Phenolic Content, Antioxidant Capacity, and Browning Impact of Apple Slices during Microwave Drying: A Chemometric Approach
Abstract
:1. Introduction
Microwave Processing
2. Materials and Methods
2.1. Experimental Design (DOE)
- the first factor, the apple cultivar with two levels: Golden (GHu) and Idared (IRo);
- the second factor, the sample with two levels: CTRL and MW, denoting the untreated samples (CTRL) and the treated samples (MW);
- the third factor, the phase with three levels: INI, MW, and OV, representing the three types of applied treatments: untreated (INI), microwave treatment (MW), and convection drying treatment (OV);
- the fourth factor, the microwave treatment time period with three active levels: t3, t4, and t5, corresponding to microwave exposure times of three, four, and five minutes; also, the untreated INI samples could have the code of t0 for this factor.
2.2. Chromatic Analysis
2.3. The Determination of the Browning Index (BI)
2.4. Total Phenolic Content (FC) and Antioxidant Capacity of MW Treated or Untreated Apple Slices
2.5. Statistical Analysis
3. Results and Discussions
3.1. Univariate Analysis
3.2. Nonlinear Regression
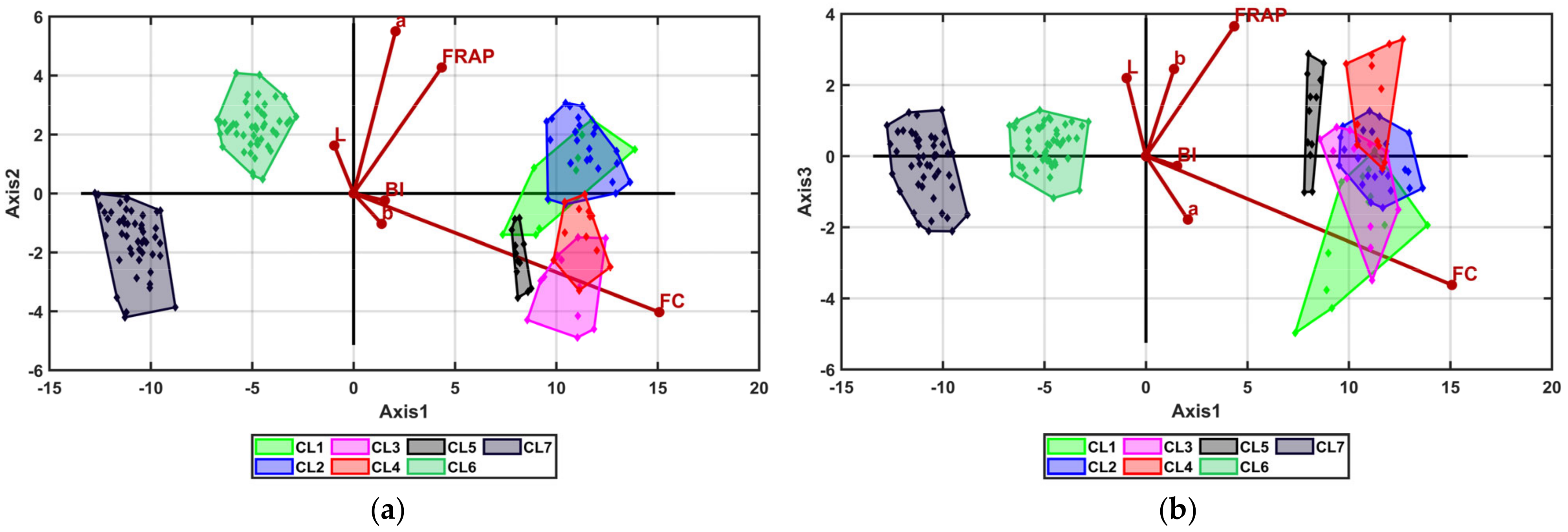
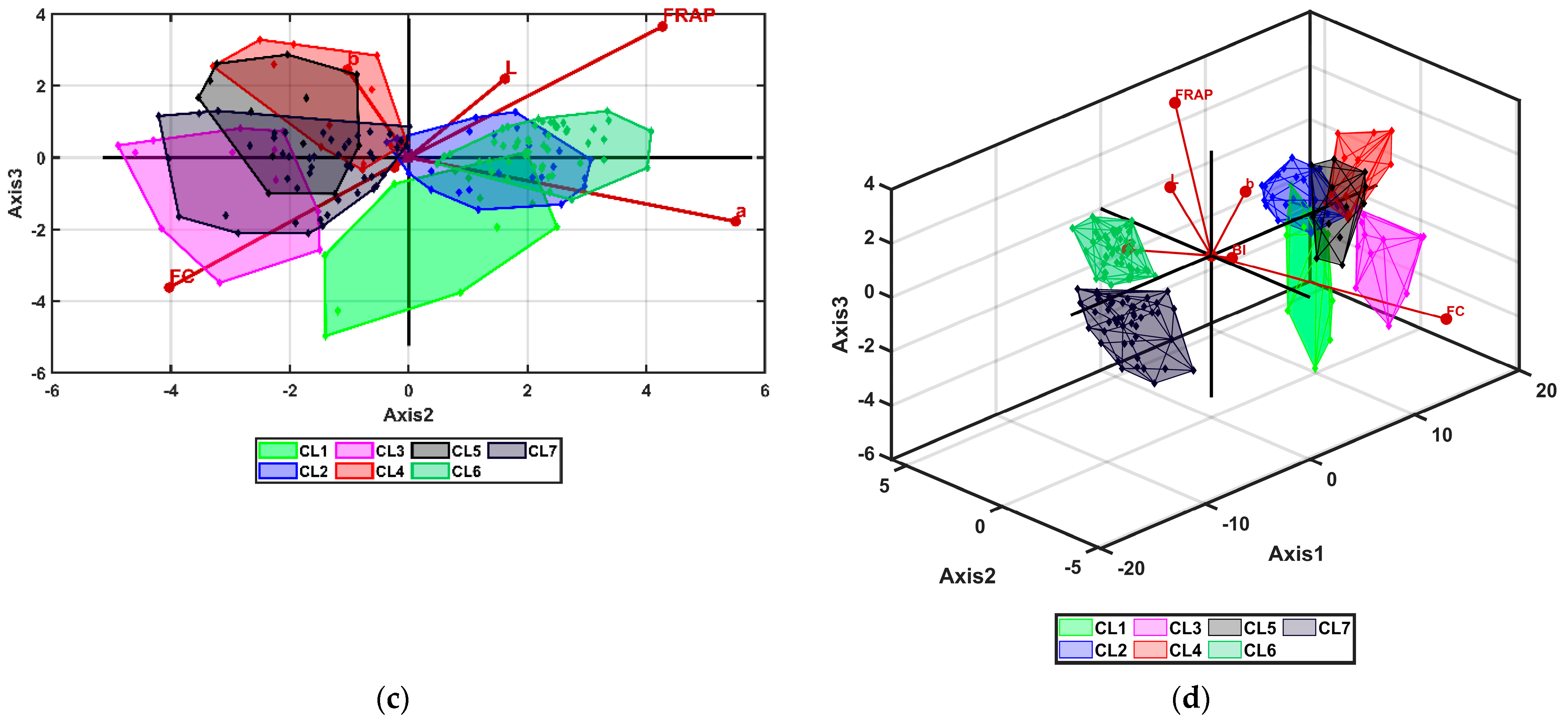
3.3. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al Juhaimi, F.; Uslu, N.; Bozkurt, D.; Ghafoor, K.; Babiker, E.E.; Özcan, M.M. Effects of oven and microwave drying on phenolic contents and antioxidant activities in four apple cultivars. Qual. Assur. Saf. Crop. Foods 2016, 8, 51–55. [Google Scholar] [CrossRef]
- Szychowski, P.J.; Lech, K.; Sendra-Nadal, E.; Hernández, F.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, A.A. Kinetics, biocompounds, antioxidant activity, and sensory attributes of quinces as affected by drying method. Food Chem. 2018, 255, 157–164. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Lagnika, C.; Song, J.; Li, D.; Liu, C.; Jiang, N.; Zhang, M.; Duan, X. A comparative study of drying methods onphysical characteristics, nutritional properties and antioxidant capacity of broccoli. Dry. Technol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef]
- Wang, R.; Ding, S.; Zhao, D.; Wang, Z.; Wu, J.; Hu, X. Effect of dehydration methods on antioxidant activities, phenolic contents, cyclic nucleotides, and volatiles of jujube fruits. Food Sci. Biotechnol. 2016, 25, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Punathil, L.; Basak, T. A theoretical analysis on the effect of containers on the microwave heating of materials. Int. Communic. Heat Mass Transf. 2017, 82, 145–153. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, phsicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef]
- Doymaz, I. Effect of citric acid and blanching pre-treatments on drying and rehydration of Amasya red apples. Food Bioprod. Process. 2010, 88, 124–132. [Google Scholar] [CrossRef]
- Kingsly, R.P.; Goyal, R.K.; ManiKaantan, M.R.; Ilyas, S.M. Effects of pre-treatments and drying air temperature on drying behaviour of peach slice. Int. J. Food Sci. Tecnol. 2007, 42, 65–69. [Google Scholar] [CrossRef]
- Onwude, D.I.; Hashim, N.; Chen, G. Recent advances of novel thermal combined hot air drying of agricultural crops. Trends Food Sci. Technol. 2016, 57, 132–145. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Sun, D.W.; Han, Z.; Cheng, J.H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency. A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Fathi-Achachlouei, B.; Azadmard-Damirchi, S.; Zahedi, Y.; Shaddel, R. Microwave pretreatment as a promising strategy for increment of nutraceutical content and extraction yield of oil from milk thistle seed. Ind. Crop. Prod. 2019, 128, 527–533. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Chahbani, A.; Fakhfakh, N.; Balti, M.A.; Mabrouk, M.; El-Hatmi, H.; Zouari, N.; Kechaou, N. Microwave drying effects on drying kinetics, bioactive compounds and antioxidant activity of green peas (Pisum sativum L.). Food Biosci. 2018, 25, 32–38. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Lillford, P.J. Structure property relationships in foods. In Food Materials Science; Springer: Berlin/Heidelberg, Germany, 2008; pp. 229–253. [Google Scholar]
- López-Fernández, O.; Bohrer, B.M.; Munekata, P.E.S.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Improving oxidative stability of foods with apple-derived polyphenols. Compr. Rev. Food Sci. Food Saf. 2022, 21, 296–320. [Google Scholar] [CrossRef] [PubMed]
- Talebzadeh, S.L.; Fatemi, H.; Azizi, M.; Kaveh, M.; Nik, A.S.; Szymanek, M.; Kulig, R. Interaction of Different Drying Methods and Storage on Appearance, Surface Structure, Energy, and Quality of Berberis vulgaris var. asperma. Foods 2022, 11, 3003. [Google Scholar] [CrossRef]
- Sablani, S. Drying of Fruits and Vegetables: Retention of Nutritional/Functional Quality. Dry. Technol. 2006, 24, 123–135. [Google Scholar] [CrossRef]
- Barsa, C.; Normand, M.; Peleg, M. On Models of the Temperature Effect on the Rate of Chemical Reaction and Biological Processes in Foods. Food Eng. Rev. 2012, 4, 191–202. [Google Scholar] [CrossRef]
- Defraeye, T. Towards more efficient intermittent drying of fruit: Insight from combined hygrothermal-quality modelling. Innov. Food Sci. Emerg. Technol. 2016, 38, 262–271. [Google Scholar] [CrossRef]
- Indiarto, R.; Asyifaa, A.H.; Adiningsih, F.C.A.; Aulia, G.A.; Achmad, S.R. Conventional And Advanced Food-Drying Technology: A Current Review. Int. J. Sci. Technol. Res. 2021, 10, 99–107. [Google Scholar]
- Rahman, M.S. Handbook of Food Preservation, 3rd ed.; Taylor & Francis Group: Abingdon, UK, 2020; ISBN 978-1-4987-4048-7. [Google Scholar]
- Sinha, N.K.; Sidhu, J.; Barta, J.; Wu, J.; Pilar Cano, M. Handbook of Fruits and Fruit Processing, 2nd ed.; Wiley-Blackwell: South Gate, CA, USA, 2012; ISBN 978-081308949. [Google Scholar]
- Önal, B.; Adiletta, G.; Di Matteo, M.; Russo, P.; Ramos, I.N.; Silva, C.L.M. Microwave and Ultrasound Pre-Treatments for Drying of the “Rocha” Pear: Impact on Phytochemical Parameters, Color Changes and Drying Kinetics. Foods 2021, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Metaxas, A.C.; Meredith, R.J. Industrial Microwave Heating; Peter Peregrinus Ltd. (IEE): London, UK, 1983. [Google Scholar]
- Decareau, R.V.; Peterson, R.A. Microwave Processing and Engineering; Ellis Horwood Ltd. & VCH Publisher: Deerfield Beach, FL, USA, 1986; ISBN 978-3527262106. [Google Scholar]
- Prusky, D. Reduction of incidence of postharvest quality losses, and future prospects. Food Secur. 2011, 3, 463–474. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Bandici, L.; Teuşdea, A.; Milian Pavel, O.; Ramona Memete, A.; Ioana Vicas, S.; Vasile Timar, A.; Emil Bandici, G. The Use of Microwaves in the Process of Reducing the Browning of Apple Slices. In Proceedings of the International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 10–11 June 2021. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bandici, L.; Teusdea, A.C.; Soproni, V.D.; Hathazi, F.I.; Arion, M.N.; Molnar, C.O.; Vicas, S.I. The Influence of Microwave Treatments on Bioactive Compounds and Antioxidant Capacity of Mentha piperita L. Materials 2022, 15, 7789. [Google Scholar] [CrossRef]
- Lunadei, L.; Diezma Iglesias, B.; Lleó García, L.; Galleguillos, P. Enzymatic Browning in Fresh-Cut Apple Slices Measured by Different Kinds of Image Algorithms. In Proceedings of the Vi Congreso Iberico de Agrolngenieria, Évora, Portugal, 5–7 September 2011. [Google Scholar]
- Lu, S.; Luo, Y.; Turner, E.; Feng, H. Efficacy of sodium chlorite as an inhibitor of enzymatic browning in apple slices. Food Chem. 2007, 104, 824–829. [Google Scholar] [CrossRef]
- Memete, A.R.; Sărac, I.; Teusdea, A.C.; Budău, R.; Bei, M.; Vicas, S.I. Bioactive Compounds and Antioxidant Capacity of Several Blackberry (Rubus spp.) Fruits Cultivars Grown in Romania. Horticulturae 2023, 9, 556. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R. Effects of Microwave Pretreatment of Apple Raw Material on the Nutrients and Antioxidant Activities of Apple Juice. J. Food Proc. 2014, 2014, e824050. [Google Scholar] [CrossRef]
- Hayat, K.; Zhang, X.; Farooq, U.; Abbas, S.; Xia, S.; Jia, C.; Zhong, F.; Zhang, J. Effect of Microwave Treatment on Phenolic Content and Antioxidant Activity of Citrus Mandarin Pomace. Food Chem. 2010, 123, 423–429. [Google Scholar] [CrossRef]
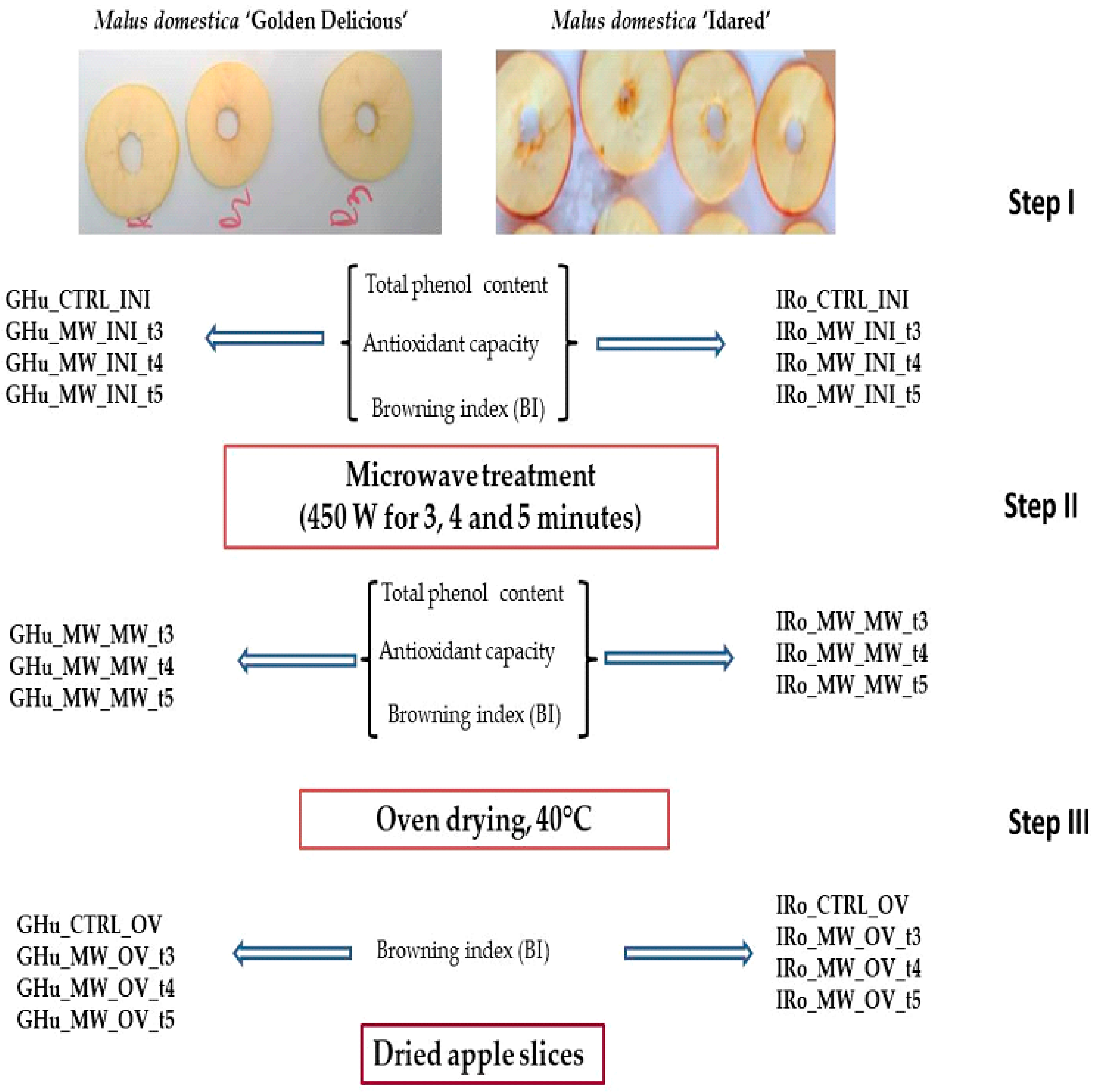



| Phase | INI | MW | OV | Explanation |
|---|---|---|---|---|
| Sample Index | ||||
| GHu_CTRL_INI | x | - | - | untreated GHu, CTRL sample |
| GHu_CTRL_OV | - | - | x | OV treated GHu, CTRL sample |
| GHu_MW_INI | x | - | - | untreated GHu, MW sample |
| GHu_MW_MW_tX | - | x | - | MW treated GHu sample with tX minutes |
| GHu_MW_OV_tX | - | x | x | MW treated GHu sample with tX minutes and after OV treatment |
| IRo_CTRL_INI | x | - | - | untreated IRo, CTRL sample |
| IRo_CTRL_OV | - | - | x | OV treated IRo, CTRL sample |
| IRo_MW_INI | x | - | - | untreated IRo, MW sample |
| IRo_MW_MW_tX | - | x | - | MW treated IRo sample with tX minutes |
| IRo_MW_OV_tX | - | x | x | MW treated IRo sample with tX minutes, and after OV treatment |
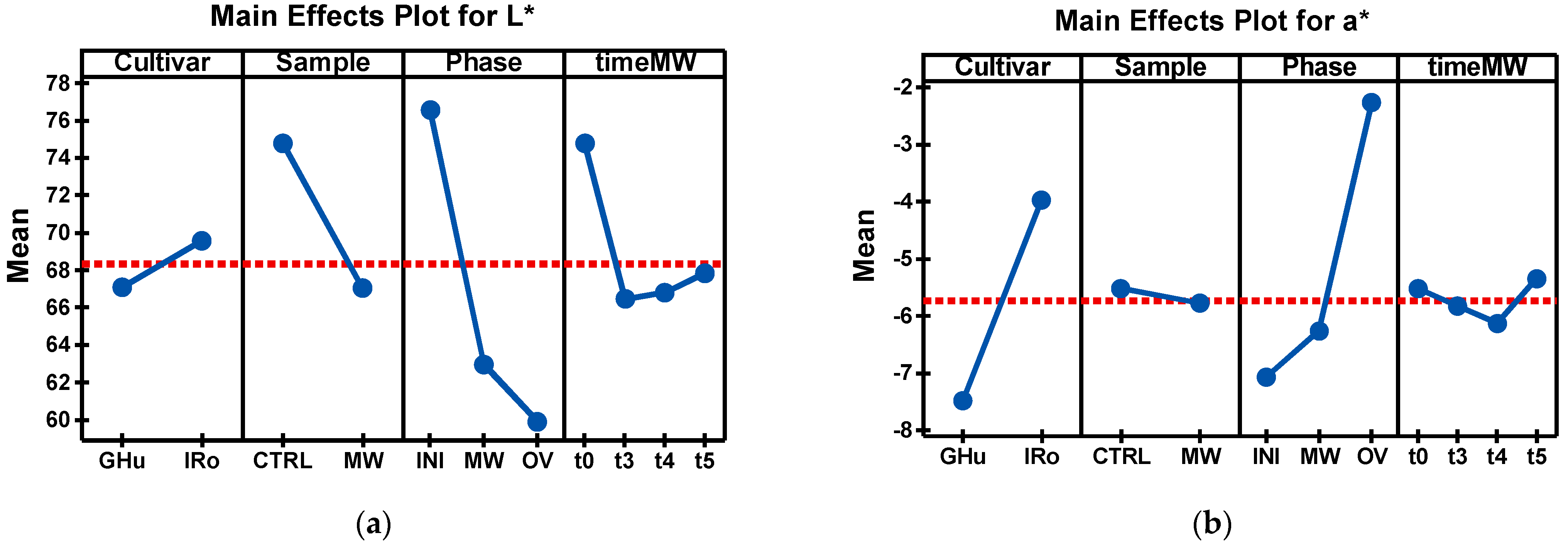
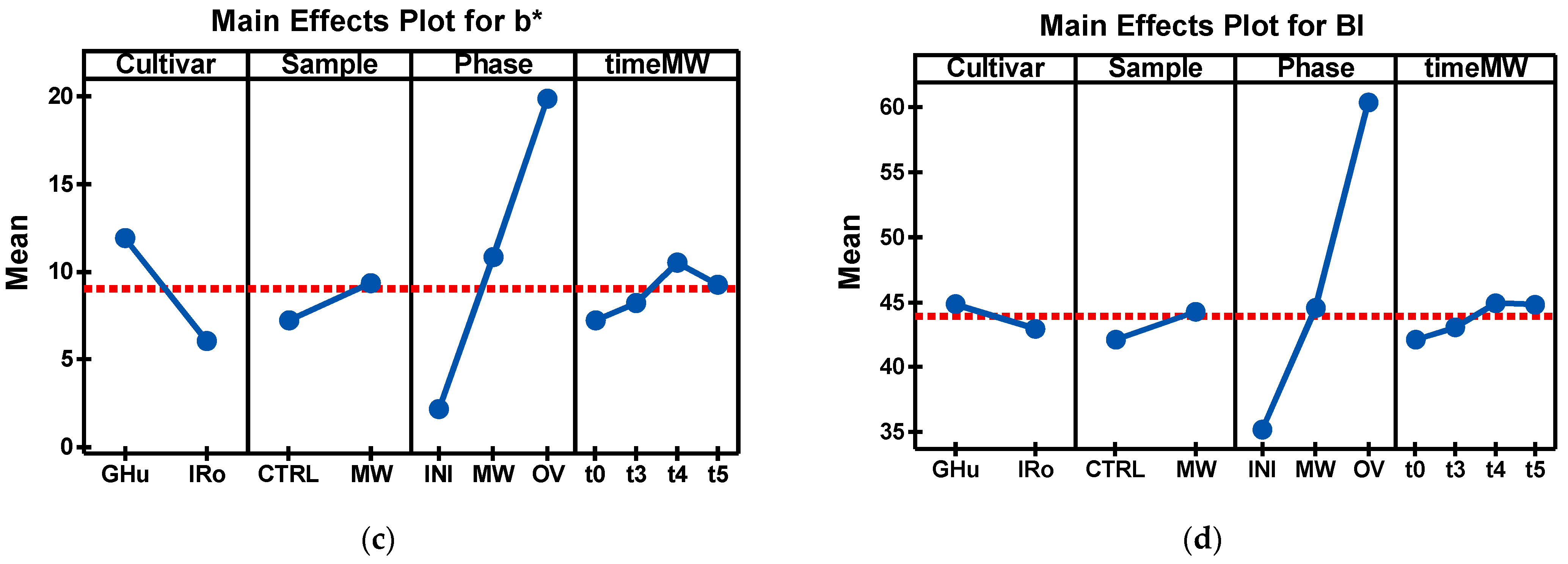
| Cultivar*Sample* Phase*Time MW | R | G | B | L* | a* | b* | BI |
|---|---|---|---|---|---|---|---|
| GHu_CTRL_INI | 213.90 a ± 9.82 | 196.20 a ± 7.82 | 144.62 a ± 10.58 | 79.30 a ± 3.20 | −9.37 h ± 1.23 | 1.95 d,e,f ± 3.45 | 32.41 h ± 2.88 |
| GHu_MW_INI_t3 | 205.89 a,b ± 13.60 | 187.50 a,b ± 12.49 | 136.19 a,b ± 15.36 | 75.88 a,b ± 5.00 | −8.67 h ± 1.10 | 3.28 d,e,f ± 4.72 | 34.48 g,h ± 4.12 |
| GHu_MW_INI_t4 | 199.43 a,b ± 16.09 | 182.17 a,b ± 15.16 | 129.45 b ± 17.57 | 73.72 a,b ± 6.02 | −9.01 h ± 0.90 | 4.73 d,e ± 3.77 | 35.46 e,f,g,h ± 4.23 |
| GHu_MW_INI_t5 | 203.30 a,b ± 8.90 | 184.49 a ± 8.19 | 135.24 a,b ± 6.95 | 74.69 a,b ± 3.29 | −8.16 g,h ± 1.01 | 2.57 d,e,f ± 1.60 | 34.58 f,g,h ± 1.90 |
| GHu_MW_MW_t3 | 161.46 e,f,g ± 6.16 | 145.37 d,e,f ± 5.47 | 88.89 d ± 14.74 | 60.49 e,f ± 2.20 | −8.32 g,h ± 0.97 | 12.67 b,c ± 7.12 | 43.70 d,e,f,g ± 7.75 |
| GHu_MW_MW_t4 | 166.60 e,f,g ± 6.95 | 147.44 d,e,f ± 5.12 | 82.73 d ± 12.96 | 61.43 e,f ± 2.11 | −7.95 f,g,h ± 1.00 | 17.62 b ± 6.59 | 49.47 c,d,e ± 7.14 |
| GHu_MW_MW_t5 | 171.16 d,e,f ± 6.00 | 150.75 d,e,f ± 3.58 | 88.55 d ± 11.17 | 62.84 d,e,f ± 1.55 | −7.35 e,f,g,h ± 1.20 | 15.76 b ± 6.17 | 48.17 c,d,e,f ± 6.21 |
| GHu_CTRL_OV | 200.26 a,b ± 6.43 | 170.75 b,c ± 7.65 | 98.48 c,d ± 12.59 | 70.86 b,c ± 2.66 | −5.65 c,d,e,f ± 1.79 | 19.41 a,b ± 4.34 | 53.07 b,c,d ± 5.81 |
| GHu_MW_OV_t3 | 143.13 g ± 3.91 | 119.05 g ± 6.96 | 49.83 e ± 20.06 | 50.95 h ± 2.44 | −4.19 c,d ± 1.41 | 24.75 a ± 8.95 | 64.41 a,b ± 12.54 |
| GHu_MW_OV_t4 | 156.98 f,g ± 7.21 | 129.21 g ± 9.01 | 51.02 e ± 22.85 | 55.15 g,h ± 3.37 | −3.94 c,d ± 1.00 | 28.52 a ± 9.02 | 67.78 a ± 11.08 |
| GHu_MW_OV_t5 | 153.97 f,g ± 11.96 | 126.46 g ± 14.70 | 55.70 e ± 29.86 | 54.14 g,h ± 5.48 | −3.21 b,c ± 1.71 | 24.82 a ± 11.60 | 65.55 a ± 15.43 |
| IRo_CTRL_INI | 211.72 a,b ± 8.19 | 183.97 a,b ± 9.66 | 137.17 a,b ± 10.36 | 75.92 a,b ± 3.40 | −4.44 c,d ± 2.01 | 2.63 d,e,f ± 3.03 | 38.81 e,f,g,h ± 5.34 |
| IRo_MW_INI_t3 | 215.61 a,b ± 5.42 | 190.59 a ± 6.77 | 144.43 a ± 8.85 | 78.13 a,b ± 2.35 | −5.77 c,d,e ± 1.17 | 0.71 f ± 2.90 | 35.16 g,h ± 4.09 |
| IRo_MW_INI_t4 | 213.25 a,b ± 6.26 | 188.71 a ± 6.86 | 143.37 a ± 7.52 | 77.41 a ± 2.44 | −5.77 d,e,f,g ± 1.27 | 0.46 f ± 2.04 | 34.97 g,h ± 3.66 |
| IRo_MW_INI_t5 | 213.83 a ± 6.33 | 188.21 a ± 9.09 | 142.57 a,b ± 10.29 | 77.34 a ± 3.04 | −5.36 c,d,e ± 1.81 | 0.94 e,f ± 2.95 | 35.95 e,f,g,h ± 5.04 |
| IRo_MW_MW_t3 | 172.44 d,e ± 8.15 | 150.17 d,e,f ± 7.14 | 107.90 c ± 10.11 | 63.08 d,e,f ± 2.88 | −4.52 c,d ± 1.09 | 4.25 d,e ± 4.31 | 39.78 d,e,f,g,h ± 4.79 |
| IRo_MW_MW_t4 | 176.95 d,e ± 5.66 | 155.31 c,d,e ± 3.37 | 107.61 c ± 5.87 | 64.67 c,d,e,f ± 1.64 | −5.41 c,d,e ± 1.18 | 6.61 c,d ± 2.86 | 41.12 d,e,f,g,h ± 2.69 |
| IRo_MW_MW_t5 | 182.51 c,d ± 5.48 | 155.93 c,d ± 5.73 | 106.52 c ± 7.92 | 65.55 c,d,e ± 1.97 | −3.82 c,d ± 1.72 | 8.34 c,d ± 3.92 | 45.38 d,e,f,g,h ± 5.48 |
| IRo_CTRL_OV | 192.28 b,c ± 18.34 | 153.83 c,d ± 18.80 | 96.01 c,d ± 17.95 | 65.59 c,d ± 6.86 | 0.25 a ± 1.97 | 15.47 b ± 3.12 | 58.31 a,b,c ± 6.06 |
| IRo_MW_OV_t3 | 172.34 d,e ± 9.71 | 137.24 f,g ± 13.92 | 83.73 d ± 23.68 | 59.33 f,g,h ± 4.94 | 0.44 a ± 2.43 | 15.09 b ± 9.02 | 59.03 a,b,c ± 13.65 |
| IRo_MW_OV_t4 | 172.74 d,e ± 12.08 | 139.37 e,f,g ± 14.16 | 80.76 d ± 24.45 | 59.82 e,f,g ± 5.26 | −0.85 a,b ± 1.40 | 17.42 b ± 8.82 | 59.72 a,b,c ± 11.86 |
| IRo_MW_OV_t5 | 179.25 d,e ± 6.45 | 145.98 d,e,f ± 8.57 | 91.86 d ± 16.65 | 62.39 d,e,f ± 3.06 | −0.96 a ± 0.84 | 13.80 b ± 6.85 | 55.34 a,b,c ± 8.39 |
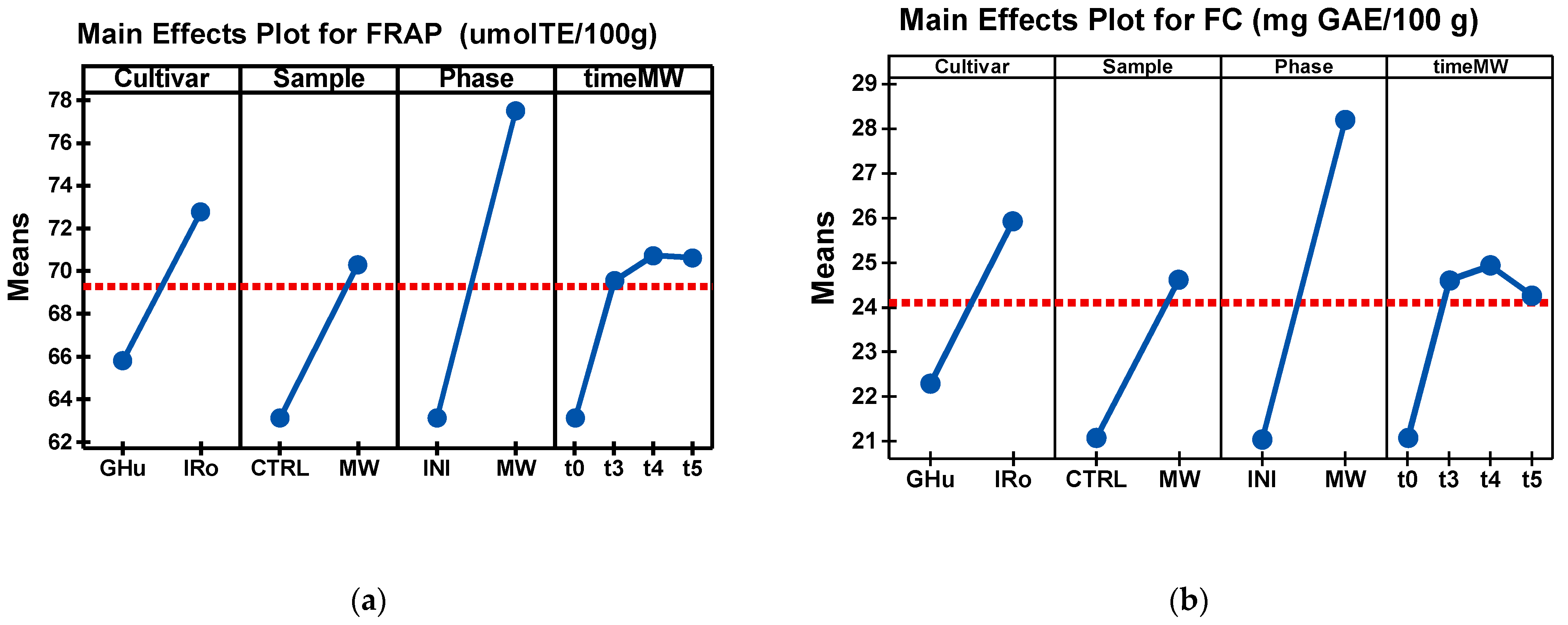
| Cultivar*Sample *Phase*Time MW | FRAP (µmol TE/100 g) | FC (mg GAE/100 g) |
|---|---|---|
| GHu_CTRL_INI | 62.42 g ± 3.12 | 18.47 e ± 0.96 |
| GHu_MW_INI_t3 | 62.61 g ± 6.89 | 18.30 e ± 0.91 |
| GHu_MW_INI_t4 | 62.58 g ± 2.92 | 18.47 e ± 0.96 |
| GHu_MW_INI_t5 | 62.42 g ± 5.87 | 18.30 e ± 0.91 |
| GHu_MW_MW_t3 | 75.75 c,d ± 1.80 | 27.77 c ± 2.11 |
| GHu_MW_MW_t4 | 76.35 b,c ± 1.45 | 27.44 c ± 0.15 |
| GHu_MW_MW_t5 | 78.24 a,b ± 1.12 | 27.30 b,c ± 1.73 |
| IRo_CTRL_INI | 68.83 e,f ± 1.55 | 18.61 d ± 2.04 |
| IRo_MW_INI_t3 | 68.67 f ± 1.94 | 18.44 d ± 2.63 |
| IRo_MW_INI_t4 | 68.79 e,f ± 0.69 | 18.49 d ± 3.41 |
| IRo_MW_INI_t5 | 68.92 d,e ± 1.45 | 18.63 d ± 1.99 |
| IRo_MW_MW_t3 | 76.24 a ± 4.75 | 28.64 a,b ± 0.58 |
| IRo_MW_MW_t4 | 79.20 a ± 0.60 | 29.52 a ± 0.84 |
| IRo_MW_MW_t5 | 79.41 a,b ± 1.30 | 28.36 a,b,c ± 1.19 |
| FRAP (umol TE/100 g) | GHu | IRo |
|---|---|---|
| Inverse Gaussian Function | Y = Ymax − k1 × exp ((−1) × X2/(k22)) | |
| Best-fit values | ||
| Ymax | 77.73 | 80.33 |
| k1 | 14.78 | 9.782 |
| k2 | 2.218 | 3.043 |
| Std. Error | ||
| Ymax | 0.3854 | 1.283 |
| k1 | 0.5482 | 1.397 |
| k2 | 0.1467 | 0.5572 |
| Goodness of fit | ||
| Degrees of freedom | 45 | 45 |
| R square | 0.9529 | 0.6799 |
| Adjusted R square | 0.9509 | 0.6656 |
| FC (mg GAE/100 g) | GHu | IRo |
|---|---|---|
| Inverse Gaussian function | Y = Ymax − k1 × exp((−1) × X2/(k22)) | |
| Best-fit values | ||
| Ymax | 28.6 | 28.95 |
| k1 | 10.14 | 8.012 |
| k2 | 1.667 | 1.795 |
| Std. Error | ||
| Ymax | 0.1555 | 0.1087 |
| k1 | 0.2785 | 0.1842 |
| k2 | 0.06465 | 0.06026 |
| Goodness of fit | ||
| Degrees of freedom | 57 | 57 |
| R square | 0.9589 | 0.9708 |
| Adjusted R square | 0.9574 | 0.9698 |
| BI | GHu | IRo |
|---|---|---|
| Inverse Gaussian function | Y = Ymax − k1 ×exp ((−1) × X2/(k22)) | |
| Best-fit values | ||
| Ymax | 50.38 | 45.230 |
| k1 | 16.25 | 9.250 |
| k2 | 2.985 | 3.881 |
| Std. Error | ||
| Ymax | 1.392 | 1.159 |
| k1 | 2.051 | 1.455 |
| k2 | 0.4572 | 0.679 |
| Goodness of fit | ||
| Degrees of freedom | 57 | 57 |
| R square | 0.5435 | 0.4407 |
| Adjusted R square | 0.5275 | 0.4211 |
| TimeMW (min) | GHu | IRo |
|---|---|---|
| FRAP | 6.57 | 6.87 |
| FC | 4.75 | 4.95 |
| BI | 8.34 | 10.15 |
| MANOVA, p-Values | GHu_CTRL_INI | GHu_MW_INI_t3 | GHu_MW_INI_t4 | GHu_MW_INI_t5 | GHu_MW_MW_t3 | GHu_MW_MW_t4 | GHu_MW_MW_t5 | IRo_CTRL_INI | IRo_MW_INI_t3 | IRo_MW_INI_t4 | IRo_MW_INI_t5 | IRo_MW_MW_t3 | IRo_MW_MW_t4 | IRo_MW_MW_t5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GHu_CTRL_INI | 0.3366 | 0.0416 | 0.0837 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| GHu_MW_INI_t3 | 0.3366 | 0.7590 | 0.7478 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| GHu_MW_INI_t4 | 0.0416 | 0.7590 | 0.4628 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| GHu_MW_INI_t5 | 0.0837 | 0.7478 | 0.4628 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| GHu_MW_MW_t3 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0004 | 0.0030 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| GHu_MW_MW_t4 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0004 | 0.0019 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| GHu_MW_MW_t5 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0030 | 0.0019 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0009 | 0.0002 | |
| IRo_CTRL_INI | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.4279 | 0.4349 | 0.7655 | 0.0000 | 0.0000 | 0.0000 | |
| IRo_MW_INI_t3 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.4279 | 0.9947 | 0.9893 | 0.0000 | 0.0000 | 0.0000 | |
| IRo_MW_INI_t4 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.4349 | 0.9947 | 0.9920 | 0.0000 | 0.0000 | 0.0000 | |
| IRo_MW_INI_t5 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.7655 | 0.9893 | 0.9920 | 0.0000 | 0.0000 | 0.0000 | |
| IRo_MW_MW_t3 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0081 | 0.0034 | |
| IRo_MW_MW_t4 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0009 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0081 | 0.0560 | |
| IRo_MW_MW_t5 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0034 | 0.0560 |
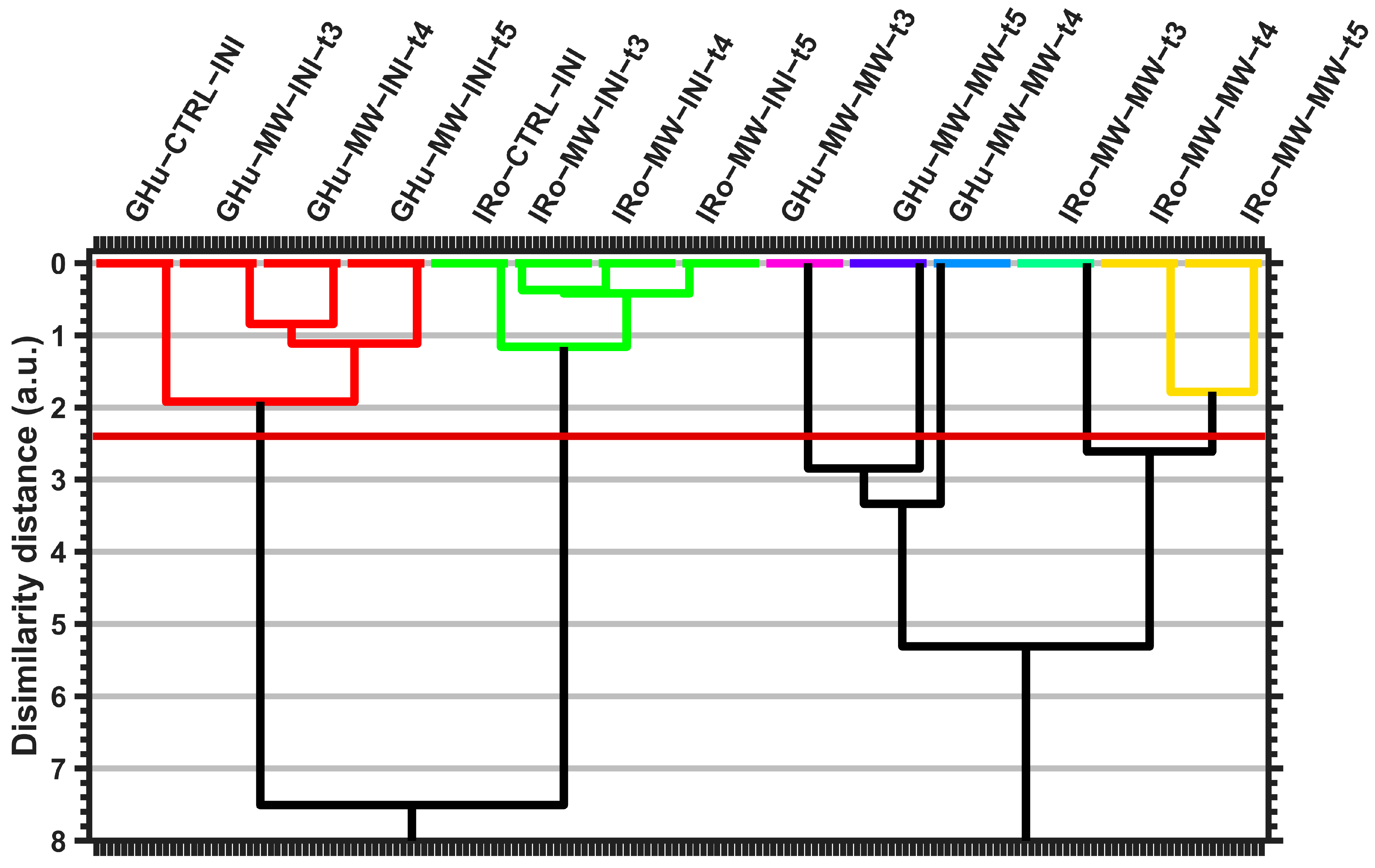
| Cluster_1 | Cluster_2 | Cluster_3 | Cluster_4 | Cluster_5 | Cluster_6 | Cluster_7 | ||
|---|---|---|---|---|---|---|---|---|
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t3 | IRo_MW_MW_t4 | GHu_MW_MW_t3 | GHu_MW_MW_t5 | GHu_MW_MW_t4 | IRo_CTRL_INI | IRo_MW_INI_t4 | GHu_CTRL_INI | GHu_MW_INI_t4 |
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
| IRo_MW_MW_t5 | IRo_MW_INI_t3 | IRo_MW_INI_t5 | GHu_MW_INI_t3 | GHu_MW_INI_t5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soproni, V.D.; Bandici, L.; Teusdea, A.C.; Hathazi, F.I.; Arion, M.N.; Molnar, C.O.; Leuca, T.; Dalea, C.A.; Vicas, S.I. Phenolic Content, Antioxidant Capacity, and Browning Impact of Apple Slices during Microwave Drying: A Chemometric Approach. Processes 2023, 11, 1601. https://doi.org/10.3390/pr11061601
Soproni VD, Bandici L, Teusdea AC, Hathazi FI, Arion MN, Molnar CO, Leuca T, Dalea CA, Vicas SI. Phenolic Content, Antioxidant Capacity, and Browning Impact of Apple Slices during Microwave Drying: A Chemometric Approach. Processes. 2023; 11(6):1601. https://doi.org/10.3390/pr11061601
Chicago/Turabian StyleSoproni, Vasile Darie, Livia Bandici, Alin Cristian Teusdea, Francisc Ioan Hathazi, Mircea Nicolae Arion, Carmen Otilia Molnar, Teodor Leuca, Claudia Andrada Dalea, and Simona Ioana Vicas. 2023. "Phenolic Content, Antioxidant Capacity, and Browning Impact of Apple Slices during Microwave Drying: A Chemometric Approach" Processes 11, no. 6: 1601. https://doi.org/10.3390/pr11061601






