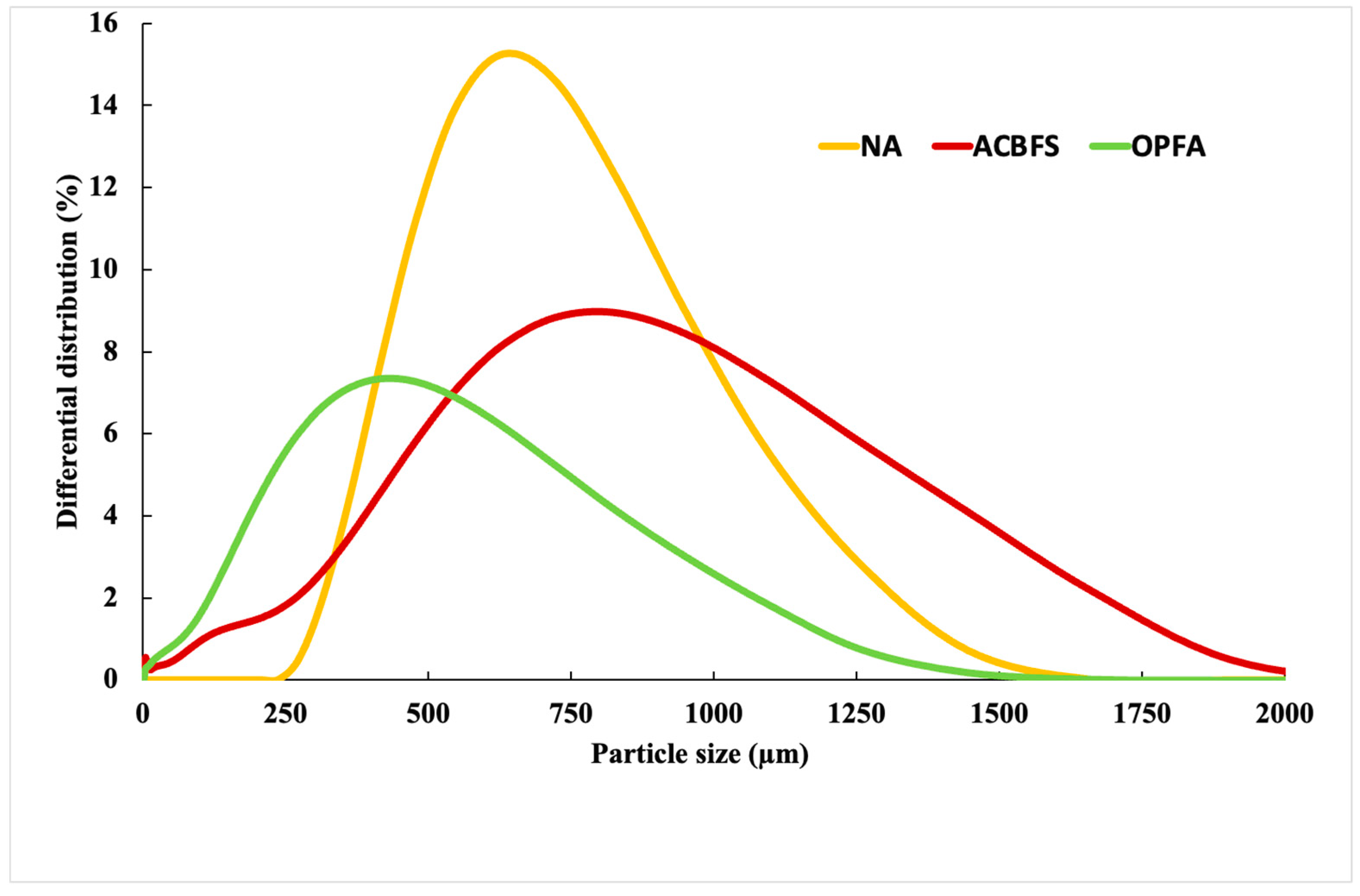
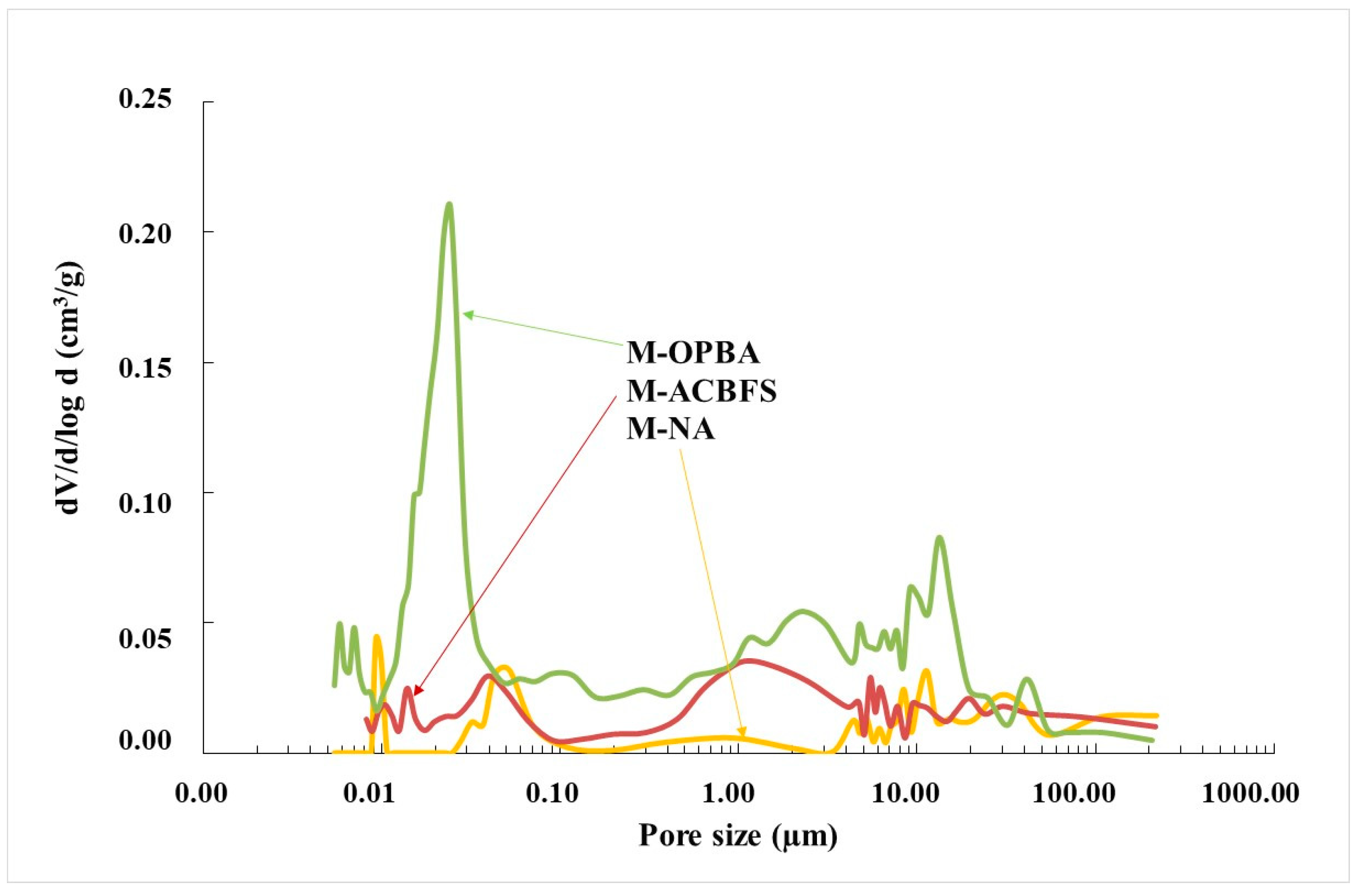
3.1. Physical and Mechanical Properties
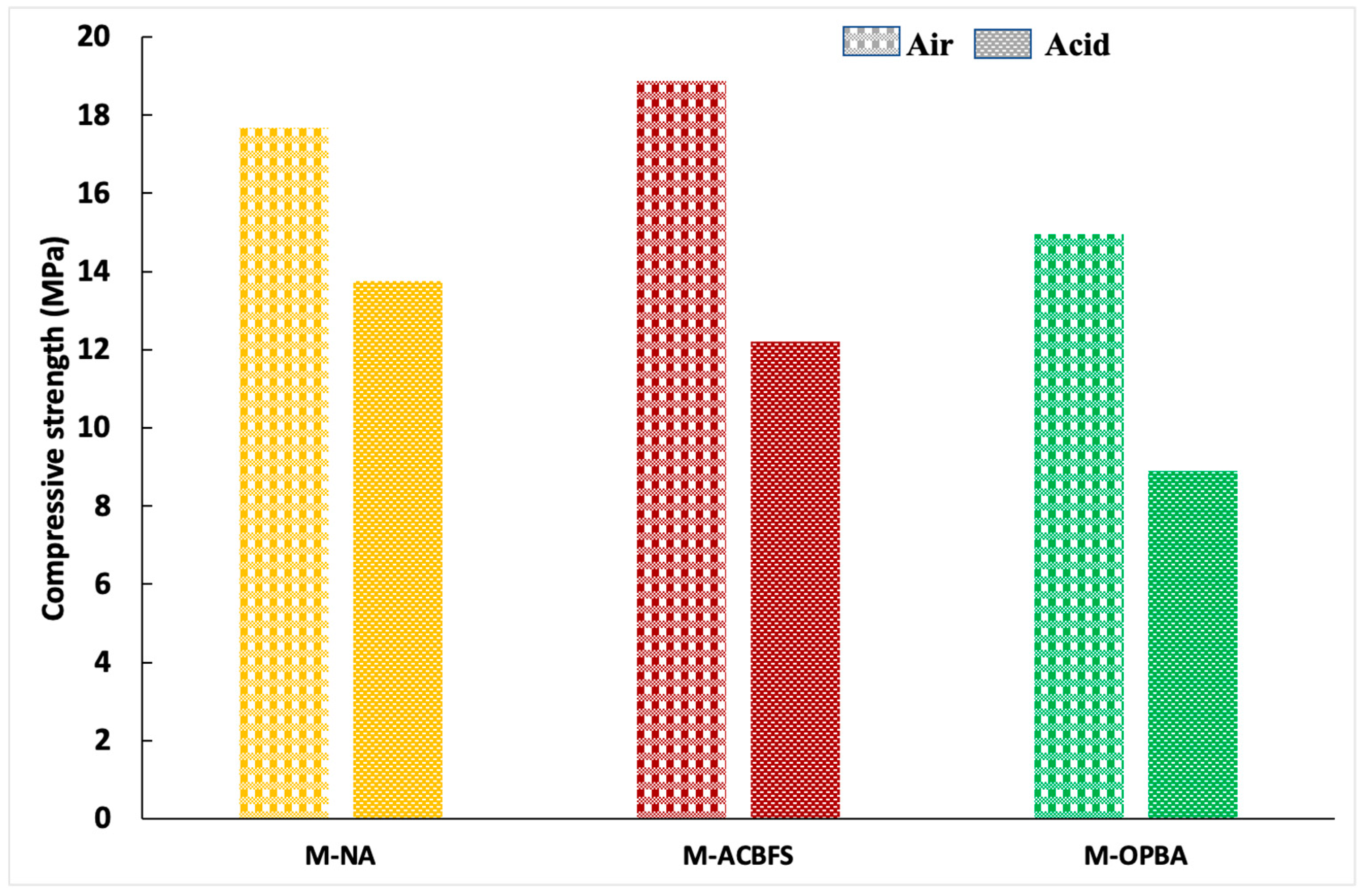
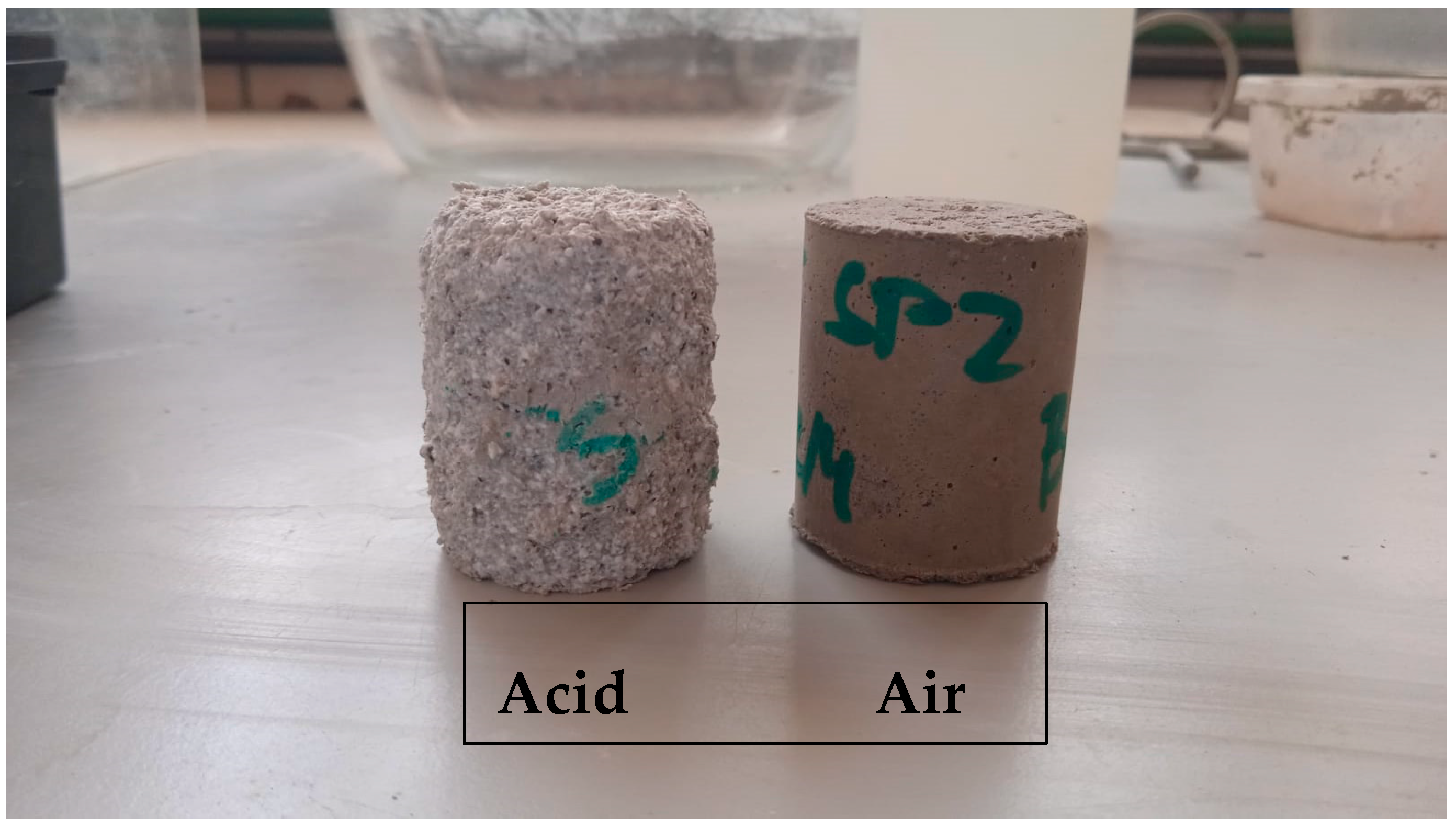
3.2. Acid Attack Test
3.3. Leaching Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peceño, B.; Bakit, J.; Cortes, N.; Alonso-Fariñas, B.; Bonilla, E.; Leiva, C. Assessing Durability Properties and Economic Potential of Shellfish Aquaculture Waste in the Construction Industry: A Circular Economy Perspective. Sustainability 2022, 14, 8383. [Google Scholar] [CrossRef]
- Alonso-Fariñas, B.; Rodríguez-Galán, M.; Arenas, C.; Torralvo, F.A.; Leiva, C. Sustainable management of spent fluid catalytic cracking catalyst from a circular economy approach. Waste Manag. 2020, 110, 10–19. [Google Scholar] [CrossRef]
- Lu, X.; Liu, B.; Zhang, Q.; Wen, Q.; Wang, S.; Xiao, K.; Zhang, S. Recycling of Coal Fly Ash in Building Materials: A Review. Minerals 2023, 13, 25. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. Effect of source materials on geopolymerisation. Eng. Chem. Res. 2003, 42, 1698–1716. [Google Scholar] [CrossRef]
- Luna-Galiano, Y.; Leiva, C.; Arenas, C.; Arroyo, F.; Vilches, L.F.; Villegas, R.; Fernández-Pereira, C. Behaviour of Fly Ash-Based Geopolymer Panels Under Fire. Waste Biomass Valorization 2017, 8, 2485–2494. [Google Scholar] [CrossRef]
- Luna Galiano, Y.; Fernández Pereira, C.; Pérez, C.M.; Suarez, P. Influence of BFS content in the mechanical properties and acid attack resistance of fly ash based geopolymers. Key Eng. 2016, 663, 50–61. [Google Scholar] [CrossRef]
- Lu, N.; Ran, X.; Pan, Z.; Korayem, A.H. Use of Municipal Solid Waste Incineration Fly Ash in Geopolymer Masonry Mortar Manufacturing. Materials 2022, 15, 8689. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Yan, C.; Yang, J.; Wu, Z. Design and Properties of Coal Gangue-Based Geopolymer Mortar. Buildings 2022, 12, 1932. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Bignozzi, M.C.; Sandrolini, F. New blended cement from polishing and grazing ceramic sludge. Int. J. Appl. Ceram. 2010, 7, 546–555. [Google Scholar] [CrossRef]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical stability of geopolymers containing municipal solid waste incinerator fly ash. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef]
- Rapazote, J.G.; Laginhas, C.; Teixeira-Pinto, A. Developed of building materials through alkaline activation of construction and demolition waste (CDW)-Resistance to acid attack. Adv. Sci. Technol. 2010, 69, 159–163. [Google Scholar] [CrossRef]
- Vaou, V.; Panias, D. Thermal insulating foamy geopolymers from perlite. Miner. Eng. 2010, 23, 1146–1151. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Dasu, J.; Murthy, Y.R.; Kapure, G.; Pal, A.R.; Filippov, L.O. Utilization perspective on water quenched and air-cooled blast furnace slags. J. Clean. Prod. 2020, 262, 121354. [Google Scholar] [CrossRef]
- Ríos, J.D.; Vahí, A.; Leiva, C.; Martínez-De la Concha, A.M.; Cifuentes, H. Analysis of the utilization of air-cooled blast furnace slag as industrial waste aggregates in self-compacting concrete. Sustainability 2019, 11, 1702. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.D.; Arenas, C.; Cifuentes, H.; Vilches, L.F.; Leiva, C. Development of a paste for passive fire protection mainly composed of granulated blast furnace slag. Environ. Prog. Sustain. Energy 2020, 39, e13382. [Google Scholar] [CrossRef]
- Azad, N.M.; Samarakoon, S.M.S.M.K. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Abdel-Ghania, N.T.; El-Sayedb, H.A.; El-Habak, A.A. Utilization of by-pass cement kiln dust and air-cooled blast-furnace steel slag in the production of some “green” cement products. HBRC J. 2018, 14, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Tole, I.; Rajczakowska, M.; Humad, A.; Kothari, A.; Cwirzen, A. Geopolymer Based on Mechanically Activated Air-cooled Blast Furnace Slag. Materials 2020, 13, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, B.-H.; Lee, S.-J.; Park, C.-G. Physical and Mechanical Properties of Rural-Road Pavement Concrete in South Korea Containing Air-Cooled Blast-Furnace Slag Aggregates. Appl. Sci. 2021, 11, 5645. [Google Scholar] [CrossRef]
- Arenas, C.; Ríos, J.D.; Cifuentes, H.; Vilches, L.F.; Leiva, C. Sound absorbing porous concretes composed of different solid wastes. Eur. J. Environ. Civ. Eng 2020, 26, 3805–3817. [Google Scholar] [CrossRef]
- Skevi, L.; Baki, V.A.; Feng, Y.; Valderrabano, M.; Ke, X. Biomass Bottom Ash as Supplementary Cementitious Material: The Effect of Mechanochemical Pre-Treatment and Mineral Carbonation. Materials 2022, 15, 8357. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Eliche-Quesada, D.; Carrasco-Hurtado, B.; Sánchez-Soto, P.J. Valorization of Olive Biomass Fly Ash for Production Eco Friendly Ceramic Bricks. Encycl. Renew. Sustain. Mater. 2020, 5, 285–294. [Google Scholar] [CrossRef]
- Eliche-Quesada, J.; Leite-Costa, J. Use of bottom ash from olive pomace combustion in the production of eco-friendly fired clay bricks. Waste Manag. 2016, 48, 323–333. [Google Scholar] [CrossRef]
- Rosales, M.; Rosales, J.; Agrela, F.; de Rojas, M.I.S.; Cabrera, M. Design of a new eco-hybrid cement for concrete pavement, made with processed mixed recycled aggregates and olive biomass bottom ash as supplementary cement materials. Constr. Build. Mater. 2022, 358, 129417. [Google Scholar] [CrossRef]
- Peceño, B.; Hurtado-Bermudez, S.; Alonso-Fariñas, B.; Villa-Alfageme, M.; Más, J.L.; Leiva, C. Recycling Bio-Based Wastes into Road-Base Binder: Mechanical, Leaching, and Radiological Implications. Appl. Sci. 2023, 13, 1644. [Google Scholar] [CrossRef]
- Cabrera, M.; Díaz-López, J.L.; Agrela, F.; Rosales, J. Eco-Efficient Cement-Based Materials Using Biomass Bottom Ash: A Review. Appl. Sci. 2020, 10, 8026. [Google Scholar] [CrossRef]
- Beltrán, M.G.; Barbudo, A.; Agrela, F.; Jiménez, J.R.; de Brito, J. Mechanical performance of bedding mortars made with olive biomass bottom ash. Constr. Build. Mater. 2016, 112, 699–707. [Google Scholar] [CrossRef]
- Leiva, C.; Gómez-Barea, A.; Vilches, L.F.; Ollero, P.; Vale, J.; Fernández-Pereira, C. Use of biomass gasification fly ash in lightweight plasterboard. Energy Fuels 2007, 21, 361–367. [Google Scholar] [CrossRef]
- Nogales, R.; Delgado, G.; Quirantes, M.; Romero, M.; Romero, E.; Molina-Alcaide, E. Characterization of Olive Waste Ashes as Fertilizers. In Recycling of Biomass Ashes; Insam, H., Knapp, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. Spanish Association for Standardization and Certification: Madrid, Spain, 2018.
- Available online: http://www.icdd.com (accessed on 16 May 2023).
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- El-Mir, A.; El-Hassan, H.; El-Dieb, A.; Alsallamin, A. Development and Optimization of Geopolymers Made with Desert Dune Sand and Blast Furnace Slag. Sustainability 2022, 14, 7845. [Google Scholar] [CrossRef]
- EN 1936; Natural Stone Test Methods—Determination of Real Density and Apparent Density, and of Total and Open Porosity. Spanish Association for Standardization and Certification: Madrid, Spain, 2007.
- EN 1015-11; Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. Spanish Association for Standardization and Certification: Madrid, Spain, 2020.
- Leiva, C.; Arenas, L.F.V.; Vilches, L.F.; Arroyo, F.; Luna-Galiano, Y. Assessing durability properties of noise barriers made of concrete incorporating bottom ash as aggregates. Eur. J. Environ. Civ. Eng. 2019, 23, 1485–1496. [Google Scholar] [CrossRef]
- EN 998-2; Specification for Mortar for Masonry—Part 2: Masonry Mortar. Spanish Association for Standardization and Certification: Madrid, Spain, 2018.
- EN 12457-4; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 4: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size below 10 mm (without or with Size Reduction). Spanish Association for Standardization and Certification: Madrid, Spain, 2003.
- US EPA. Toxicity Characteristics Leaching Procedure, Method 1311. Test Methods for the Evaluation of Solid Waste. 1992. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/1311.pd (accessed on 10 March 2023).
- NEN 7375; Leaching Characteristics—Determination of the Leaching of Inorganic Components from Moulded or Monolitic Materials with a Diffusion Test—Solid Earthy and Stony Materials. NEN (Netherlands Standardization Institute): Delf, The Netherlands, 2005.
- US EPA. Method 1315: Mass Transfer Rates of Constituents in Monolithic or Compacted Granular Materials Using a Semi-Dynamic Tank Leaching Procedure. In Test Methods for Evaluating Solid Waste, Physical/Chemical Methods; US Environmental Protection Agency: Washington, DC, USA, 1986. [Google Scholar]
- Council Directive 1999/31/EC of 26 April 1999 on the landfill of Waste. Official Journal L 182, 16/07/1999 P. 0001–0019. European Commission, 1999. Available online: http://data.europa.eu/eli/dir/1999/31/oj (accessed on 8 March 2022).
- DL 183/2009; Waste Disposal at Landfills. Transposition to the Portuguese Law of Council. Directive 1999/31/CE, April 26. Portuguese Official Journal, Portuguese Mint and Official Printing Office: Lisbon, Portugal, 2009.
- Ministero dell’Ambiente e Della Tutela Del Territorio. Decreto 5 Aprile 2006, n 186. Regolamento Recante Modifiche al Decreto Ministeriale 5 Febbraio 1998 «Individuazione Dei Rifiuti Non Pericolosi Sottoposti Alle Procedure Semplificate Di Recupero, ai Sensi Degli Articoli 31 e 33 Del Decreto Legislativo 5 Febbraio 1997, n. 22». Gazzeta Ufficiale, GU Serie Generale n.115 Del 19-05-2006, Italia, Roma. 2006. Available online: https://www.gazzettaufficiale.it/eli/id/2006/05/19/006G0202/sg (accessed on 10 March 2023).
- Decreto 100/2018 de Valorización de Escorias en la Comunidad Autónoma de Cantabria. Cantabria, Spain, 2019. Available online: https://boc.cantabria.es/boces/verAnuncioAction.do?idAnuBlob=333876 (accessed on 8 March 2022).
- Decreto 34 del País Vasco por el que se Regula la Valorización y Posterior Utilización de Escorias Procedentes de la Fabricación de Acero en Hornos de arco Eléctrico, en el Ámbito de la Comunidad Autónoma del País Vasco. País Vasco, Spain, 2003. Available online: https://www.legegunea.euskadi.eus/eli/es-pv/d/2003/02/18/34/dof/spa/html/webleg00-contfich/es/ (accessed on 8 March 2022).
- Soares, I.; Nobre, F.X.; Vasconcelos, R.; Ramírez, M.A. Study of Metakaolinite Geopolymeric Mortar with Plastic Waste Replacing the Sand: Effects on the Mechanical Properties, Microstructure, and Efflorescence. Materials 2022, 15, 8626. [Google Scholar] [CrossRef] [PubMed]
- ASTM C270-19; Standard Specification for Mortar for Unit Masonry. ASTM International: West Conshohocken, PA, USA, 2019.




| Chemical Composition (% Weight) | BFS | Natural Aggregate | ACBFS | OPBA |
|---|---|---|---|---|
| CaO | 43.46 | 0.59 | 42.14 | 16.5 |
| SiO2 | 35.82 | 86.5 | 34.76 | 45.4 |
| Al2O3 | 11.60 | 5.83 | 9.12 | 10.4 |
| MgO | 7.59 | 0.13 | 6.06 | 5.0 |
| SO3 | - | 0.04 | 1.77 | - |
| TiO2 | - | 0.13 | 0.76 | - |
| K2O | 0.36 | 2.37 | 0.54 | 17.2 |
| Fe2O3 | 1.01 | 1.33 | 0.42 | 4.2 |
| MnO2 | - | - | 0.41 | - |
| Na2O | 0.21 | 0.87 | 0.19 | 1.7 |
| BaO | - | - | 0.11 | - |
| P2O5 | - | 0.07 | - | - |
| MnO | - | 0.03 | - | - |
| Specific gravity (g/ | 2.93 | 2.71 | 2.91 | 2.05 |
| Loss on ignition (%) | 1.47 | 1.34 | 1.49 | 9.30 |
| Fine Aggregates | NaOH/BFS | Fine Aggregate/BFS | SP/BFS | H2O/BFS |
|---|---|---|---|---|
| M-NA | 0.3 | 3 | 0.078 | 0.027 |
| M-CBFS | 0.3 | 3 | 0.078 | 0.168 |
| M-OPBA | 0.3 | 3 | 0.078 | 0.503 |
| Fine Aggregates | Density (kg/m3) | Compressive Strength (MPa) | Flexural Strength (MPa) |
|---|---|---|---|
| M-NA | 2313 ± 24 | 17.6 ± 1.1 | 2.5 ± 0.2 |
| M-CBFS | 2316 ± 34 | 18.9 ± 0.9 | 2.7 ± 0.1 |
| M-OPBA | 1712 ± 15 | 14.9 ± 0.7 | 2.0 ± 0.1 |
| BFS | NA | OPBA | ACBFS | Inert Waste | Non-Hazardous Waste | Hazardous Waste | |
|---|---|---|---|---|---|---|---|
| As | <0.2 | <0.2 | 1.8 | <0.2 | 0.5 | 2 | 25 |
| Zn | <0.25 | <0.25 | <0.25 | <0.25 | 4 | 50 | 200 |
| V | 0.19 | - | - | 0.16 | - | - | - |
| Sn | <0.25 | - | - | <0.25 | - | - | - |
| Se | <0.04 | <0.04 | 0.4 | 0.07 | 0.1 | 0.5 | 7 |
| Sb | <0.05 | <0.05 | 0.2 | <0.05 | 0.06 | 0.7 | 5 |
| Pb | <0.2 | <0.2 | 9.3 | <0.2 | 0.5 | 10 | 50 |
| Ni | <0.05 | <0.05 | 1.3 | <0.05 | 0.4 | 10 | 40 |
| Mo | <0.2 | <0.2 | 1.8 | <0.2 | 0.5 | 10 | 30 |
| Hg | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | 0.2 | 2 |
| Cu | <0.1 | <0.1 | 6.7 | <0.1 | 2 | 50 | 100 |
| Cr | <0.1 | <0.1 | <0.1 | <0.1 | 0.5 | 10 | 70 |
| Co | <0.02 | <0.02 | <0.02 | <0.02 | - | - | - |
| Cd | <0.02 | <0.02 | <0.02 | <0.02 | 0.04 | 1 | 5 |
| Ba | 13.1 | 0.82 | 0.3 | 0.7 | 20 | 100 | 300 |
| Element | Portugal [43] | Italy [44] | Cantabria [45] | Basque Country [46] | BFS | OPBA | ACBFS |
|---|---|---|---|---|---|---|---|
| Zn | 4 | 0.03 | 4 | 1.2 | <0.25 | <0.25 | <0.25 |
| V | - | - | - | 1.3 | 0.19 | - | 0.16 |
| Sn | - | - | - | - | <0.25 | - | <0.25 |
| Se | 0.1 | 0.1 | 0.1 | 0.007 | <0.04 | 0.4 | 0.07 |
| Sb | 0.06 | - | 0.06 | - | <0.05 | 0.2 | <0.05 |
| Pb | 0.5 | 0.5 | 0.5 | - | <0.2 | 9.3 | <0.2 |
| Ni | 0.4 | 0.1 | 0.4 | 0.8 | <0.05 | 1.3 | <0.05 |
| Mo | 0.5 | - | 0.5 | 1.3 | <0.2 | 1.8 | <0.2 |
| Hg | 0.01 | 0.01 | 0.01 | - | <0.01 | <0.01 | <0.01 |
| Cu | 2 | 0.5 | 2 | - | <0.1 | 6.7 | <0.1 |
| Cr (total) | 0.5 | 0.5 | 0.5 | 2.6 | <0.1 | <0.1 | <0.1 |
| Co | - | - | - | - | <0.02 | <0.02 | <0.02 |
| Cd | 0.04 | 0.05 | 0.04 | 0.009 | <0.02 | <0.02 | <0.02 |
| Ba | 20 | 10 | 20 | 17 | 13.1 | 0.3 | 0.7 |
| As | 0.5 | 0.5 | 0.5 | - | <0.2 | 1.8 | <0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Galiano, Y.; Leiva Fernández, C.; Villegas Sánchez, R.; Fernández-Pereira, C. Development of Geopolymer Mortars Using Air-Cooled Blast Furnace Slag and Biomass Bottom Ashes as Fine Aggregates. Processes 2023, 11, 1597. https://doi.org/10.3390/pr11061597
Luna-Galiano Y, Leiva Fernández C, Villegas Sánchez R, Fernández-Pereira C. Development of Geopolymer Mortars Using Air-Cooled Blast Furnace Slag and Biomass Bottom Ashes as Fine Aggregates. Processes. 2023; 11(6):1597. https://doi.org/10.3390/pr11061597
Chicago/Turabian StyleLuna-Galiano, Yolanda, Carlos Leiva Fernández, Rosario Villegas Sánchez, and Constantino Fernández-Pereira. 2023. "Development of Geopolymer Mortars Using Air-Cooled Blast Furnace Slag and Biomass Bottom Ashes as Fine Aggregates" Processes 11, no. 6: 1597. https://doi.org/10.3390/pr11061597






