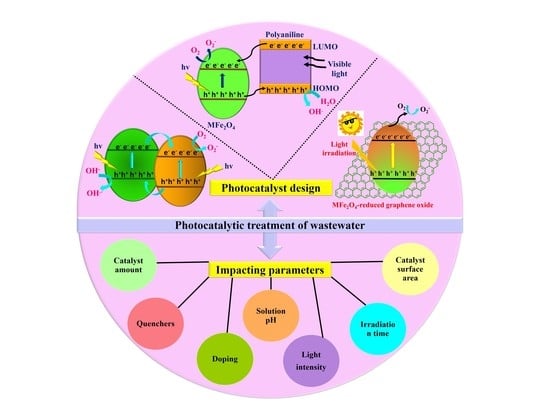
A Review on Impacting Parameters for Photocatalytic Degradation of Organic Effluents by Ferrites and Their Nanocomposites
Abstract
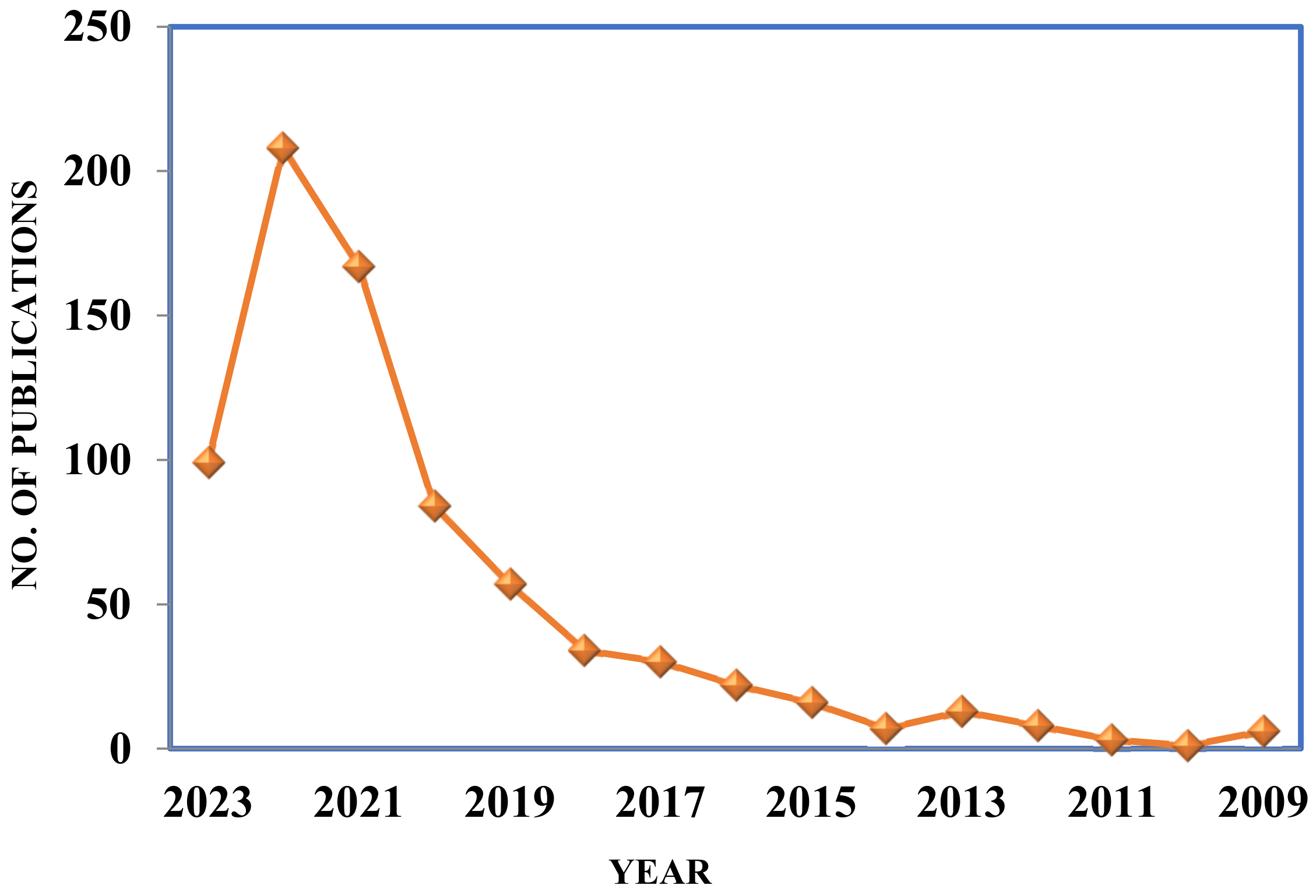
:1. Introduction
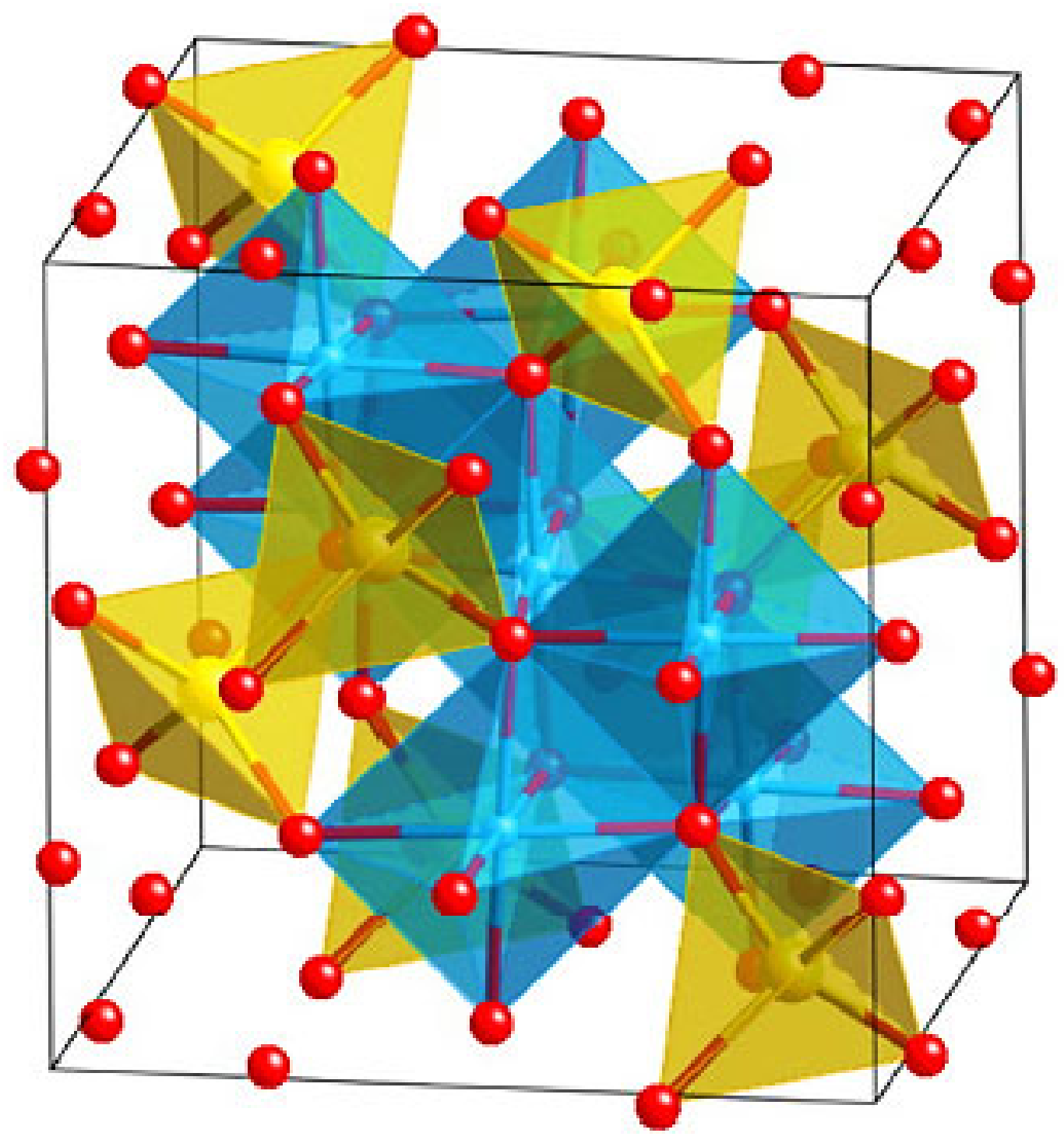
2. Structure of Ferrites
3. Methods of Synthesis of Ferrites and Their Nanocomposites
| S.No. | Compound | Method | Size (nm) | Band Gap (ev) | Surface Area | Pollutant | Catalyst Amount (mg) | Degradation %ge | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1. | ZnFe2O4 | Solvothermal Microwave Sol-gel | 77 40 34.8 | 1.65 1.9 1.6 | 51.81 6.0 - | RhB MB OI | 80 60 400 | 100 32 36.8 | [65] [66] [67,68] |
| 2. | MnFe2O4 | Microwave | 39 | 1.56 | - | IC | 50 | 94 | [69] |
| 3. | CaFe2O4 | Solution combustion | 100 | 1.82 | 79.3 | MB | 50 | 100 | [70] |
| 4. | MgFe2O4 | Solid state | - | 2 | 5 | MB | 50 | 90 | [71] |
| 5. | CuFe2O4 | Co-precipitation Solution combustion | 26 8 | 1.92 1.9 | - - | 4-CP MG | 10 40 | 62 82 | [72] [73] |
| 6. | NiFe2O4 | Solution combustion Co-precipitation | 14 29 | 2 1.78 | - 13.48 | MG MO | 40 100 | 94.5 83.2 | [73] [74] |
| 7. | BaFe2O4 | Sol-gel | - | - | - | BY BV MB AO | 10,000 | 100 | [75] |
| 8. | CoFe2O4 | Precipitation Solvothermal Sol-gel | 17 40 40 | 3.05 2.33 1.15 | - - - | MO MB RhB | 100 50 50 | 77 93 78 | [76] [77] [78] |
| 9. | CdFe2O4 | Pyrolysis | - | 2.06 | 15.27 | CS | 30 | 55.5 | [79] |
| 10. | SrFe2O4 | Sol-gel | - | 1.75 | - | RhB | 20 | 89.3 | [80] |
4. Impacting Parameters for Photocatalytic Activity of Ferrites and Their Nanocomposites
4.1. Effect of pH
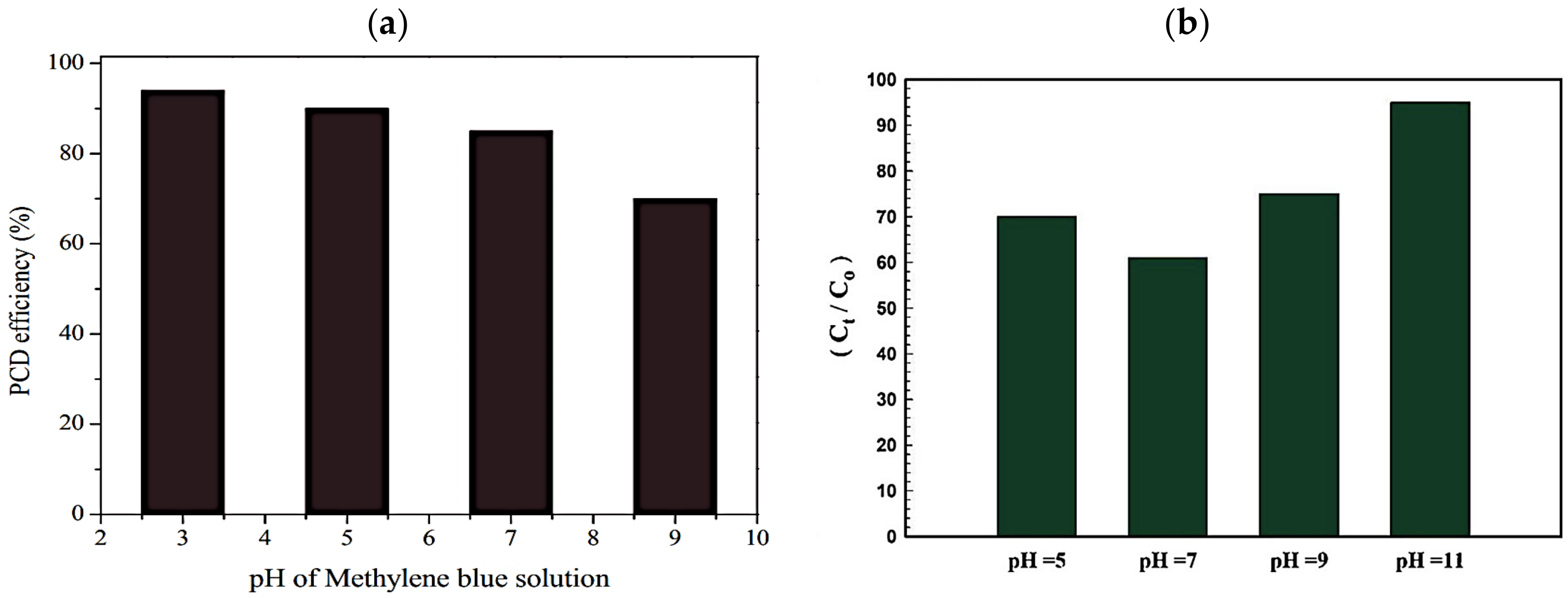
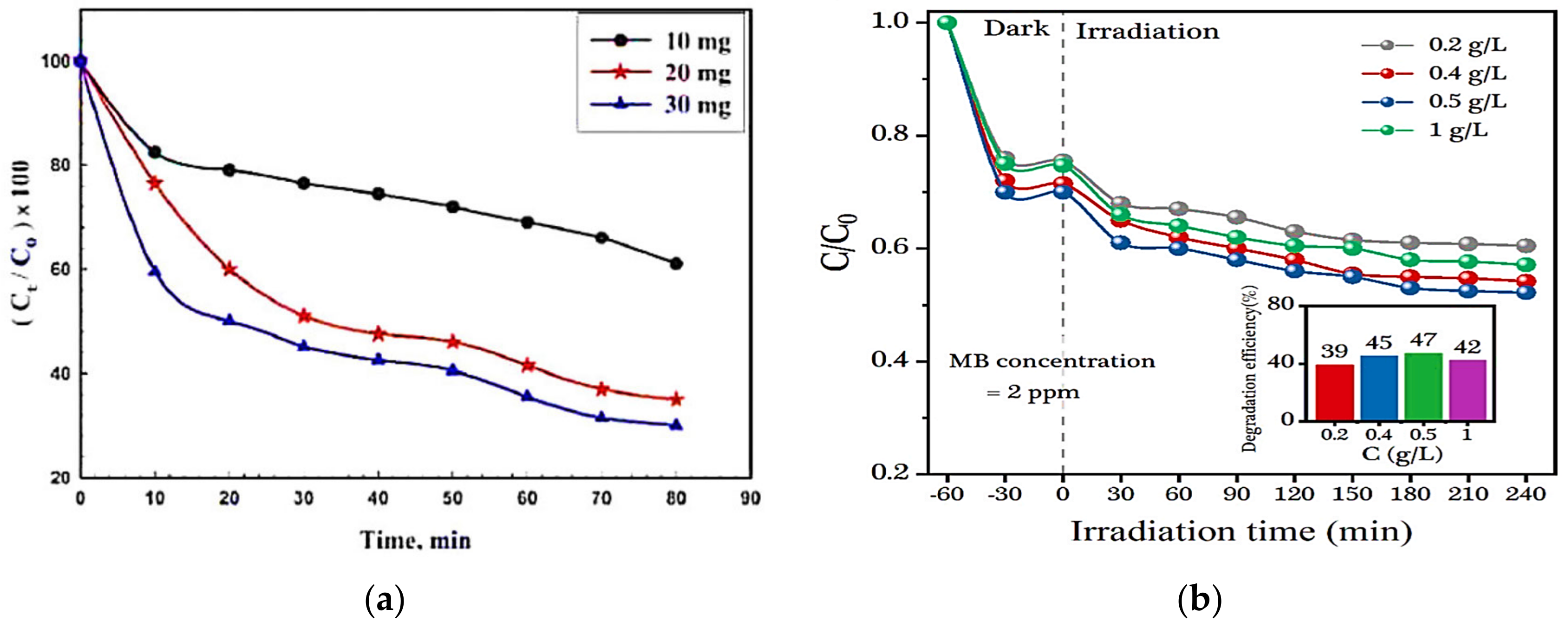
4.2. Effect of Catalyst Dose
4.3. Effect of Surface Area of Photocatalyst
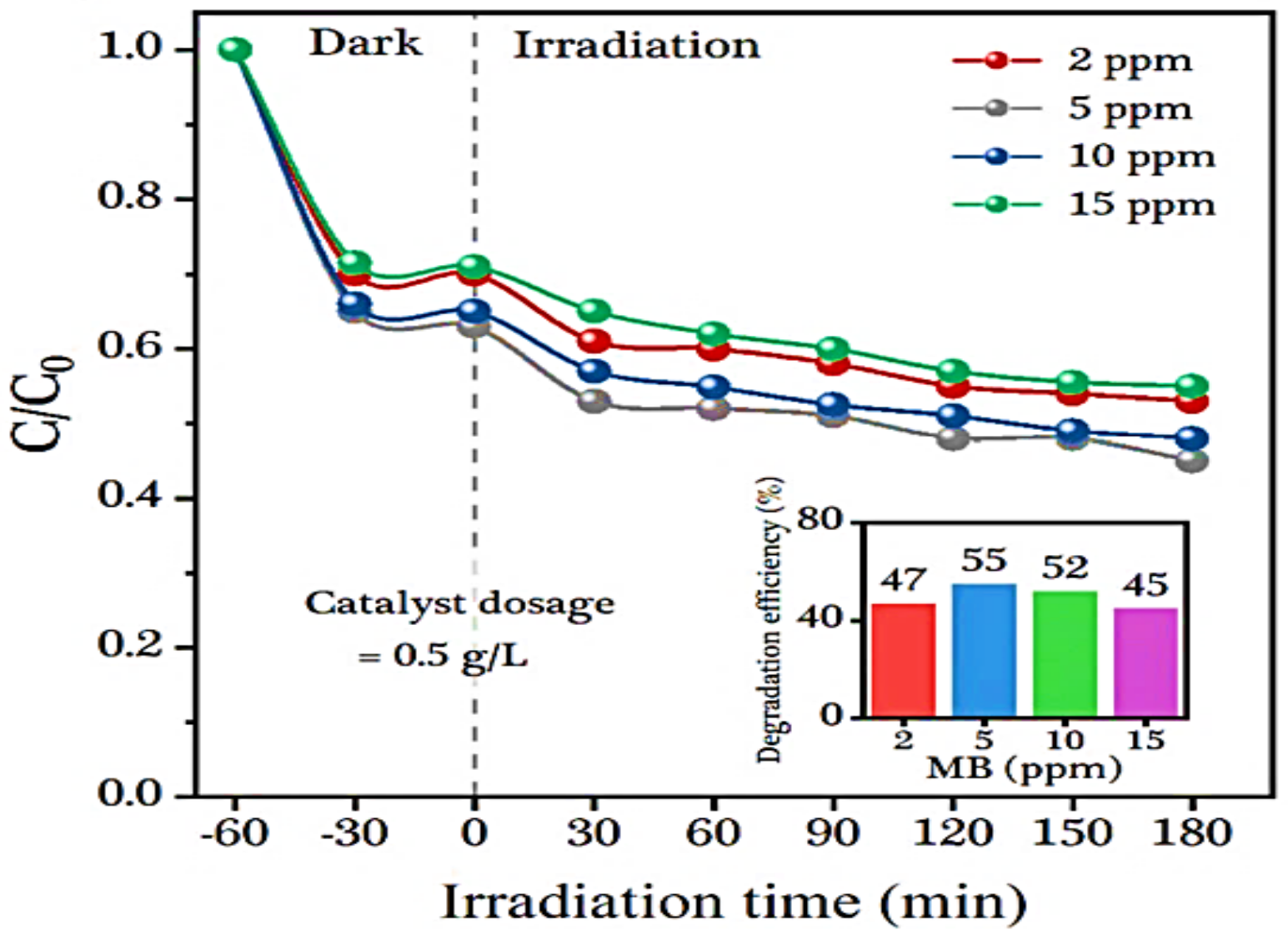
4.4. Effect of Substrate Concentration
4.5. Effect of Irradiation Intensity and Time
4.6. Effect of Doping
4.7. Effect of Quenchers

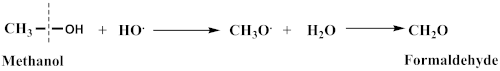
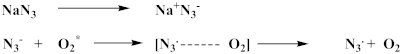
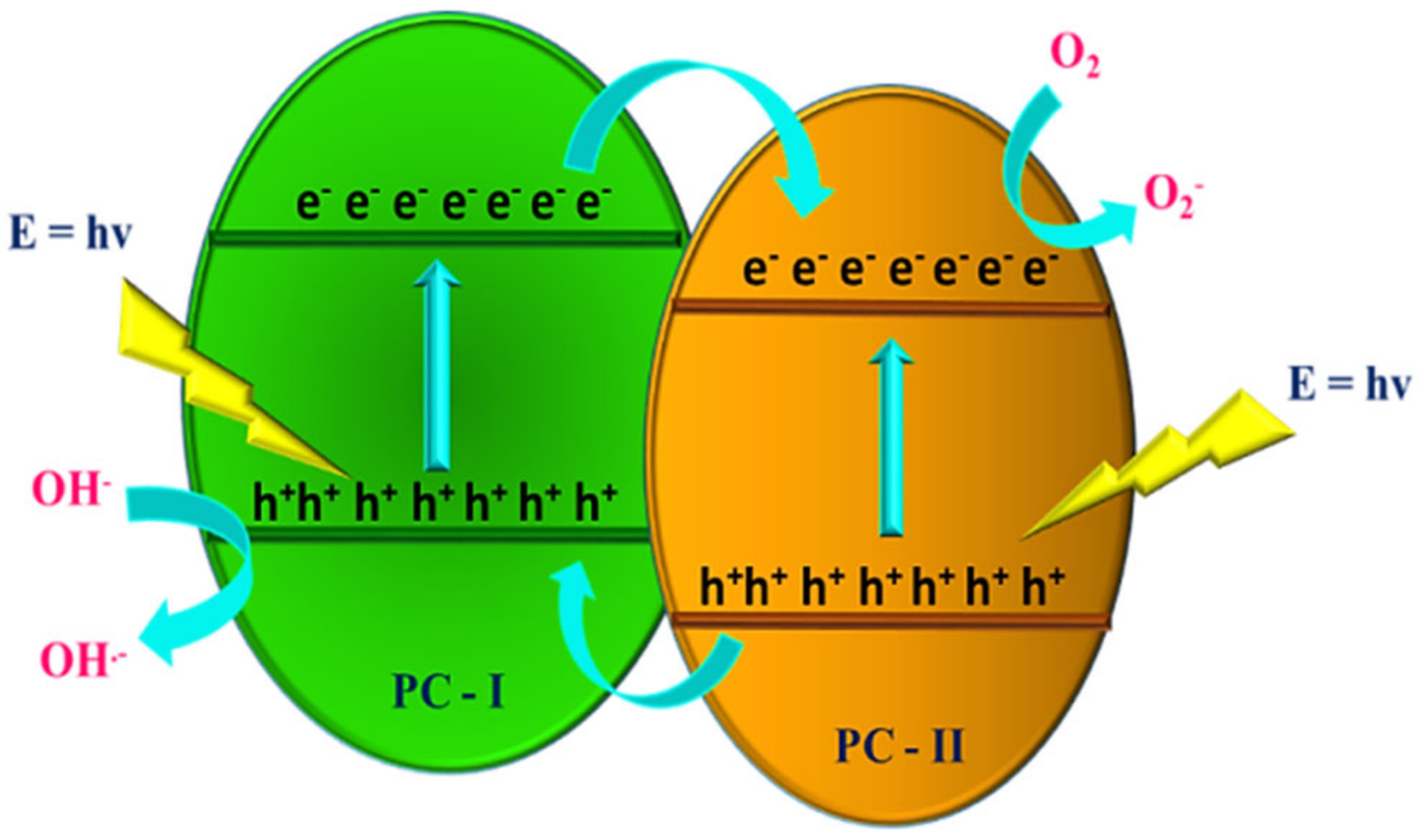
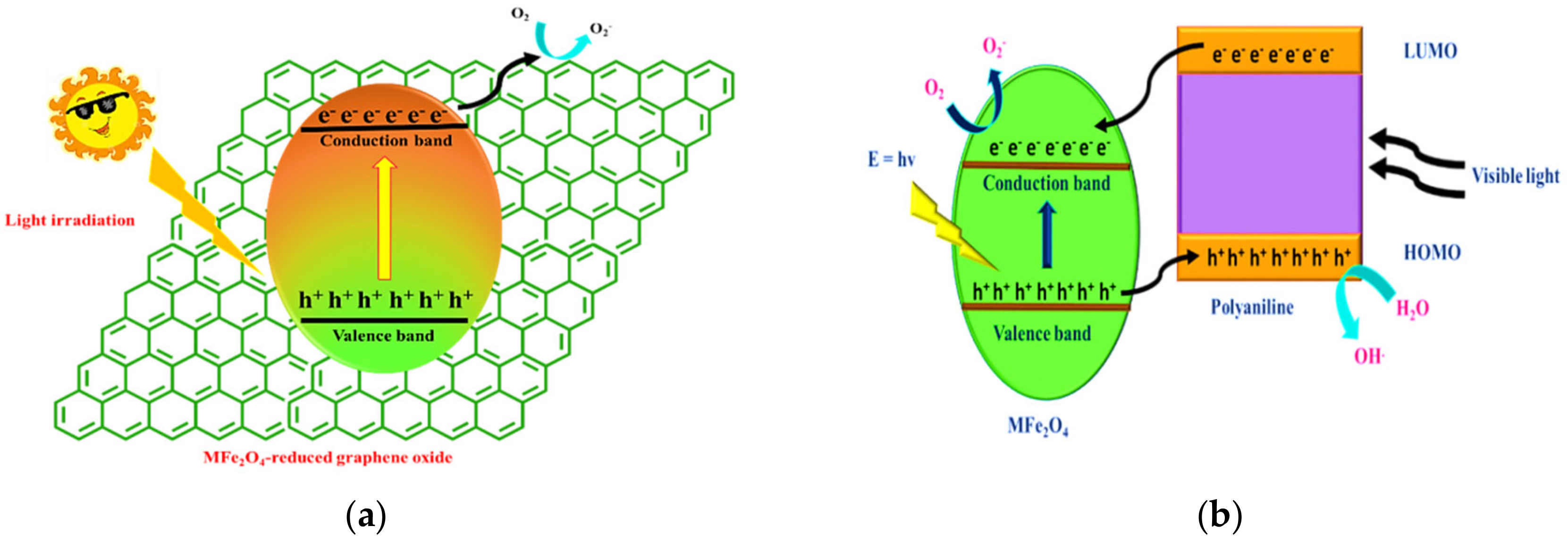
4.8. Photodegradation Mechanism
4.9. Reusability and Stability
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2020, 764, 142865. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L.; Ooi, C.W.; Anuar, M.S.; Ariff, A.; Yusof, Y.A.; Chen, S.K.; Annuar, M.S.M.; Ling, T.C. Recovery of lipase derived from Burkholderiacenocepacia ST8 using sustainable aqueous two-phase flotation composed of recycling hydrophilic organic solvent and inorganic salt. Sep. Purif. Technol. 2013, 110, 112–118. [Google Scholar] [CrossRef]
- Sakhare, P.A.; Pawar, S.S.; Bhat, T.S.; Yadav, S.D.; Patil, G.R.; Patil, P.S.; Sheikh, A.D. Magnetically Recoverable BiVO4/NiFe2O4 Nanocomposite Photocatalyst for Efficient Detoxification of Polluted Water Under Collected Sunlight. Mater. Res. Bull. 2020, 129, 110908. [Google Scholar] [CrossRef]
- Saxena, G.; Bharagava, R. Organic and Inorganic Pollutants in Industrial Wastes: Ecotoxicological Effects, Health Hazards, and Bioremediation Approaches. In Environmental Pollutants and Their Bioremediation Approaches; Bharagava, R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 23–56. ISBN 9781138628892. [Google Scholar]
- Uday, U.; Mahata, N.; Sasmal, S.; Bandyopadhyay, T.; Mondal, A.; Bhunia, B. Dyes Contamination in the Environment: Ecotoxicological Effects, Health Hazards, and Biodegradation and Bioremediation Mechanisms for Environmental Cleanup. In Environmental Pollutants and Their Bioremediation Approaches; Bharagava, R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 127–176. ISBN 9781138628892. [Google Scholar]
- Koduru, J.; Shankar, S.; More, N.; Shikha; Lingamdinne, L.; Singh, J. Toxic Metals Contamination in the Environment: Toxi-cological Effects and Bioremediation Approaches for Environmental Cleanup. In Environmental Pollutants and Their Bioremediation Approaches; Bharagava, R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 209–240. ISBN 9781138628892. [Google Scholar]
- Karimi-Maleh, H.; Alizadeh, M.; Orooji, Y.; Karimi, F.; Baghayeri, M.; Rouhi, J.; Tajik, S.; Beitollahi, H.; Agarwal, S.; Gupta, V.K.; et al. Guanine-Based DNA Biosensor Amplified with Pt/SWCNTs Nanocomposite as Analytical Tool for Nanomolar Determination of Daunorubicin as an Anticancer Drug: A Docking/Experimental Investigation. Ind. Eng. Chem. Res. 2021, 60, 816–823. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Kyzas, G.Z.; Matis, K.A. Various flotation techniques for metal ions removal. J. Mol. Liq. 2016, 225, 260–264. [Google Scholar] [CrossRef]
- Yu, K.L.; Show, P.L.; Ong, H.C.; Ling, T.C.; Lan, J.C.-W.; Chen, W.-H.; Chang, J.-S. Microalgae from wastewater treatment to biochar—Feedstock preparation and conversion technologies. Energy Convers. Manag. 2017, 150, 1–13. [Google Scholar] [CrossRef]
- Chua, S.F.; Nouri, A.; Ang, W.L.; Mahmoudi, E.; Mohammad, A.W.; Benamor, A.; Ba-Abbad, M. The emergence of multifunctional adsorbents and their role in environmental remediation. J. Environ. Chem. Eng. 2021, 9, 104793. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.-Y.; Zhang, Q.; Liu, H. Overview of recent developments of resource recovery from wastewater via electrochemistry-based technologies. Sci. Total Environ. 2021, 757, 143901. [Google Scholar] [CrossRef]
- Hansima, M.A.C.K.; Makehelwala, M.; Jinadasa, K.B.S.N.; Wei, Y.; Nanayakkara, K.G.N.; Herath, A.C.; Weerasooriya, R. Fouling of ion exchange membranes used in the electrodialysis reversal advanced water treatment: A review. Chemosphere 2021, 263, 127951. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [Green Version]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Al-Wakel, S.F.A.; Assi, H.A.; Naji, L.A.; Naushad, M. Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J. Water Process Eng. 2020, 33, 101112. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar] [CrossRef]
- Chen, C.; Duan, F.; Zhao, S.; Wang, W.; Yang, F.; Nuansing, W.; Zhang, B.; Qin, Y.; Knez, M. Porous Fe2O3 nanotubes with α-γ phase junction for enhanced charge separation and photocatalytic property produced by molecular layer deposition. Appl. Catal. B Environ. 2019, 248, 218–225. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, M.; Wang, L.; Fei, Y.; Wang, S.; Núñez-Delgado, A.; Bokhari, A.; Race, M.; Khataee, A.; Klemeš, J.J.; et al. Photocatalytic degradation of xanthate in flotation plant tailings by TiO2/graphene nanocomposites. Chem. Eng. J. 2022, 431, 134104. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A.; Abdulrasheed, A.A. A review on recent progression of photocatalytic desulphurization study over decorated photocatalysts. J. Ind. Eng. Chem. 2019, 74, 172–186. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Chakkunny, A.H.; Thomas, B.; Alexander, L.K. Influence of pH and salinity on the photocatalytic dye degradation and heavy metal ion reduction with cobalt ferrite photocatalysts. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1187, 012018. [Google Scholar] [CrossRef]
- Helmy, E.T.; Soliman, U.A.; Elbasiony, A.M.; Nguyen, B.-S. CuCe-Ferrite/TiO2 Nanocomposite as an Efficient Magnetically Separable Photocatalyst for Dye Pollutants Decolorization. Top. Catal. 2023, 66, 53–63. [Google Scholar] [CrossRef]
- Padmapriya, G.; Manikandan, A.; Krishnasamy, V.; Jaganathan, S.K.; Antony, S.A. Spinel Ni Zn1Fe2O4 (0.0 ≤ x ≤ 1.0) nano-photocatalysts: Synthesis, characterization and photocatalytic degradation of methylene blue dye. J. Mol. Struct. 2016, 1119, 39–47. [Google Scholar] [CrossRef]
- Haruna, A.; Abdulkadir, I.; Idris, S.O. Photocatalytic activity and doping effects of BiFeO3 nanoparticles in model organic dyes. Heliyon 2020, 6, e03237. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.P.; Marques, G.N.; Castro, M.A.M.; Costa, R.C.V.; Rangel, J.H.G.; Rodrigues, S.F.; dos Santos, C.C.; Oliveira, M.M. Synthesis and photocatalytic investigation of ZnFe2O4 in the degradation of organic dyes under visible light. J. Mater. Res. Technol. 2020, 9, 15001–15015. [Google Scholar] [CrossRef]
- Hitam, C.; Jalil, A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef]
- Ji, J.; Huang, Y.; Yin, J.; Zhao, X.; Cheng, X.; He, S.; Li, X.; He, J.; Liu, J. Synthesis and Electromagnetic and Microwave Absorption Properties of Monodispersive Fe3O4/α-Fe2O3 Composites. ACS Appl. Nano Mater. 2018, 1, 3935–3944. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y.; Adil, S.F. Polymeric Nanocomposites of Iron–Oxide Nanoparticles (IONPs) Synthesized Using Terminalia chebula Leaf Extract for Enhanced Adsorption of Arsenic(V) from Water. Colloids Interfaces 2019, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillert, R.; Taffa, D.H.; Wark, M.; Bredow, T.; Bahnemann, D.W. Research Update: Photoelectrochemical water splitting and photocatalytic hydrogen production using ferrites (MFe2O4) under visible light irradiation. APL Mater. 2015, 3, 104001. [Google Scholar] [CrossRef] [Green Version]
- Casbeer, E.; Sharma, V.K.; Li, X.-Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Buchanan, R.C. (Ed.) Ceramic Materials for Electronics, 3rd ed.; Dekker: New York, NY, USA; Basel, Switzerland, 2004; revised expanded; ISBN 9780824740283. [Google Scholar]
- Tokubuchi, T.; Arbi, R.I.; Zhenhua, P.; Katayama, K.; Turak, A.; Sohn, W.Y. Enhanced photoelectrochemical water splitting efficiency of hematite (α-Fe2O3)-Based photoelectrode by the introduction of maghemite (γ-Fe2O3) nanoparticles. J. Photochem. Photobiol. A Chem. 2021, 410, 113179. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Kushwaha, P.; Chauhan, P. Synthesis of spherical and Rod-Like EDTA assisted α-Fe2O3 nanoparticles via Co-precipitation method. Mater. Today Proc. 2021, 44, 3086–3090. [Google Scholar] [CrossRef]
- Uma, K.; KrishnaKumar, B.; Pan, G.-T.; Yang, T.C.-K.; Lin, J.-H. Enriched silver plasmon resonance activity on the sonochemical synthesis of ZnO flowers with α-Fe2O3 as an efficient catalyst for photo-Fenton reaction and photo-oxidation of ethanol. J. Water Process Eng. 2020, 34, 101089. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Zheng, Y.; Huang, R.; Yao, J. Facile and efficient synthesis of α-Fe2O3 nanocrystals by glucose-assisted thermal decomposition method and its application in lithium ion batteries. J. Power Sour. 2019, 416, 62–71. [Google Scholar] [CrossRef]
- Tadic, M.; Panjan, M.; Tadic, B.V.; Lazovic, J.; Damnjanovic, V.; Kopani, M.; Kopanja, L. Magnetic properties of hematite (α − Fe2O3) nanoparticles synthesized by sol-gel synthesis method: The influence of particle size and particle size distribution. J. Electr. Eng. 2019, 70, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.-H.; Hu, D.-B.; Liu, H.; Li, J.-Z.; Yi, T.-F. Hydrothermal synthesis and characterization of α-Fe2O3/C using acid-pickled iron oxide red for Li-ion batteries. J. Hazard. Mater. 2019, 368, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, G. Synthesis and Characterization of α-Fe2O3 Nanoparticles by Microemulsion Method. Erzincan Üniv. Fen Bilim. Enst. Derg. 2020, 13, 890–897. [Google Scholar] [CrossRef]
- He, H.-Y. Photocatalytic degradations of dyes on magnetically separable Ni1−xCoxFe2O4 nanoparticles synthesized by a hydrothermal process. Part. Sci. Technol. 2016, 34, 143–151. [Google Scholar] [CrossRef]
- Parsons, J.G.; Lopez, M.L.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Determination of arsenic (III) and arsenic (V) binding to microwave assisted hydrothermal synthetically prepared Fe3O4, Mn3O4, and MnFe2O4 nanoadsorbents. Microchem. J. 2009, 91, 100–106. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, M. Comparative studies on impact of synthesis methods on structural and magnetic properties of magnesium ferrite nanoparticles. Process. Appl. Ceram. 2014, 8, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Routray, K.L.; Saha, S.; Behera, D. Green synthesis approach for nano sized CoFe2O4 through aloe vera mediated sol-gel auto combustion method for high frequency devices. Mater. Chem. Phys. 2019, 224, 29–35. [Google Scholar] [CrossRef]
- Zhang, D.; Pu, X.; Du, K.; Yu, Y.M.; Shim, J.J.; Cai, P.; Kim, S.I.; Seo, H.J. Combustion synthesis of magnetic Ag/NiFe2O4 composites with enhanced visible-light photocatalytic properties. Sep. Purif. Technol. 2014, 137, 82–85. [Google Scholar] [CrossRef]
- Johnson, B.F.G. Nanoparticles in Catalysis. Top. Catal. 2003, 24, 147–159. [Google Scholar] [CrossRef]
- Govan, J.; Gun’Ko, Y.K. Recent Advances in the Application of Magnetic Nanoparticles as a Support for Homogeneous Catalysts. Nanomaterials 2014, 4, 222–241. [Google Scholar] [CrossRef] [Green Version]
- Demirelli, M.; Karaoglu, E.; Baykal, A.; Sozeri, H. M-hexaferrite–APTES/Pd(0) Magnetically Recyclable Nano Catalysts (MRCs). J. Inorg. Organomet. Polym. Mater. 2013, 23, 1274–1281. [Google Scholar] [CrossRef]
- Demirelli, M.; Karaoğlu, E.; Baykal, A.; Sözeri, H.; Uysal, E.; Duygulu, O. Recyclable NiFe2O4–APTES/Pd Magnetic Nanocatalyst. J. Inorg. Organomet. Polym. Mater. 2013, 23, 937–943. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Nogueira, I.D.; Ghumman, C.A.A.; Bundaleski, N.; Santos, A.; Teodoro, O.M.N.D.; Luque, R. Catalytic applications of a versatile magnetically separable Fe–Mo (Nanocat-Fe–Mo) nanocatalyst. Green Chem. 2013, 15, 682–689. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.K.; Branco, P.S.; Nogueira, I.D.; Velhinho, A.; Shrikhande, J.J.; Indulkar, U.U.; Jayaram, R.V.; Ghumman, C.A.A.; Bundaleski, N.; et al. Regio- and Chemoselective Reduction of Nitroarenes and Carbonyl Compounds over Recyclable Magnetic Ferrite-Nickel Nanoparticles (Fe3O4-Ni) by Using Glycerol as a Hydrogen Source. Chem. Eur. J. 2012, 18, 12628–12632. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Velhinho, A.; Nogueira, I.D.; Ghumman, C.A.A.; Teodoro, O.M.N.D.; Branco, P.S. A facile synthesis of cysteine–ferrite magnetic nanoparticles for application in multicomponent reactions—A sustainable protocol. RSC Adv. 2012, 2, 6144–6149. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.; Nogueira, I.D.; Ghumman, C.A.A.; Bundaleski, N.; Teodoro, O.M.N.D.; Branco, P.S. A Recyclable Ferrite-Co Magnetic Nanocatalyst for the Oxidation of Alcohols to Carbonyl Compounds. Chempluschem 2012, 77, 865–871. [Google Scholar] [CrossRef]
- Duan, H.-Z.; Zhou, F.-L.; Cheng, X.; Chen, G.-H.; Li, Q.-L. Preparation of hollow microspheres of Ce3+ dopedNiCo ferrite with high microwave absorbing performance. J. Magn. Magn. Mater. 2017, 424, 467–471. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Yuen, C.W.M.; Jiang, S.-X.; Hu, E. Hydrothermal synthesis of magnetic CoFe2O4/graphene nanocomposites with improved photocatalytic activity. Appl. Surf. Sci. 2015, 351, 140–147. [Google Scholar] [CrossRef]
- Rehman, M.A.; Yusoff, I.; Alias, Y. Structural, morphological and magnetic investigations of CuCe0.2Fe1.8O4 graphene-supported nanocomposites. Ceram. Int. 2016, 42, 1399–1407. [Google Scholar] [CrossRef]
- Wang, B.; Si, L.; Geng, J.; Su, Y.; Li, Y.; Yan, X.; Chen, L. Controllable magnetic 3D nitrogen-doped graphene gel: Synthesis, characterization, and catalytic performance. Appl. Catal. B Environ. 2017, 204, 316–323. [Google Scholar] [CrossRef]
- Randhawa, B.S.; Dosanjh, H.S.; Kaur, M. Preparation of Ferrites from the Combustion of Metal Nitrate-Oxalyl Dihydrazide Solutions. IJEMS 2005, 12, 151–154. [Google Scholar]
- Li, X.; Hou, Y.; Zhao, Q.; Wang, L. A general, one-step and template-free synthesis of sphere-like zinc ferrite nanostructures with enhanced photocatalytic activity for dye degradation. J. Colloid Interface Sci. 2011, 358, 102–108. [Google Scholar] [CrossRef]
- Dom, R.; Subasri, R.; Radha, K.; Borse, P.H. Synthesis of solar active nanocrystalline ferrite, MFe2O4 (M: Ca, Zn, Mg) photocatalyst by microwave irradiation. Solid State Commun. 2011, 151, 470–473. [Google Scholar] [CrossRef]
- Borhan, A.I.; Samoila, P.; Hulea, V.; Iordan, A.R.; Palamaru, M.N. Photocatalytic activity of spinel ZnFe2−xCrxO4 nanoparticles on removal Orange I azo dye from aqueous solution. J. Taiwan Inst. Chem. Eng. 2014, 45, 1655–1660. [Google Scholar] [CrossRef]
- Borhan, A.I.; Samoila, P.; Hulea, V.; Iordan, A.R.; Palamaru, M.N. Effect of Al3+ substituted zinc ferrite on photocatalytic degradation of Orange I azo dye. J. Photochem. Photobiol. A Chem. 2014, 279, 17–23. [Google Scholar] [CrossRef]
- Jesudoss, S.K.; Vijaya, J.J.; Kennedy, L.J.; Rajan, P.I.; Al-Lohedan, H.A.; Ramalingam, R.J.; Kaviyarasu, K.; Bououdina, M. Studies on the efficient dual performance of Mn1–xNixFe2O4 spinel nanoparticles in photodegradation and antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 165, 121–132. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W. Solution combustion synthesis of CaFe2O4 nanocrystal as a magnetically separable photocatalyst. Mater. Lett. 2014, 133, 212–215. [Google Scholar] [CrossRef]
- Shahid, M.; Jingling, L.; Ali, Z.; Shakir, I.; Warsi, M.F.; Parveen, R.; Nadeem, M. Photocatalytic degradation of methylene blue on magnetically separable MgFe2O4 under visible light irradiation. Mater. Chem. Phys. 2013, 139, 566–571. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, X.; Zhao, Q.; Li, Y.; Sun, C.; Cao, Y. Photocatalytic performances and activities of Ag-doped CuFe2O4 nanoparticles. Mater. Res. Bull. 2013, 48, 2927–2932. [Google Scholar] [CrossRef]
- Shetty, K.; Renuka, L.; Nagaswarupa, H.P.; Nagabhushana, H.; Anantharaju, K.S.; Rangappa, D.; Prashantha, S.C.; Ashwini, K. A comparative study on CuFe2O4, ZnFe2O4 and NiFe2O4: Morphology, Impedance and Photocatalytic studies. Mater. Today Proc. 2017, 4, 11806–11815. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gadri, A. RETRACTED ARTICLE: Photocatalytic degradation of methyl orange dye by NiFe2O4 nanoparticles under visible irradiation: Effect of varying the synthesis temperature. J. Mater. Sci. Mater. Electron. 2018, 29, 7057–7067. [Google Scholar] [CrossRef]
- Roonasi, P.; Mazinani, M. Synthesis and application of barium ferrite/activated carbon composite as an effective solar photocatalyst for discoloration of organic dye contaminants in wastewater. J. Environ. Chem. Eng. 2017, 5, 3822–3827. [Google Scholar] [CrossRef]
- Talebi, R. Preparation and characterization of cobalt ferrite nanoparticles with different capping agents and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2017, 28, 9749–9754. [Google Scholar] [CrossRef]
- Talukdar, S.; Mandal, D.; Mandal, K. Surface modification of Cobalt ferrite nano-hollowspheres for inherent multiple photoluminescence and enhanced photocatalytic activities. Chem. Phys. Lett. 2017, 672, 57–62. [Google Scholar] [CrossRef]
- Parhizkar, J.; Habibi, M.H.; Mosavian, S.Y. Synthesis and Characterization of Nano CoFe2O4 Prepared by Sol-Gel Auto-Combustion with Ultrasonic Irradiation and Evaluation of Photocatalytic Removal and Degradation Kinetic of Reactive Red 195. Silicon 2019, 11, 1119–1129. [Google Scholar] [CrossRef]
- Fang, M.-M.; Shao, J.-X.; Huang, X.-G.; Wang, J.-Y.; Chen, W. Direct Z-scheme CdFe2O4/g-C3N4 hybrid photocatalysts for highly efficient ceftiofur sodium photodegradation. J. Mater. Sci. Technol. 2020, 56, 133–142. [Google Scholar] [CrossRef]
- Bo, L.; Hu, Y.; Zhang, Z.; Tong, J. Efficient photocatalytic degradation of Rhodamine B catalyzed by SrFe2O4/g-C3N4 composite under visible light. Polyhedron 2019, 168, 94–100. [Google Scholar] [CrossRef]
- Shakir, I.; Sarfraz, M.; Ali, Z.; Aboud, M.F.A.; Agboola, P.O. Magnetically separable and recyclable graphene-MgFe2O4 nanocomposites for enhanced photocatalytic applications. J. Alloys Compd. 2016, 660, 450–455. [Google Scholar] [CrossRef]
- Grewal, J.K.; Kaur, M.; Sharma, R.K.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Structural and Photocatalytic Studies on Oxygen Hyperstoichiometric Titanium-Substituted Strontium Ferrite Nanoparticles. Magnetochemistry 2022, 8, 120. [Google Scholar] [CrossRef]
- Ubhi, M.K.; Kaur, M.; Singh, D.; Javed, M.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Hierarchical Nanoflowers of MgFe2O4, Bentonite and B-,P- Co-Doped Graphene Oxide as Adsorbent and Photocatalyst: Optimization of Parameters by Box–Behnken Methodology. Int. J. Mol. Sci. 2022, 23, 9678. [Google Scholar] [CrossRef]
- Singh, E.; Kaur, M.; Sharma, S. Structural tuning of CTAB@MgFe2O4 nanocomposite as peroxidase mimic for H2O2 and glucose sensing. Mater. Chem. Phys. 2021, 271, 124851. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Singh, D.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Synthesis of CaFe2O4-NGO Nanocomposite for Effective Removal of Heavy Metal Ion and Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2021, 11, 1471. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Ubhi, M.K.; Grewal, J.K.; Singh, D. Insight into the structural, optical, adsorptive, and photocatalytic properties of MgFe2O4-bentonite nanocomposites. J. Phys. Chem. Solids 2021, 154, 110060. [Google Scholar] [CrossRef]
- Khushboo; Kaur, M.; Jeet, K. Mechanistic insight into adsorption and photocatalytic potential of magnesium ferrite-bentonite nanocomposite. J. Photochem. Photobiol. A Chem. 2022, 425, 113717. [Google Scholar] [CrossRef]
- Ubhi, M.K.; Kaur, M.; Grewal, J.K.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Insight into photocatalytic behavior of magnesium ferrite–bentonite nanocomposite for the degradation of organic contaminants. J. Mater. Res. 2022, 38, 990–1006. [Google Scholar] [CrossRef]
- Guan, X.; Kuang, J.; Yang, L.; Lu, M.; Wang, G. Membrane-Solvothermal Synthesis of Cobalt Ferrite/Reduced Graphene Oxide Nanocomposites and Their Photocatalytic and Electromagnetic Wave Absorption Properties. ChemistrySelect 2019, 4, 9516–9522. [Google Scholar] [CrossRef]
- Sheshmani, S.; Falahat, B.; Nikmaram, F.R. Preparation of magnetic graphene oxide-ferrite nanocomposites for oxidative decomposition of Remazol Black B. Int. J. Biol. Macromol. 2017, 97, 671–678. [Google Scholar] [CrossRef]
- Yoo, P.S.; Reddy, D.A.; Jia, Y.; Bae, S.E.; Huh, S.; Liu, C. Magnetic core-shell ZnFe2O4/ZnS nanocomposites for photocatalytic application under visible light. J. Colloid Interface Sci. 2017, 486, 136–143. [Google Scholar] [CrossRef]
- Middea, A.; Spinelli, L.S.; Jr, F.G.S.; Neumann, R.; Fernandes, T.L.A.P.; Gomes, O.D.F.M. Preparation and characterization of an organo-palygorskite-Fe3O4 nanomaterial for removal of anionic dyes from wastewater. Appl. Clay Sci. 2017, 139, 45–53. [Google Scholar] [CrossRef]
- Song, W.; Gao, B.; Xu, X.; Xing, L.; Han, S.; Duan, P.; Song, W.; Jia, R. Adsorption–desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater. Bioresour. Technol. 2016, 210, 123–130. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Bian, Z.; Huo, Y.; Zhang, Y.; Zhu, J.; Lu, Y.; Li, H. Aerosol-spay assisted assembly of Bi2Ti2O7 crystals in uniform porous microspheres with enhanced photocatalytic activity. Appl. Catal. B Environ. 2009, 91, 247–253. [Google Scholar] [CrossRef]
- Harraz, F.A.; Mohamed, R.M.; Rashad, M.M.; Wang, Y.C.; Sigmund, W. Magnetic nanocomposite based on titania–silica/cobalt ferrite for photocatalytic degradation of methylene blue dye. Ceram. Int. 2014, 40, 375–384. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Su, C.-C.; Chen, C.-W.; Dong, C.-D. Synthesis of magnetically recoverable ferrite (MFe2O4, M Co, Ni and Fe)-supported TiO2 photocatalysts for decolorization of methylene blue. Catal. Commun. 2015, 72, 127–132. [Google Scholar] [CrossRef]
- Nardea, S.; Gadegoneb, S.; Lanjewar, M.R.; Lanjewar, R.B. Degradation of Azo Dye Congo Red Using Ni0.6Co0.4Fe2O4 as Photocatalyst. Pharma Chem. 2017, 9, 115–120. [Google Scholar]
- Ge, M.; Liu, W.; Hu, X.-R.; Li, Z.-L. Magnetically separable Ag/AgBr/NiFe2O4 composite as a highly efficient visible light plasmonic photocatalyst. J. Phys. Chem. Solids 2017, 109, 1–8. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; El-Sayyad, G.S.; El-Khawaga, A.M.; Abd Elkodous, M.; Abokhadra, A.; Elsayed, M.A.; Gobara, M.; Soliman, L.I.; El-Bahnasawy, H.H.; Ashour, A.H. Nanostructured Mg substituted Mn-Zn ferrites: A magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. J. Hazard. Mater. 2020, 399, 123000. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, P.; Bansal, S.; Singhal, S. Enhanced photocatalytic performance of Ru-doped spinel nanoferrites for treating recalcitrant organic pollutants in wastewater. J. Sol-Gel Sci. Technol. 2019, 92, 760–774. [Google Scholar] [CrossRef]
- Tabasum, A.; Alghuthaymi, M.; Qazi, U.Y.; Shahid, I.; Abbas, Q.; Javaid, R.; Nadeem, N.; Zahid, M. UV-Accelerated Photocatalytic Degradation of Pesticide over Magnetite and Cobalt Ferrite Decorated Graphene Oxide Composite. Plants 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, M.; Taher, M.A.; Tamaddon, A.-M. Facile synthesis and characterization of magnetic nanocomposite ZnO/CoFe2O4 hetero-structure for rapid photocatalytic degradation of imidacloprid. Heliyon 2019, 5, e02870. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Fang, G.; Liu, P.; Zhou, D.; Ma, C.; Zhang, D.; Zhan, J. Fe3O4@β-CD nanocomposite as heterogeneous Fenton-like catalyst for enhanced degradation of 4-chlorophenol (4-CP). Appl. Catal. B Environ. 2016, 188, 113–122. [Google Scholar] [CrossRef]
- Boruah, P.K.; Sharma, B.; Karbhal, I.; Shelke, M.V.; Das, M.R. Ammonia-modified graphene sheets decorated with magnetic Fe3O4 nanoparticles for the photocatalytic and photo-Fenton degradation of phenolic compounds under sunlight irradiation. J. Hazard. Mater. 2016, 325, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, X.; Zhao, Q.; Chen, G. ZnFe2O4 multi-porous microbricks/graphene hybrid photocatalyst: Facile synthesis, improved activity and photocatalytic mechanism. Appl. Catal. B Environ. 2013, 142–143, 80–88. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Gao, J.; Yan, T.; Zhou, F.; Cui, S.; Bi, W. Synthesis of Fe3O4/g-C3N4 nanocomposites and their application in the photodegradation of 2,4,6-trichlorophenol under visible light. Mater. Lett. 2016, 164, 183–189. [Google Scholar] [CrossRef]
- Stoia, M.; Muntean, C.; Militaru, B. MnFe2O4 nanoparticles as new catalyst for oxidative degradation of phenol by peroxydisulfate. J. Environ. Sci. 2017, 53, 269–277. [Google Scholar] [CrossRef]
- Arifin, N.; Karim, K.M.R.; Abdullah, H.; Khan, M.R. Synthesis of Titania Doped Copper Ferrite Photocatalyst and Its Photoactivity towards Methylene Blue Degradation under Visible Light Irradiation. Bull. Chem. React. Eng. Catal. 2019, 14, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Mapossa, A.B.; Mhike, W.; Adalima, J.L.; Tichapondwa, S. Removal of Organic Dyes from Water and Wastewater Using Magnetic Ferrite-Based Titanium Oxide and Zinc Oxide Nanocomposites: A Review. Catalysts 2021, 11, 1543. [Google Scholar] [CrossRef]
- Gupta, N.K.; Ghaffari, Y.; Kim, S.; Bae, J.; Kim, K.S.; Saifuddin, M. Photocatalytic Degradation of Organic Pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) Nanoparticles at Neutral PH. Sci. Rep. 2020, 10, 4942. [Google Scholar] [CrossRef] [Green Version]
- Makofane, A.; Motaung, D.E.; Hintsho-Mbita, N.C. Photocatalytic degradation of methylene blue and sulfisoxazole from water using biosynthesized zinc ferrite nanoparticles. Ceram. Int. 2021, 47, 22615–22626. [Google Scholar] [CrossRef]
- Rashmi, S.K.; Bhojya Naik, H.S.; Jayadevappa, H.; Viswanath, R.; Patil, S.B.; Madhukara Naik, M. Solar light responsive Sm-Zn ferrite nanoparticle as efficient photocatalyst. Mater. Sci. Eng. B 2017, 225, 86–97. [Google Scholar] [CrossRef]
- Kirankumar, V.S.; Sumathi, S. Copper and cerium co-doped cobalt ferrite nanoparticles: Structural, morphological, optical, magnetic, and photocatalytic properties. Environ. Sci. Pollut. Res. 2019, 26, 19189–19206. [Google Scholar] [CrossRef]
- Mahdikhah, V.; Saadatkia, S.; Sheibani, S.; Ataie, A. Outstanding photocatalytic activity of CoFe2O4/rGO nanocomposite in degradation of organic dyes. Opt. Mater. 2020, 108, 110193. [Google Scholar] [CrossRef]
- Manikandan, A.; Hema, E.; Durka, M.; Seevakan, K.; Alagesan, T.; Arul Antony, S. Room Temperature Ferromagnetism of Magnetically Recyclable Photocatalyst of Cu1−xMnxFe2O4-TiO2 (0.0 ≤ x ≤ 0.5) Nanocomposites. J. Supercond. Nov. Magn. 2015, 28, 1783–1795. [Google Scholar] [CrossRef]
- Grewal, J.K.; Kaur, M.; Ubhi, M.K.; Oliveira, A.C.; Garg, V.K.; Sharma, V.K. Structural, magnetic, and photocatalytic properties of core–shell reversal nanocomposites of titanium-doped strontium ferrite and silica. J. Mater. Res. 2023, 38, 1019–1034. [Google Scholar] [CrossRef]
- Mohan, H.; Ramalingam, V.; Adithan, A.; Natesan, K.; Seralathan, K.-K.; Shin, T. Highly efficient visible light driven photocatalytic activity of zinc/ferrite: Carbamazepine degradation, mechanism and toxicity assessment. J. Hazard. Mater. 2021, 416, 126209. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Huang, C.P.; Doong, R.-A. Photocatalytic degradation of bisphenol A over a ZnFe2O4/TiO2 nanocomposite under visible light. Sci. Total Environ. 2019, 646, 745–756. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, L.T.H.; Manh, N.C.; Quoc, D.N.; Quang, H.N.; Nguyen, H.T.T.; Nguyen, D.C.; Bach, L.G. A Facile Synthesis, Characterization, and Photocatalytic Activity of Magnesium Ferrite Nanoparticles via the Solution Combustion Method. J. Chem. 2019, 2019, 3428681. [Google Scholar] [CrossRef]
- Sun, M.; Han, X.; Chen, S. Synthesis and photocatalytic activity of nano-cobalt ferrite catalyst for the photo-degradation various dyes under simulated sunlight irradiation. Mater. Sci. Semicond. Process. 2019, 91, 367–376. [Google Scholar] [CrossRef]
- Sundararajan, M.; John Kennedy, L.; Nithya, P.; Judith Vijaya, J.; Bououdina, M. Visible Light Driven Photocatalytic Degradation of Rhodamine B Using Mg Doped Cobalt Ferrite Spinel Nanoparticles Synthesized by Microwave Combustion Method. J. Phys. Chem. Solids 2017, 108, 61–75. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, M.; Garg, V.K.; Oliveira, A.C. Oxygen hyper stoichiometric trimetallic titanium doped magnesium ferrite: Structural and photocatalytic studies. Ceram. Int. 2022, 48, 24476–24484. [Google Scholar] [CrossRef]
- Sharma, R.; Komal; Kumar, V.; Bansal, S.; Singhal, S. Boosting the catalytic performance of pristine CoFe2O4 with yttrium (Y3+) inclusion in the spinel structure. Mater. Res. Bull. 2017, 90, 94–103. [Google Scholar] [CrossRef]
- Dhiman, M.; Chudasama, B.; Kumar, V.; Tikoo, K.B.; Singhal, S. Augmenting the photocatalytic performance of cobalt ferrite via change in structural and optical properties with the introduction of different rare earth metal ions. Ceram. Int. 2019, 45, 3698–3709. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Anandan, S.; Ashokkumar, M. CoFe2O4/TiO2 nanocatalysts for the photocatalytic degradation of Reactive Red 120 in aqueous solutions in the presence and absence of electron acceptors. Chem. Eng. J. 2013, 220, 302–310. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Kanjwal, M.A.; Chronakis, I.S.; Kim, H.Y. Influence of temperature on the photodegradation process using Ag-doped TiO2 nanostructures: Negative impact with the nanofibers. J. Mol. Catal. A Chem. 2013, 366, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.P.A. A Review on the Factors Affecting the Photocatalytic Degradation of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ubhi, M.K.; Kaur, M.; Singh, D.; Sharma, V.K. Structural and adsorptive properties of boron- and phosphorous-doped graphene oxide: Insight into effective removal of Pb (II) and as (III). J. Water Process Eng. 2023, 52, 103539. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, C.; Bi, H.; Liu, Y.; Yan, Q. Magnetically separable CuFe2O4/AgBr composite photocatalysts: Preparation, characterization, photocatalytic activity and photocatalytic mechanism under visible light. Appl. Surf. Sci. 2017, 392, 701–707. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Zafar, M.N.; Dar, Q.; Nawaz, F.; Zafar, M.N.; Iqbal, M.; Nazar, M.F. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef]
- Miri, A.; Mahabbati, F.; Najafidoust, A.; Miri, M.J.; Sarani, M. Nickel oxide nanoparticles: Biosynthesized, characterization and photocatalytic application in degradation of methylene blue dye. Inorg. Nano-Metal Chem. 2022, 52, 122–131. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, V.; Romero, R.; Sharma, K.; Singh, J. Applications of Nanoparticles in Wastewater Treatment. In Nanobiotechnology in Bioformulations; Prasad, R., Kumar, V., Kumar, M., Choudhary, D., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 395–418. ISBN 9783030170608. [Google Scholar]
- Dimapilis, E.A.S.; Hsu, C.-S.; Mendoza, R.M.O.; Lu, M.-C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Dobrucka, R. Facile Synthesis of Trimetallic Nanoparticles Au/CuO/ZnO Using Vitex Agnus-Castus Extract and Their Activity in Degradation of Organic Dyes. Int. J. Environ. Anal. Chem. 2021, 101, 2046–2057. [Google Scholar] [CrossRef]
- Kirankumar, V.S.; Sumathi, S. Photocatalytic and antibacterial activity of bismuth and copper co-doped cobalt ferrite nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 8738–8746. [Google Scholar] [CrossRef]
- Sun, F.; Zeng, Q.; Tian, W.; Zhu, Y.; Jiang, W. Magnetic MFe2O4-Ag2O (M = Zn, Co, & Ni) composite photocatalysts and their application for dye wastewater treatment. J. Environ. Chem. Eng. 2019, 7, 103011. [Google Scholar] [CrossRef]
- Babu, B.; Koutavarapu, R.; Shim, J.; Yoo, K. SnO2 quantum dots decorated NiFe2O4 nanoplates: 0D/2D heterojunction for enhanced visible-light-driven photocatalysis. Mater. Sci. Semicond. Process. 2020, 107, 104834. [Google Scholar] [CrossRef]
- Kuang, M.; Zhang, J.; Wang, W.; Chen, J.; Liu, R.; Xie, S.; Wang, J.; Ji, Z. Synthesis of octahedral-like ZnO/ZnFe2O4 heterojunction photocatalysts with superior photocatalytic activity. Solid State Sci. 2019, 96, 105901. [Google Scholar] [CrossRef]
- Emadian, S.S.; Ghorbani, M.; Bakeri, G. Magnetically separable CoFe2O4/ZrO2 nanocomposite for the photocatalytic reduction of hexavalent chromium under visible light irradiation. Synth. Met. 2020, 267, 116470. [Google Scholar] [CrossRef]
- Silva, E.D.N.; Brasileiro, I.L.O.; Madeira, V.S.; de Farias, B.A.; Ramalho, M.L.A.; Rodríguez-Aguado, E.; Rodríguez-Castellón, E. Reusable CuFe2O4–Fe2O3 catalyst synthesis and application for the heterogeneous photo-Fenton degradation of methylene blue in visible light. J. Environ. Chem. Eng. 2020, 8, 104132. [Google Scholar] [CrossRef]
- Munir, S.; Rasheed, A.; Zulfiqar, S.; Aadil, M.; Agboola, P.O.; Shakir, I.; Warsi, M.F. Synthesis, characterization and photocatalytic parameters investigation of a new CuFe2O4/Bi2O3 nanocomposite. Ceram. Int. 2020, 46, 29182–29190. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, Y.; Lin, S.; Wang, L.; Wang, C. Synthesis of magnetic ZnFe2O4/graphene composite and its application in photocatalytic degradation of dyes. J. Alloys Compd. 2013, 579, 336–342. [Google Scholar] [CrossRef]
- Behera, A.; Mansingh, S.; Das, K.K.; Parida, K. Synergistic ZnFe2O4-carbon allotropes nanocomposite photocatalyst for norfloxacin degradation and Cr (VI) reduction. J. Colloid Interface Sci. 2019, 544, 96–111. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Singh, P. Magnetically recoverable graphitic carbon nitride and NiFe2O4 based magnetic photocatalyst for degradation of oxytetracycline antibiotic in simulated wastewater under solar light. J. Environ. Chem. Eng. 2018, 6, 3874–3883. [Google Scholar] [CrossRef]
- Palanivel, B.; Ayappan, C.; Jayaraman, V.; Chidambaram, S.; Maheswaran, R.; Mani, A. Inverse spinel NiFe2O4 deposited g-C3N4 nanosheet for enhanced visible light photocatalytic activity. Mater. Sci. Semicond. Process. 2019, 100, 87–97. [Google Scholar] [CrossRef]
- Wang, Q.; Hui, J.; Li, J.; Cai, Y.; Yin, S.; Wang, F.; Su, B. Photodegradation of methyl orange with PANI-modified BiOCl photocatalyst under visible light irradiation. Appl. Surf. Sci. 2013, 283, 577–583. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Dong, H.; Yan, M.; Wang, J.; Hu, W.; Wang, J.; Zhou, Y.; Tang, J. Enhanced visible light photocatalytic performance of polyaniline modified mesoporous single crystal TiO2 microsphere. Appl. Surf. Sci. 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Kong, X.; He, F.; Zhao, R.; Wu, R.; Wei, T.; Wang, L.; Feng, J. A novel P-N heterojunction with staggered energy level based on ZnFe2O4 decorating SnS2 nanosheet for efficient photocatalytic degradation. Appl. Surf. Sci. 2020, 510, 145442. [Google Scholar] [CrossRef]
- Huang, S.; Xu, Y.; Zhou, T.; Xie, M.; Ma, Y.; Liu, Q.; Jing, L.; Xu, H.; Li, H. Constructing magnetic catalysts with in-situ solid-liquid interfacial photo-Fenton-like reaction over Ag3PO4@NiFe2O4 composites. Appl. Catal. B Environ. 2018, 225, 40–50. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhang, L.; Zhuo, S. A facile route to the synthesis of magnetically separable BiOBr/NiFe2O4 composites with enhanced photocatalytic performance. Appl. Surf. Sci. 2017, 419, 586–594. [Google Scholar] [CrossRef]
- Eskandari, N.; Nabiyouni, G.; Masoumi, S.; Ghanbari, D. Preparation of a new magnetic and photo-catalyst CoFe2O4–SrTiO3 perovskite nanocomposite for photo-degradation of toxic dyes under short time visible irradiation. Compos. Part B Eng. 2019, 176, 107343. [Google Scholar] [CrossRef]
- Jia, J.; Du, X.; Zhang, Q.; Liu, E.; Fan, J. Z-scheme MgFe2O4/Bi2MoO6 heterojunction photocatalyst with enhanced visible light photocatalytic activity for malachite green removal. Appl. Surf. Sci. 2019, 492, 527–539. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Deng, F.; Luo, X.; Dionysiou, D.D. Rapid toxicity elimination of organic pollutants by the photocatalysis of environment-friendly and magnetically recoverable step-scheme SnFe2O4/ZnFe2O4 nano-heterojunctions. Chem. Eng. J. 2020, 379, 122264. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Kumar, P.S.; Vo, D.-V.N.; Cornejo-Ponce, L. Recent advancements of spinel ferrite based binary nanocomposite photocatalysts in wastewater treatment. Chemosphere 2021, 274, 129734. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.H.; Jennings, B.D.; Akinoglu, G.E.; Selkirk, A.; Gatensby, R.; Mokarian-Tabari, P. Enhanced Dye Degradation through Multi-Particle Confinement in a Porous Silicon Substrate: A Highly Efficient, Low Band Gap Photocatalyst. Adv. Opt. Mater. 2021, 9, 2002238. [Google Scholar] [CrossRef]
- Wilson, A.; Mishra, S.R.; Gupta, R.; Ghosh, K. Preparation and photocatalytic properties of hybrid core–shell reusable CoFe2O4–ZnO nanospheres. J. Magn. Magn. Mater. 2012, 324, 2597–2601. [Google Scholar] [CrossRef]
- Suwarnkar, M.B.; Dhabbe, R.S.; Kadam, A.N.; Garadkar, K.M. Enhanced photocatalytic activity of Ag doped TiO2 nanoparticles synthesized by a microwave assisted method. Ceram. Int. 2014, 40, 5489–5496. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, F.; Wang, M.; Ding, J.; Sun, S.; Bao, J.; Gao, C. Facile synthesis, structure and visible light photocatalytic activity of recyclable ZnFe2O4/TiO2. Appl. Surf. Sci. 2014, 319, 83–89. [Google Scholar] [CrossRef]
- Gupta, V.K.; Eren, T.; Atar, N.; Yola, M.L.; Parlak, C.; Karimi-Maleh, H. CoFe2O4@TiO2 decorated reduced graphene oxide nanocomposite for photocatalytic degradation of chlorpyrifos. J. Mol. Liq. 2015, 208, 122–129. [Google Scholar] [CrossRef]
- Masunga, N.; Mmelesi, O.K.; Kefeni, K.K.; Mamba, B.B. Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: Review. J. Environ. Chem. Eng. 2019, 7, 103179. [Google Scholar] [CrossRef]
- Rani, M.; Rachna; Shanker, U. Efficient photocatalytic degradation of Bisphenol A by metal ferrites nanoparticles under sunlight. Environ. Technol. Innov. 2020, 19, 100792. [Google Scholar] [CrossRef]


| Type | Methods | Advantages | Disadvantages |
|---|---|---|---|
| Physical | Ball Milling |
|
|
| Chemical | Sol-gel |
|
|
| Co-precipitation |
|
| |
| Combustion |
|
| |
| Micro emulsion |
|
| |
| Microwave and Hydrothermal |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, G.; Ubhi, M.K.; Jeet, K.; Singla, C.; Kaur, M. A Review on Impacting Parameters for Photocatalytic Degradation of Organic Effluents by Ferrites and Their Nanocomposites. Processes 2023, 11, 1727. https://doi.org/10.3390/pr11061727
Singh G, Ubhi MK, Jeet K, Singla C, Kaur M. A Review on Impacting Parameters for Photocatalytic Degradation of Organic Effluents by Ferrites and Their Nanocomposites. Processes. 2023; 11(6):1727. https://doi.org/10.3390/pr11061727
Chicago/Turabian StyleSingh, Gurpinder, Manpreet Kaur Ubhi, Kiran Jeet, Chetan Singla, and Manpreet Kaur. 2023. "A Review on Impacting Parameters for Photocatalytic Degradation of Organic Effluents by Ferrites and Their Nanocomposites" Processes 11, no. 6: 1727. https://doi.org/10.3390/pr11061727