A Review on the Full Chain Application of 3D Printing Technology in Precision Medicine
Abstract
:1. Introduction
2. Silicon Models
2.1. Three-Dimensional Printing Technology of Silicon
2.1.1. Vat Photopolymerization
2.1.2. Material Jetting
2.1.3. Extrusion Processes
2.2. Training and Surgical Planning
3. Surgical Navigation Template
3.1. Osteotomy Guide Plate
3.2. Guide Plate for Nail Placement
4. Invisible Aligners
5. Implants
5.1. PEEK Implants
5.2. Titanium Alloy Implants
6. Discussion and Prospects
- (1)
- Strength issue: Compared with traditional manufacturing, products manufactured by means of 3D printing have certain differences in many aspects such as strength, hardness, and flexibility. The manufacturing process of 3D printing is additive, layer-by-layer production, which makes it difficult to match the material properties achieved by traditional molding techniques, even if the layers are bonded tightly. Currently, 3D-printed products cannot be used on a large scale as functional parts.
- (2)
- Accuracy issue: Due to the layer-by-layer production method of 3D printing, there is a common “stair-step effect”, especially when manufacturing objects with curved surfaces, which inevitably leads to deviations in accuracy. In addition, many 3D-printed objects require secondary processing such as sanding and high-temperature heating. The produced objects can easily warp due to material shrinkage, further reducing their accuracy.
- (3)
- It is difficult to achieve functional medical models. Although 3D-printed personalized medical models can solve the problem of the scarcity and high cost of medical training models, and provide medical staff with a three-dimensional understanding of patients’ anatomical structures, these models are still static. Currently, silicone medical models can simulate the flexibility of human organs, but lack functional simulation, which means that 3D-printed models cannot truly simulate the physiological characteristics of the human body. In complex minimally invasive surgeries such as those involving the heart, major blood vessels, and neurovasculature, functional medical models will provide precise surgical guidance to medical personnel, thereby achieving high-precision medical outcomes.
- (4)
- It is difficult to establish uniform quality testing standards. Surgical navigation templates, invisible orthodontic appliances, and human implants can all be considered medical auxiliary devices. Due to the differentiated physiological characteristics of the human body, medical devices manufactured through 3D printing are personalized rather than standardized. Establishing quality assessment standards for these personalized auxiliary devices is therefore challenging. Without quality testing standards, it will be difficult to determine the performance of these devices, increasing clinical application costs and reducing medical efficacy. This deficiency will also severely hinder the application and promotion of 3D printing technology in the market.
- (5)
- Static auxiliary devices cannot adapt to complex biomechanical characteristics. Invisible orthodontic appliances need to move with the upper and lower jawbones and be subject to dynamic tooth forces, while implants in the human body need to be in contact with bone tissue and withstand certain stresses. Three-dimensionally printed internal auxiliary devices in the human body will experience complex and variable stress conditions, but the devices cannot change with changing surrounding conditions. This lack of dynamic biological characteristics makes it difficult for these auxiliary devices to adapt to complex human tissue structures, thereby reducing their therapeutic effectiveness.
- (1)
- Developing application standards for 3D printing medical auxiliary devices. The major obstacle for promoting the application of 3D printing medical devices is the lack of corresponding quality testing standards. Traditional inspection standards for manufactured parts are relatively straightforward to develop, as common indicators can be summarized for mass-produced parts. However, 3D printing medical devices are personalized and their application cases are also individualized, making it difficult to find uniform quality testing standards to evaluate their printing quality and clinical effectiveness. In order to establish a sound set of quality testing standards for 3D printing medical devices, application standards for 3D printing can be added to existing standards, such as material standards and surface quality standards.
- (2)
- Conducting applications of 4D printing technology. The human body can be understood as a complex and variable biological field, making it difficult for static and unchanging internal assistive devices to adapt to this dynamic environment. Research and development of assistive devices that can adapt to changes in working conditions can greatly improve medical effectiveness. These devices can respond to changes in the surrounding physical field, and their structure also correspondingly changes to meet the compatibility with other tissues and avoid the impact of biologic forces. Four-dimensional printing technology can achieve the effect of corresponding structural changes due to changes in temperature, humidity, and mechanics, etc., and has good adaptability in the human body. For example, an invisible orthodontic appliance manufactured using 4D printing technology can adapt to new mechanical conditions as the force field between the appliance and teeth changes due to changes in biting or alignment. However, controlling its structure is still a challenge that requires extensive research.
- (3)
- Development of new materials and new processes. Biocompatibility is an important performance of internal assistive devices. Traditional single implant materials cannot meet the biological characteristics of human tissues, including biomechanical properties and biocompatibility. The clinical demand for implants with excellent biocompatibility will greatly increase. The development of new composite materials and their printing processes can improve the level of medical treatment and enhance the effectiveness of precision medicine. This is an important research direction for 3D printing of human implants. For example, tantalum metal has excellent biocompatibility and has become a research hotspot for orthopedic implants. However, the clinical application of 3D-printed porous tantalum is limited, and there is a lack of supporting design theories and manufacturing processes. Related studies are not yet systematic. Therefore, research on new materials, composite materials and their 3D printing processes is an important breakthrough for achieving precise 3D printing of medical implants.
- (4)
- Intelligent manufacturing applications. Traditional 3D printing of medical models cannot record information such as the force, position, and motion trajectory of the operator’s actions, and the level of skill and training effectiveness cannot be quantified. By combining multi-sensor technology with medical models and using big data to simulate the real surgical environment, it is possible to achieve the quantitative evaluation of skill levels, such as by incorporating pressure sensors for quantitatively evaluating operating pressure. This can create an objective evaluation method system for surgical procedures and achieve a quantitative score for surgical skills.
- (5)
- Three-dimensional bioprinting technology. Three-dimensional bioprinting technology can create disease models with highly simulated physiological structures by controlling the spatial arrangement of tissue cells. Many functions of the body’s tissues are essential, such as the multilayer barrier function that controls transdermal drug delivery. Researchers can replicate this function by creating 3D-printed biological tissues to improve drug testing. Furthermore, bio-3D printing technology can achieve the reconstruction of human tissue organs for repairing or transplanting human tissue organs. Therefore, the development of 3D bioprinting technology can bring new breakthroughs to precision medicine.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, F.S.; Varmus, H. A New Initiative on Precision Medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.J.; Cui, Z.W.; Sun, F.; Liu, H.B.; Tang, Z.M. Superalloy GH4169 complicated components prepared by selective laser melting forming technique. Powder Met. Technol. 2016, 34, 368. [Google Scholar]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Saggiomo, V.; Velders, A. Simple 3D Printed Scaffold-Removal Method for Fabrication of Intricate Microfluidic Devices. Adv. Sci. 2015, 2, 1500125. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.Y.; Homan, K.A.; Robinson, S.S.; Kolesky, D.B.; Duarte, N.; Moisan, A.; Lewis, J.A. Renal Reabsorption in 3D vascularized proximal tubule models. Proc. Natl. Acad. Sci. USA 2019, 116, 5399–5404. [Google Scholar] [CrossRef] [Green Version]
- Davoodi, E.; Montazerian, H.; Haghniaz, R.; Rashidi, A.; Ahadian, S.; Sheikhi, A.; Chen, J.; Khademhosseini, A.; Milani, A.S.; Hoorfar, M.; et al. 3D-Printed Ultra-Robost Surface-Doped Porous Silicone Sensors for Wearable Biomonitoring. ACS Nano 2020, 14, 1520–1532. [Google Scholar] [CrossRef]
- Morris, C.; Barber, R.; Day, R. Orofacial prosthesis design and fabrication using stereolithography. Aust. Dent. J. 2000, 45, 250–253. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, B.; Bi, Y.; Zhao, Y. Selective laser sintering technology for customized fabrication of facial prostheses. J. Prosthet. Dent. 2008, 100, 56–60. [Google Scholar] [CrossRef]
- Yoshioka, F.; Ozawa, S.; Okazaki, S.; Tanaka, Y. Fabrication of an Orbital Prosthesis Using a Noncontact Three-Dimensional Digitizer and Rapid-Prototyping System. J. Prosthodont. 2010, 19, 598–600. [Google Scholar] [CrossRef]
- Al Mardini, M.; Ercoli, C.; Graser, G.N. A technique to produce a mirror-image wax pattern of an ear using rapid prototyping technology. J. Prosthet. Dent. 2005, 94, 195–198. [Google Scholar] [CrossRef]
- Chandra, A.; Watson, J.; Rowson, J.; Holland, J.; Harris, R.; Williams, D. Application of rapid manufacturing techniques in support of maxillofacial treatment: Evidence of the requirements of clinical applications. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2005, 219, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Reitelshofer, S.; Landgraf, M.; Graf, D.; Bugert, L.; Franke, J. A new production process for soft actuators and sensors based on dielectric elastomers intended for safe human robot interaction. In Proceedings of the 2015 IEEE/SICE International Symposium on System Integration (SII), Nagoya, Japan, 11–13 December 2015; pp. 51–56. [Google Scholar] [CrossRef]
- McCoul, D.; Rosset, S.; Schlatter, S.; Shea, H. Inkjet 3D printing of UV and thermal cure silicone elastomers for dielectric elastomer actuators. Smart Mater. Struct. 2017, 26, 125022. [Google Scholar] [CrossRef] [Green Version]
- Stieghorst, J.; Doll, T. Rheological behavior of PDMS silicone rubber for 3D printing of medical implants. Addit. Manuf. 2018, 24, 217–223. [Google Scholar] [CrossRef]
- Liravi, F.; Vlasea, M. Powder bed binder jetting additive manufacturing of silicone structures. Addit. Manuf. 2018, 21, 112–124. [Google Scholar] [CrossRef]
- Liravi, F.; Toyserkani, E. A hybrid additive manufacturing method for the fabrication of silicone bio-structures: 3D printing optimization and surface characterization. Mater. Des. 2017, 138, 46–61. [Google Scholar] [CrossRef]
- Liravi, F.; Jacob-John, V.; Toyserkani, A.; Vlasea, M. A Hybrid Method for Additive Manufacturing of Silicone Structures. In Proceedings of the 2017 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 7–9 August 2017; pp. 1897–1917. [Google Scholar]
- In, E.; Walker, E.; Naguib, H.E. Novel development of 3D printable UV-curable silicone for multimodal imaging phantom. Bioprinting 2017, 7, 19–26. [Google Scholar] [CrossRef]
- Kim, D.S.D.; Suriboot, J.; Grunlan, M.A.; Tai, B.L. Feasibility study of silicone stereolithography with an optically created dead zone. Addit. Manuf. 2019, 29, 100793. [Google Scholar] [CrossRef]
- Liravi, F.; Toyserkani, E. Additive manufacturing of silicone structures: A review and prospective. Addit. Manuf. 2018, 24, 232–242. [Google Scholar] [CrossRef]
- Kim, D.S.D.; Tai, B.L. Hydrostatic support-free fabrication of three-dimensional soft structures. J. Manuf. Process. 2016, 24, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, N.; Parra-Cabrera, C.; Kim, Y.T.; Kuo, A.P.; Folch, A. Desktop-Stereolithography 3D-Printing of a Poly (dimethylsiloxane)-Based Material with Sylgard-184 Properties. Adv. Mater. 2018, 30, 1800001. [Google Scholar] [CrossRef]
- Wallin, T.J.; Simonsen, L.E.; Pan, W.; Wang, K.; Giannelis, E.; Shepherd, R.F.; Mengüç, Y. 3D printable tough silicone double networks. Nat. Commun. 2020, 11, 4000. [Google Scholar] [CrossRef] [PubMed]
- Reitelshöfer, S.; Göttler, M.; Schmidt, P.; Treffer, P.; Landgraf, M.; Franke, J. Aerosol-Jet-Printing silicone layers and electrodes for stacked dielectric elastomer actuators in one processing device. In Electroactive Polymer Actuators and Devices (EAPAD); SPIE: Bellingham, WA, USA, 2016. [Google Scholar]
- Yang, H.; He, Y.; Tuck, C.; Wildman, R.; Ashcroft, I.; Dickens, P.; Hague, R. High Viscosity Jetting System for 3D Reactive Inkjet Printing; University of Texas at Austin: Austin, TX, USA, 2013. [Google Scholar]
- Unkovskiy, A.; Spintzyk, S.; Brom, J.; Huettig, F.; Keutel, C. Direct 3D printing of silicone facial prostheses: A preliminary experience in digital workflow. J. Prosthet. Dent. 2018, 120, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, T.E.; Hatch, S.E.; Colton, M.B.; Thomson, S.L. 3D printing low-stiffness silicone within a curable support matrix. Addit. Manuf. 2020, 37, 101681. [Google Scholar] [CrossRef] [PubMed]
- Haghiashtiani, G.; Qiu, K.; Sanchez, J.D.Z.; Fuenning, Z.J.; Nair, P.; Ahlberg, S.E.; Iaizzo, P.A.; McAlpine, M.C. 3D printed patient-specific aortic root models with internal sensors for minimally invasive applications. Sci. Adv. 2020, 6, eabb4641. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Gao, Q.; Fu, J.Z.; Chen, Q.Y.; Zhu, J.P.; Sun, Y.; He, Y. Multi-Material 3D Printing of Highly Stretchable Silicone Elastomer. ACS Appl. Mater. Interfaces 2019, 11, 23573–23583. [Google Scholar] [CrossRef]
- O’bryan, C.S.; Bhattacharjee, T.; Niemi, S.R.; Balachandar, S.; Baldwin, N.; Ellison, S.T.; Taylor, C.R.; Sawyer, W.G.; Angelini, T.E. Three-dimensional printing with sacrificial materials for soft matter manufacturing. MRS Bull. 2017, 42, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [Green Version]
- Duraivel, S.; Laurent, D.; Rajon, D.A.; Scheutz, G.M.; Shetty, A.M.; Sumerlin, B.S.; Banks, S.A.; Bova, F.J.; Angelini, T.E. A silicone-based support material eliminates interfacial instabilities in 3D silicone printing. Science 2023, 379, 1248–1252. [Google Scholar] [CrossRef]
- Casas-Murillo, C.; Zuñiga-Ruiz, A.; Lopez-Barron, R.E.; Sanchez-Uresti, A.; Gogeascoechea-Hernandez, A.; Muñoz-Maldonado, G.E.; Salinas-Chapa, M.; Elizondo-Riojas, G.; Negreros-Osuna, A.A. 3D-printed anatomical models of the cystic duct and its variants, a low-cost solution for an in-house built simulator for laparoscopic surgery training. Surg. Radiol. Anat. 2021, 43, 537–544. [Google Scholar] [CrossRef]
- Li, A.; Tang, R.; Rong, Z.; Zeng, J.; Xiang, C.; Yu, L.; Zhao, W.; Dong, J. The Use of Three-Dimensional Printing Model in the Training of Choledochoscopy Techniques. World J. Surg. 2018, 42, 4033–4038. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xia, J.; Zhang, J.; Mao, J.; Chen, H.; Lin, H.; Jiang, P.; He, X.; Xu, X.; Yin, M.; et al. Validity of a soft and flexible 3D-printed Nissen fundoplication model in surgical training. Bioprint 2022, 8, 61–69. [Google Scholar] [CrossRef]
- Wei, F.; Wang, W.; Gong, H.; Cao, J.; Chen, J.; Chen, H.; Wang, Z. Reusable modular 3D-printed dry lab training models to simulate minimally invasive choledocho jejunostomy. J. Gastrointest. Surg. 2021, 25, 1899–1901. [Google Scholar] [CrossRef]
- Engelhardt, S.; Sauerzapf, S.; Preim, B.; Karck, M.; Wolf, I.; De Simone, R. Flexible and comprehensive patient-specific mitral valve silicone models with chordae tendineae made from 3D-printable molds. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Imbrie-Moore, A.M.; Paullin, C.C.; Paulsen, M.J.; Grady, F.; Wang, H.; Hironaka, C.E.; Farry, J.M.; Lucian, H.J.; Woo, Y.J. A novel 3D-Printed preferential posterior mitral annular dilation device delineates regurgitation onset threshold in an ex vivo heart simulator. Med. Eng. Phys. 2020, 77, 10–18. [Google Scholar] [CrossRef]
- Zelis, J.M.; Meiburg, R.; Roijen, J.J.; Janssens, K.L.; van’t Veer, M.; Pijls, N.H.; Johnson, N.P.; van de Vosse, F.N.; Tonino, P.A.; Rutten, M.C. 3D-printed stenotic aortic valve model to simulate physiology before, during, and after transcatheter aortic valve implantation. Int. J. Cardiol. 2020, 313, 32–34. [Google Scholar] [CrossRef]
- Michael, B. Consequences of Malalignment in Total Knee Arthroplasty: Few if Any-Opposes. J. Arthroplast. 2010, 21, 99–101. [Google Scholar]
- Gan, Y.; Ding, J.; Xu, Y.; Hou, C. Accuracy and efficacy of osteotomy in total knee arthroplasty with patient-specific navigational template. Int. J. Clin. Exp. Med. 2015, 8, 12192–12201. [Google Scholar]
- Kwon, O.R.; Kang, K.T.; Son, J.; Choi, Y.J.; Suh, D.S.; Koh, Y.G. The Effect of Femoral Cutting Guide Design Improvements for Patient-Specific Instruments. BioMed Res. Int. 2015, 2015, 978686. [Google Scholar] [CrossRef] [Green Version]
- Asada, S.; Mori, S.; Matsushita, T.; Nakagawa, K.; Tsukamoto, I.; Akagi, M. Comparison of MRI-and CT-based patient-specific guides for total knee arthroplasty. Knee 2014, 21, 1238–1243. [Google Scholar] [CrossRef]
- Hafez, M.A.; Chelule, K.L.; Seedhom, B.B.; Sherman, K.P. Computer-assisted total knee arthroplasty using patient-specific templating. Clin. Orthop. Relat. Res. 2006, 444, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Ikram, N.; Batra, A.V. Accuracy of bone resection in total knee arthroplasty using CT assisted-3D printed patient specific cutting guides. SICOT J. 2018, 4, 29. [Google Scholar]
- Yu, J.; Shi, Q. Efficacy Evaluation of 3D Navigational Template for Salter Osteotomy of DDH in Children. BioMed Res. Int. 2021, 2021, 8832617. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.; Paulsen, R.; Babuska, J.M.; Rajpal, S.; Burneikiene, S.; Nelson, E.L.; Villavicencio, A.T. The accuracy of pedicle screw placement using intraoperative image guidance system. J. Neurosurg. Spine 2014, 20, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matjaz, M.; Igor, D.; Matjaz, V.; Tomaz, B.; Tomaz, F.; Gregor, R. Error rate of multilevel rapid prototyping trajectories for pedicles screw placement in lumbar and sacral sping. Chin. J. Traumatol. 2014, 17, 261–266. [Google Scholar]
- Gelalis, I.D.; Stafilas, K.S.; Korompilias, A.V.; Zacharis, K.C.; Beris, A.E.; Xenakis, T.A. Decompressive surgery for degenerative lumbar spinal stenosis: Long-term results. Int. Orthop. 2006, 30, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Yuan, Z.S.; Kepler, C.K.; Albert, T.J.; Xie, H.; Yuan, J.B.; Dong, W.X.; Wang, C.T. Deviation analysis of atlantoaxial pedicle screws assisted by a drill template. Orthopedics 2014, 5, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell inter. Methods Mol. Biol. 2011, 695, 17–39. [Google Scholar]
- Sugawara, T.; Higashiyama, N.; Kaneyama, S.; Takabatake, M.; Watanabe, N.; Uchida, F.; Sumi, M.; Mizoi, K. Multistep pedicle screw insertion procedure with patient-specific lamina fit-and-lock templates for the thoracic spine: Clinical article. J. Neurosurg. Spine 2013, 19, 185–190. [Google Scholar] [CrossRef]
- Liang, B.; Chen, Q.; Liu, S.; Chen, S.; Yao, Q.; Wei, B.; Xu, Y.; Tang, C.; Wang, L. A feasibility study of individual 3D-printed navigation template for the deep external fixator pin position on the iliac crest. BMC Musculoskelet. Disord. 2020, 21, 478. [Google Scholar] [CrossRef]
- Shi, W.; Aierken, G.; Wang, S.; Abuduwali, N.; Xia, Y.; Rezhake, R.; Zhao, S.; Zhou, M.; Sheng, W.; Rexiti, P. Application study of three-dimensional printed navigation template between traditional and novel cortical bone trajectory on osteoporosis lumbar spine. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2021, 85, 41–48. [Google Scholar] [CrossRef]
- Ryu, J.-H.; Kwon, J.-S.; Jiang, H.B.; Cha, J.-Y.; Kim, K.-M. Effects of thermoforming on the physical and mechanical properties of thermoplastic materials for transparent orthodontic aligners. Korean J. Orthod. 2018, 48, 316–325. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Mapelli, A.; Maspero, C.; Santaniello, T.; Serafin, M.; Farronato, M.; Caprioglio, A. Direct 3D Printing of Clear Orthodontic Aligners: Current State and Future Possibilities. Materials 2021, 14, 1799. [Google Scholar] [CrossRef]
- Edelmann, A.; English, J.D.; Chen, S.J.; Kasper, F.K. Analysis of the thickness of 3-dimensional-printed orthodontic aligners. Am. J. Orthod. Dentofac. Orthop. 2020, 158, e91–e98. [Google Scholar] [CrossRef]
- Bartkowiak, T.; Walkowiak-Śliziuk, A. 3D printing technology in orthodontics-review of current applications. J. Stomatol. 2018, 71, 356–364. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Phillips, B.T.; Allder, J.; Bolan, G.; Nagle, R.S.; Redington, A.; Hellebrekers, T.; Borden, J.; Pawlenko, N.; Licht, S. Additive manufacturing aboard a moving vessel at sea using passively stabilized stereolithography (SLA) 3D printing. Addit. Manuf. 2020, 31, 100969. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Ronsivalle, V.; Grippaudo, C.; Lucchese, A.; Muraglie, S.; Lagravère, M.O.; Isola, G. One Step before 3D Printing-Evaluation of Imaging Software Accuracy for 3-Dimensional Analysis of the Mandible: A Comparative Study Using a Surface-to-Surface Matching Technique. Materials 2020, 13, 2798. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Ortensi, L.; Farronato, M.; Lucchese, A.; Lo Castro, E.; Isola, G. The step further smile virtual planning: Milled versus prototyped mock-ups for the evaluation of the designed smile characteristics. BMC Oral Health 2020, 20, 165. [Google Scholar] [CrossRef]
- Nasef, A.A.; El-Beialy, A.R.; Mostafa, Y.A. Virtual techniques for designing and fabricating a retainer. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 394–398. [Google Scholar] [CrossRef]
- Jindal, P.; Juneja, M.; Siena, F.L.; Bajaj, D.; Breedon, P. Mechanical and geometric properties of thermoformed and 3D printed clear dental aligners. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Zinelis, S.; Panayi, N.; Polychronis, G.; Papageorgiou, S.N.; Eliades, T. Comparative analysis of mechanical properties of orthodontic aligners produced by different contemporary 3D printers. Orthod. Craniofacial Res. 2022, 25, 336–341. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.C.; Chen, S.J.; English, J.D.; Kasper, F. Effect of print orientation and duration of ultraviolet curing on the dimensional accuracy of a 3-dimensionally printed orthodontic clear aligner design. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Aravind Shanmugasundaram, S.; Razmi, J.; Mian, M.J.; Ladani, L. Mechanical Anisotropy and Surface Roughness in Additively Manufactured Parts Fabricated by Stereolithography (SLA) Using Statistical Analysis. Materials 2020, 13, 2496. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T. 3D printing of PEEK and its composite to increase biointerfaces as a biomedical material—A review. Colloids Surf. B Biointerfaces 2021, 203, 111726. [Google Scholar] [CrossRef]
- Anandhapadman, A.; Venkateswaran, A.; Jayaraman, H.; Veerabadran Ghone, N. Advances in 3D printing of composite scaffolds for the repairment of bone tissue associated defects. Biotechnol. Prog. 2022, 38, e3234. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.; Yang, C.; Spintzyk, S.; Scheideler, L.; Li, P.; Li, D.; Geis-Gerstorfer, J.; Rupp, F. Carbon Fiber Reinforced PEEK Composites Based on 3D-Printing Technology for Orthopedic and Dental Applications. J. Clin. Med. 2019, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Haleem, A.; Javaid, M. Polyether ether ketone (PEEK) and its manufacturing of customised 3D printed dentistry parts using additive manufacturing. Clin. Epidemiol. Glob. Health 2019, 7, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Zeng, Z.; Peng, B.; Yan, S.; Ke, W. Mechanical Properties Optimization of Poly-Ether-Ether-Ketone via Fused Deposition Modeling. Materials 2018, 11, 216. [Google Scholar] [CrossRef] [Green Version]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T.; Bowoto, O.K.; Olawumi, M.A.; Muhammad, M.A. 3D printing of PEEK–cHAp scaffold for medical bone implant. Bio-Des. Manuf. 2021, 4, 44–59. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Ding, S.; Li, L.; Huang, C. Effects of FDM-3D printing parameters on mechanical properties and microstructure of CF/PEEK and GF/PEEK. Chin. J. Aeronaut. 2020, 34, 236–246. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, H.; Dong, E.; Kang, J.; Liu, C.; Sun, C.; Li, D.; Wang, L. Additively-manufactured PEEK/HA porous scaffolds with highly-controllable mechanical properties and excellent biocompatibility. Mater. Sci. Eng. C 2021, 2, 112333. [Google Scholar] [CrossRef]
- Mounir, M.; Shalash, M.; Mounir, S.; Nassar, Y.; El Khatib, O. Assessment of three dimensional bone augmentation of severely atrophied maxillary alveolar ridges using prebent titanium mesh vs customized poly-ether-ether-ketone (PEEK) mesh: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 960–967. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, J.; Zheng, J.; Wang, L.; Li, D.; Liu, S. 3D-printed PEEK implant for mandibular defects repair—A new method. J. Mech. Behav. Biomed. Mater. 2021, 116, 104335. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, J.; Jia, Y.G.; Lu, B.; Ren, L. TiO2 and PEEK reinforced 3D printing PMMA composite resin for dental denture base applications. Nanomaterials 2019, 9, 1049. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.C.; Klemm, D.; Eckert, J.; Hao, Y.L.; Sercombe, T.B. Manufacture by selective laser melting andmechanical behavior of a biomedical Ti–24Nb–4Zr–8Sn alloy. Scr. Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Edwards, P.; Ramulu, M. Effect of build direction on the fracture toughness andfatigue crack growth in selective laser melted Ti-6Al-4v. Fatigue Fract. Eng. Mater. Struct. 2015, 38, 1228–1236. [Google Scholar] [CrossRef]
- Chen, L.Y.; Huang, J.C.; Lin, C.H.; Pan, C.T.; Chen, S.Y.; Yang, T.L.; Lin, D.Y.; Lin, H.K.; Jang, J.S.C. Anisotropic response of Ti-6Al-4V alloyfabricated by 3D printing selective laser melting. Mater. Sci. Eng. A 2017, 682, 389–395. [Google Scholar] [CrossRef]
- Dekker, T.J.; Steele, J.R.; Federer, A.E.; Hamid, K.S.; Adams, S.B., Jr. Use of patient-specific 3D-printed titanium implants for complex foot and ankle limb salvage, deformity correction and arthrodesis procedures. Foot Ankle Int. 2018, 8, 916–921. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Parr, W.C.; Choy, W.J.; McEvoy, A.; Walsh, W.R.; Phan, K. Anterior Lumbar Interbody Fusion (ALIF) using a personalised approach: Is custom the future of implants for ALIF surgery? World Neurosurg. 2019, 124, 1–23. [Google Scholar] [CrossRef]
- Belvedere, C.; Siegler, S.; Fortunato, A.; Caravaggi, P.; Liverani, E.; Durante, S.; Ensini, A.; Konow, T.; Leardini, A. New comprehensive procedure forcustom-made total ankle replacements: Medical imaging, joint modeling, prosthesis design and 3D printing. J. Orthop. Res. 2019, 37, 760–768. [Google Scholar] [CrossRef]
- Xu, L.; Qin, H.; Tan, J.; Cheng, Z.; Luo, X.; Tan, H.; Huang, W. Clinical study of 3D printed personalized prosthesis in the treatment of bone defect after pelvic tumor resection. J. Orthop. Translat. 2021, 29, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Lu, S.; Chen, T.; Zhao, Y.; Chen, H.; Tang, Z. Fabrication and characterization of selective laser melting printed Ti–6Al–4V alloys subjected to heat treatment for customized implants design. Prog. Nat. Sci. Mater. Int. 2016, 26, 671–677. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Zhang, Y.; Yao, Y.; Mo, Z.; Wang, L.; Fan, Y. Diagonal-symmetrical and Midline-symmetrical Unit Cells with Same Porosity for Bone Implant: Mechanical Properties Evaluation. J. Bionic Eng. 2019, 16, 468–479. [Google Scholar] [CrossRef]
- Epasto, G.; Palomba, G.; Di Bella, S.; Mineo, R.; Guglielmino, E.; Traina, F. Experimental investigation of rhombic dodecahedron micro-lattice structures manufactured by Electron Beam Melting. Mater. Today Proc. 2019, 7 Pt 1, 578–585. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Luo, S.; Nai, M.L.S.; Ding, J.; Wei, J. Additively manufactured heterogeneously porous metallic bone with biostructural functions and bone-like mechanical properties. J. Mater. Sci. Technol. 2021, 62, 173–179. [Google Scholar] [CrossRef]
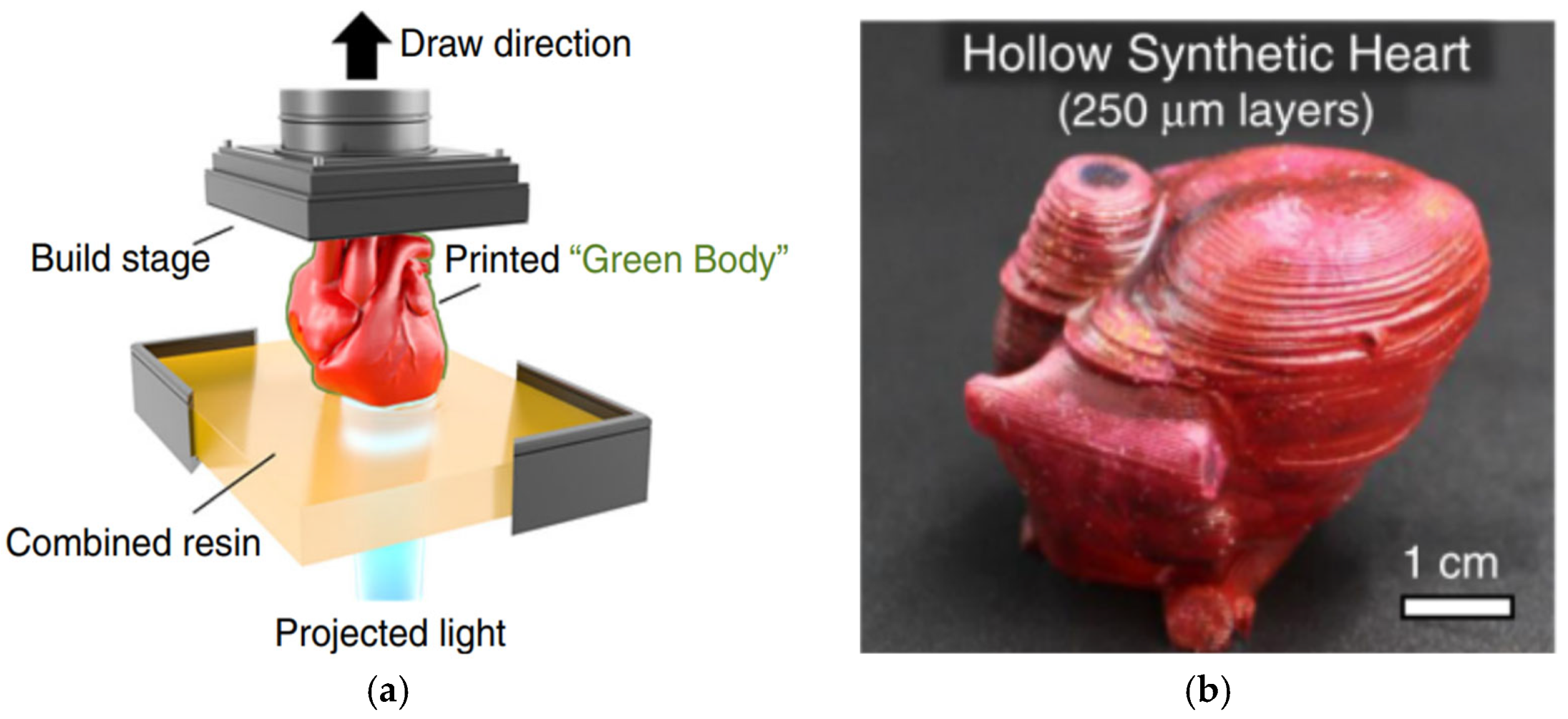
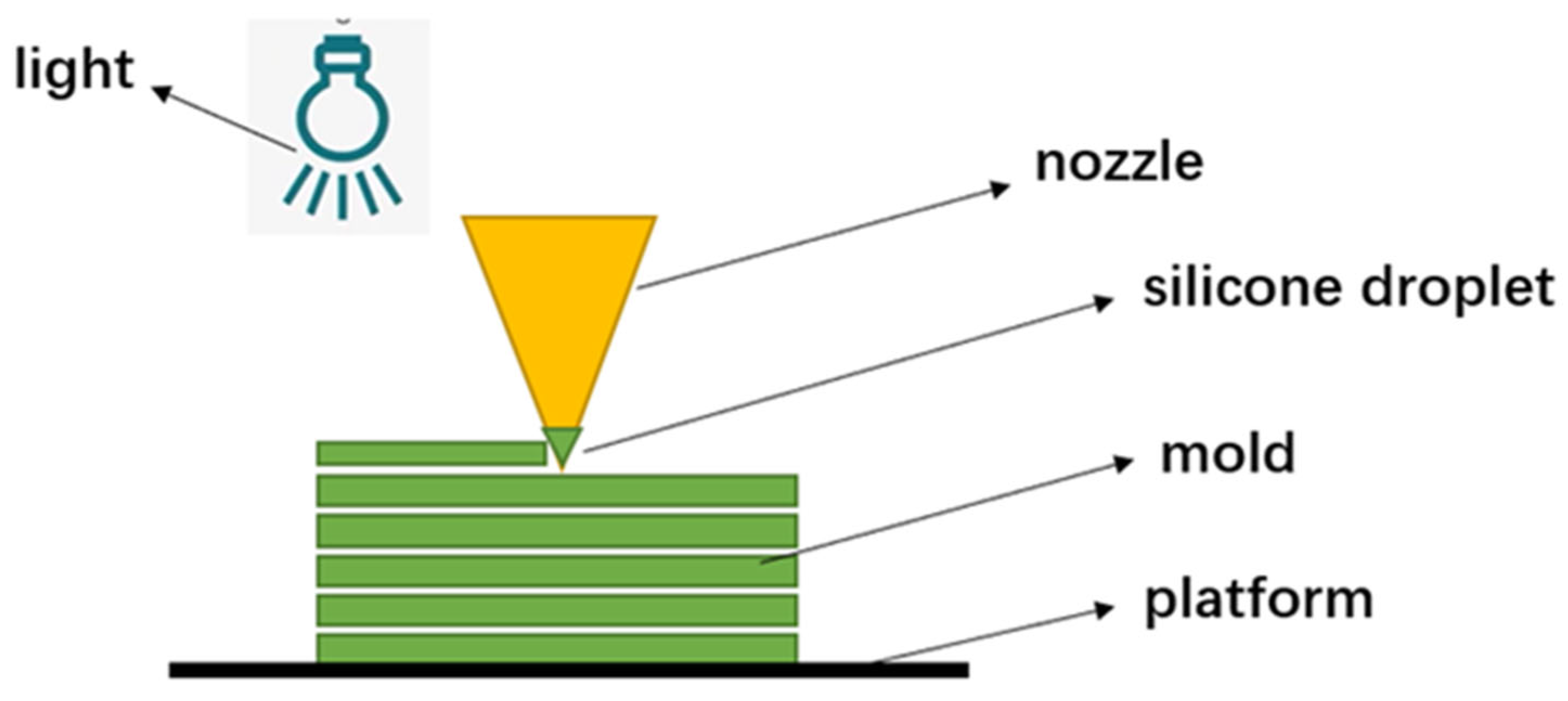

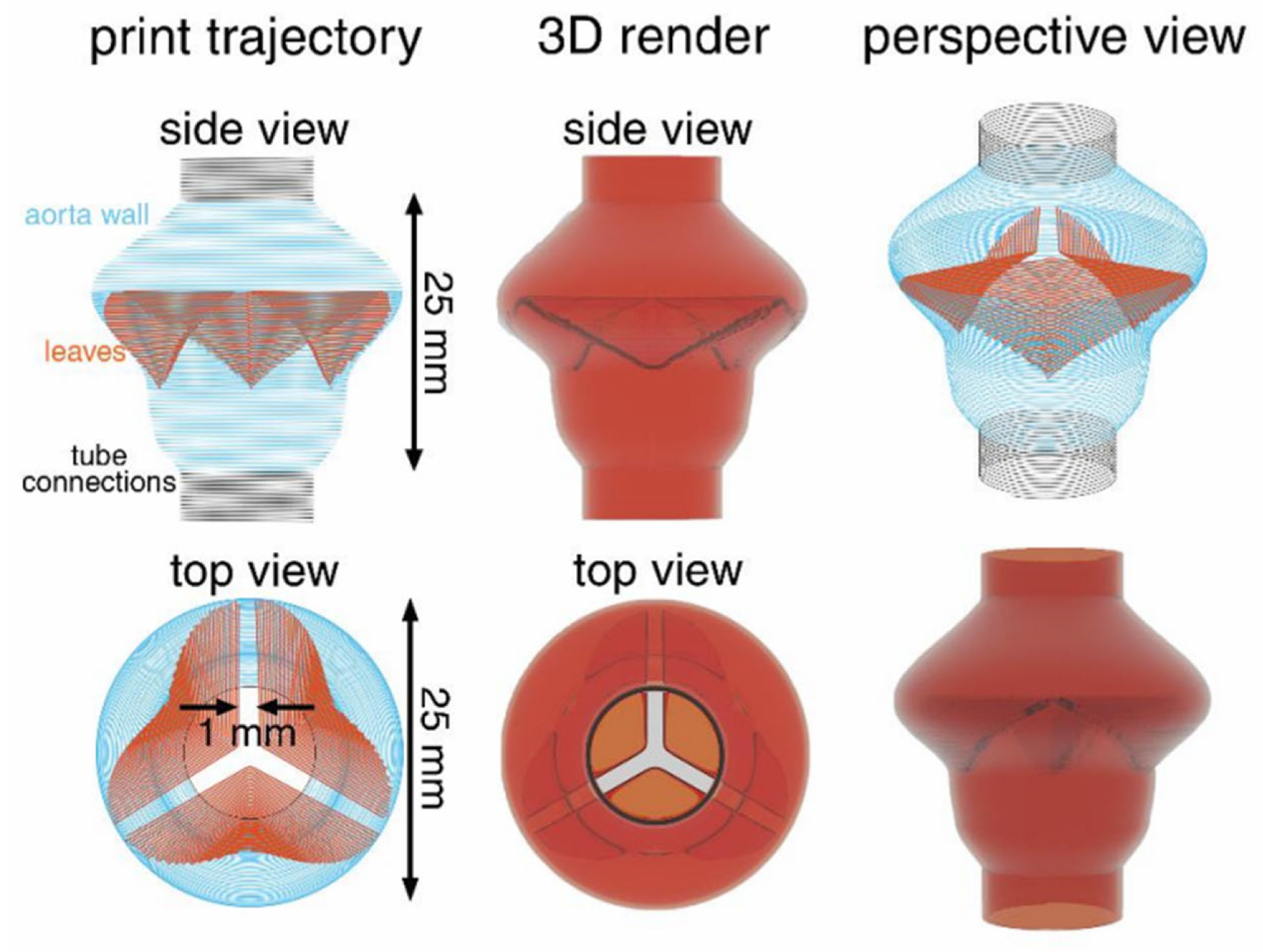




| Stage | Medical Subdivision Fields |
|---|---|
| Relatively mature and commercialized development | Medical model, surgical guide plate, dental application |
| Clinical data accumulation research | High-performance orthopedic implants |
| Laboratory research stage | Functional tissue organ |
| 3D Printing Method | Curing System | Advantages | Shortcomings |
|---|---|---|---|
| Vat Photopolymerization | UV | High resolution | Limit to the low-viscosity silicon |
| Material Jetting | UV or HTV | High speed Multi-color printing | Complex device structure |
| Direct Ink Writing | UV or HTV | Simple structure | Poor equality and difficult to control |
| Removable Embedded 3D printing | UV or HTV | Low-viscosity ultra-soft materials printing | High interfacial tension |
| Complete Matrix-Cure Embedded 3D Printing | UV or HTV | Complex structure printing | Tedious steps |
| Materials | Characteristics | 3D Technology | Applications |
|---|---|---|---|
| Co-Cr Alloy | Strong corrosion resistance, high hardness, excellent mechanical properties, and low cost | Selective laser melting Electron beam selective melting | Prosthesis, fixation screw, bone plate, denture, cobalt-chromium alloy porcelain-fused-to-metal crown |
| 316L Stainless Steel | Excellent mechanical properties, favorable biocompatibility, good corrosion resistance, and low cost | Selective laser melting Electron beam selective melting | Fracture internal fixation, cardiovascular intervention therapy, dental implant and periodontitis treatment stent |
| Tantalum | Corrosion resistance, good plasticity, excellent biocompatibility | Selective laser melting Electron beam selective melting | Spinal fusion surgery, cranial shaping surgery, ankle surgery, tumor reconstruction surgery |
| Titanium Alloy | Low material density, low elastic modulus, good mechanical properties, corrosion resistance, biocompatibility | Selective laser melting Electron beam selective melting | Joint replacement surgery, percutaneous coronary intervention (PCI), dental implantation, periodontal scaffold |
| Magnesium Alloy | Similar to bone density, enhances osteoblast proliferation, promotes bone growth and healing | Selective laser melting Electron beam selective melting | Biodegradable cardiovascular stent, bone plate, fixed screw |
| PLA | Biocompatible and biodegradable | Fused deposition modeling Selective laser sintering | Soft tissue repair, bone repair |
| PMMA | Good plasticity, stable chemical structure, and good mechanical properties | Fused deposition modeling Selective laser sintering | Fracture repair, joint replacement surgery, spinal surgery, denture, dental crown restoration, cochlear implants |
| PEEK | Good biocompatibility and chemical stability, elastic modulus similar to human bone | Fused deposition modeling Selective laser sintering | Spinal fusion device, artificial intervertebral disc, bone plate, tissue scaffold |
| Hydrogel | Good hydrophilicity, biocompatibility, and biodegradability | Stereolithography Embedded 3D printing Material jetting | Tissue scaffold, heart valve, vascular and dermal tissue, drug delivery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zeng, J.; Li, H.; Han, C.; Wu, W.; Zeng, W.; Tang, L. A Review on the Full Chain Application of 3D Printing Technology in Precision Medicine. Processes 2023, 11, 1736. https://doi.org/10.3390/pr11061736
Wu S, Zeng J, Li H, Han C, Wu W, Zeng W, Tang L. A Review on the Full Chain Application of 3D Printing Technology in Precision Medicine. Processes. 2023; 11(6):1736. https://doi.org/10.3390/pr11061736
Chicago/Turabian StyleWu, Shenglin, Jinbin Zeng, Haoxin Li, Chongyang Han, Weibin Wu, Wenyi Zeng, and Luxin Tang. 2023. "A Review on the Full Chain Application of 3D Printing Technology in Precision Medicine" Processes 11, no. 6: 1736. https://doi.org/10.3390/pr11061736
APA StyleWu, S., Zeng, J., Li, H., Han, C., Wu, W., Zeng, W., & Tang, L. (2023). A Review on the Full Chain Application of 3D Printing Technology in Precision Medicine. Processes, 11(6), 1736. https://doi.org/10.3390/pr11061736







