Recycling Silicon Waste from the Photovoltaic Industry to Prepare Yolk–Shell Si@void@C Anode Materials for Lithium–Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
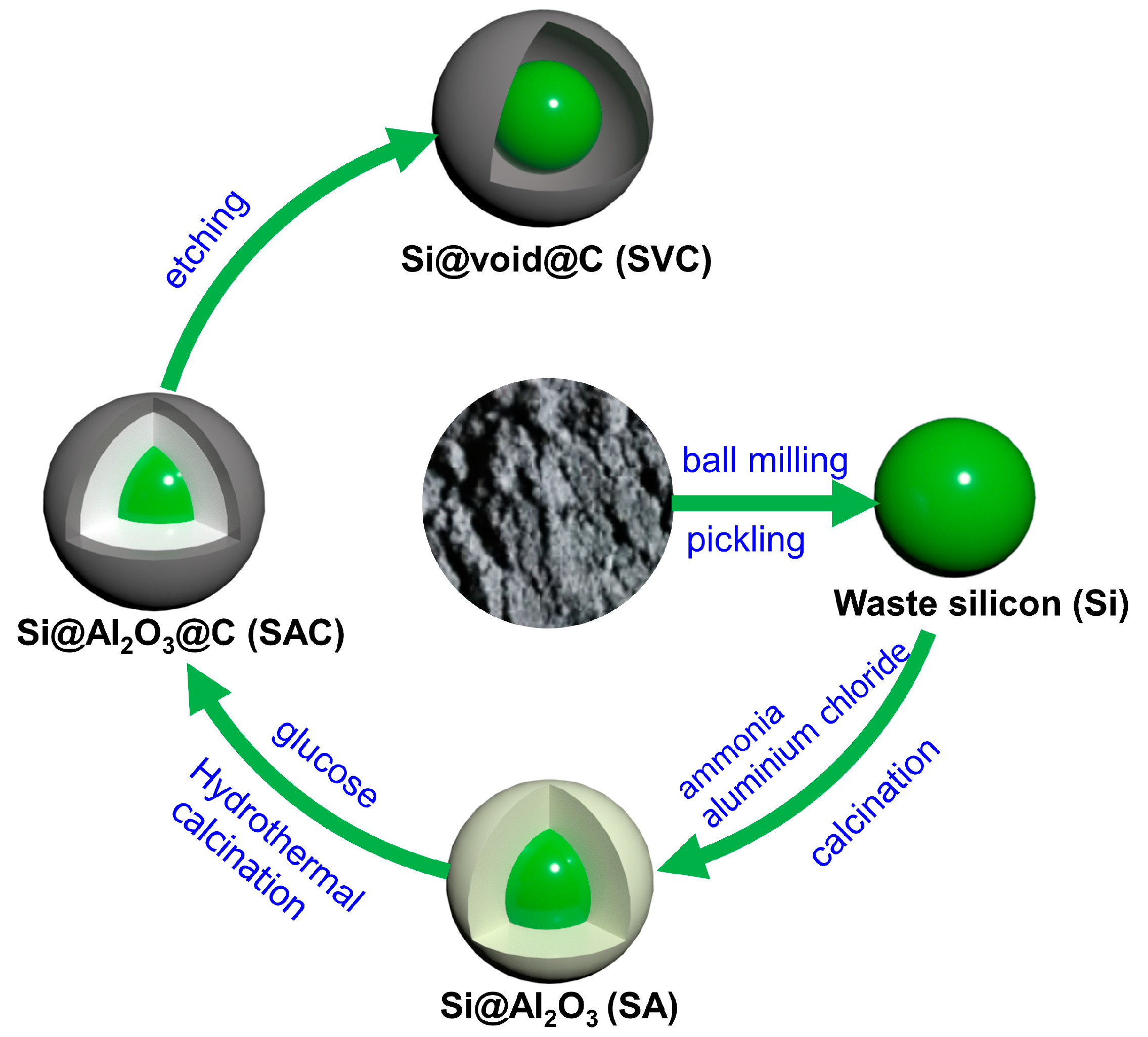
2.1. Pretreatment of KL Si Waste
2.2. Synthesis of Si@void@C
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results
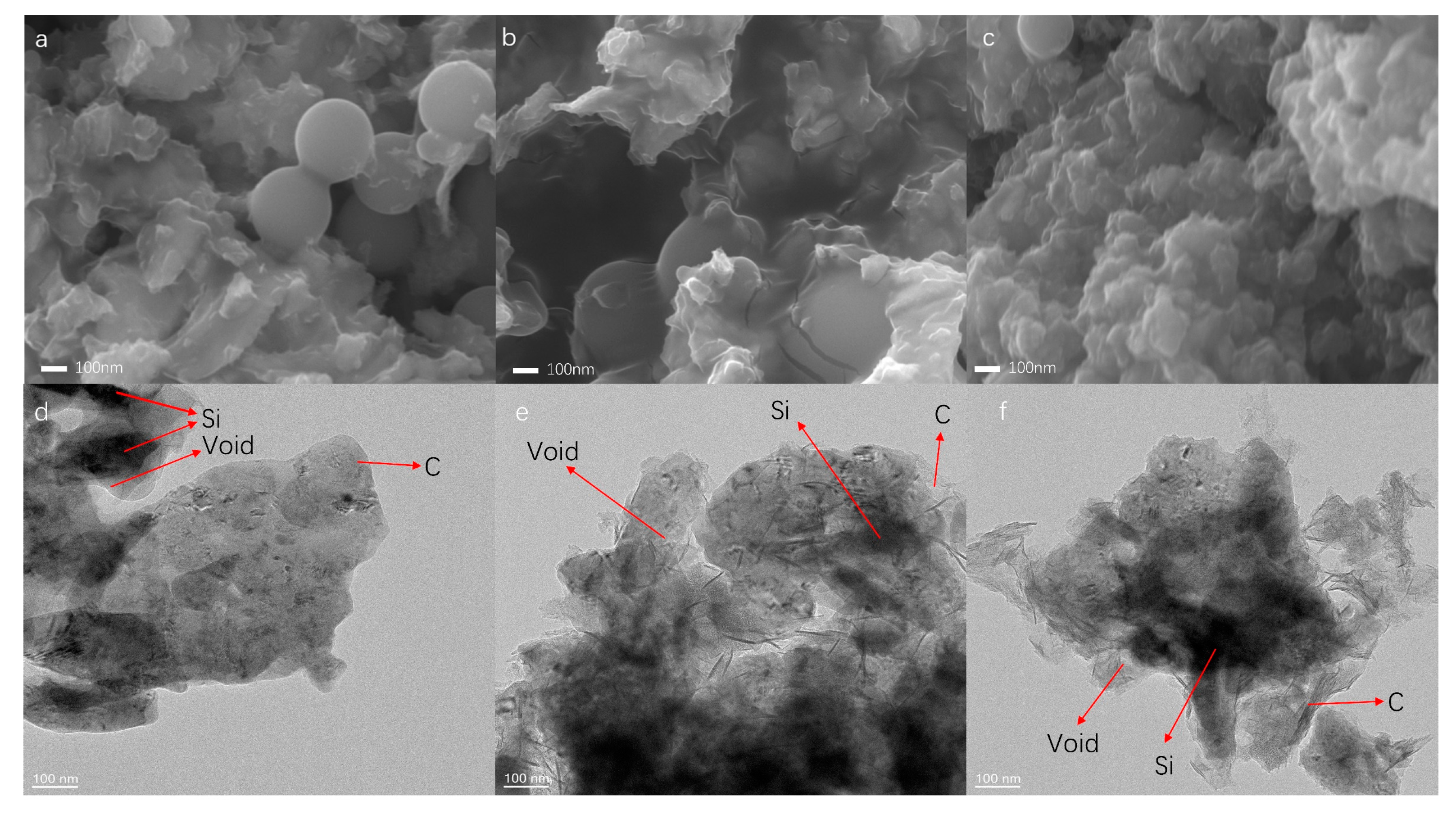
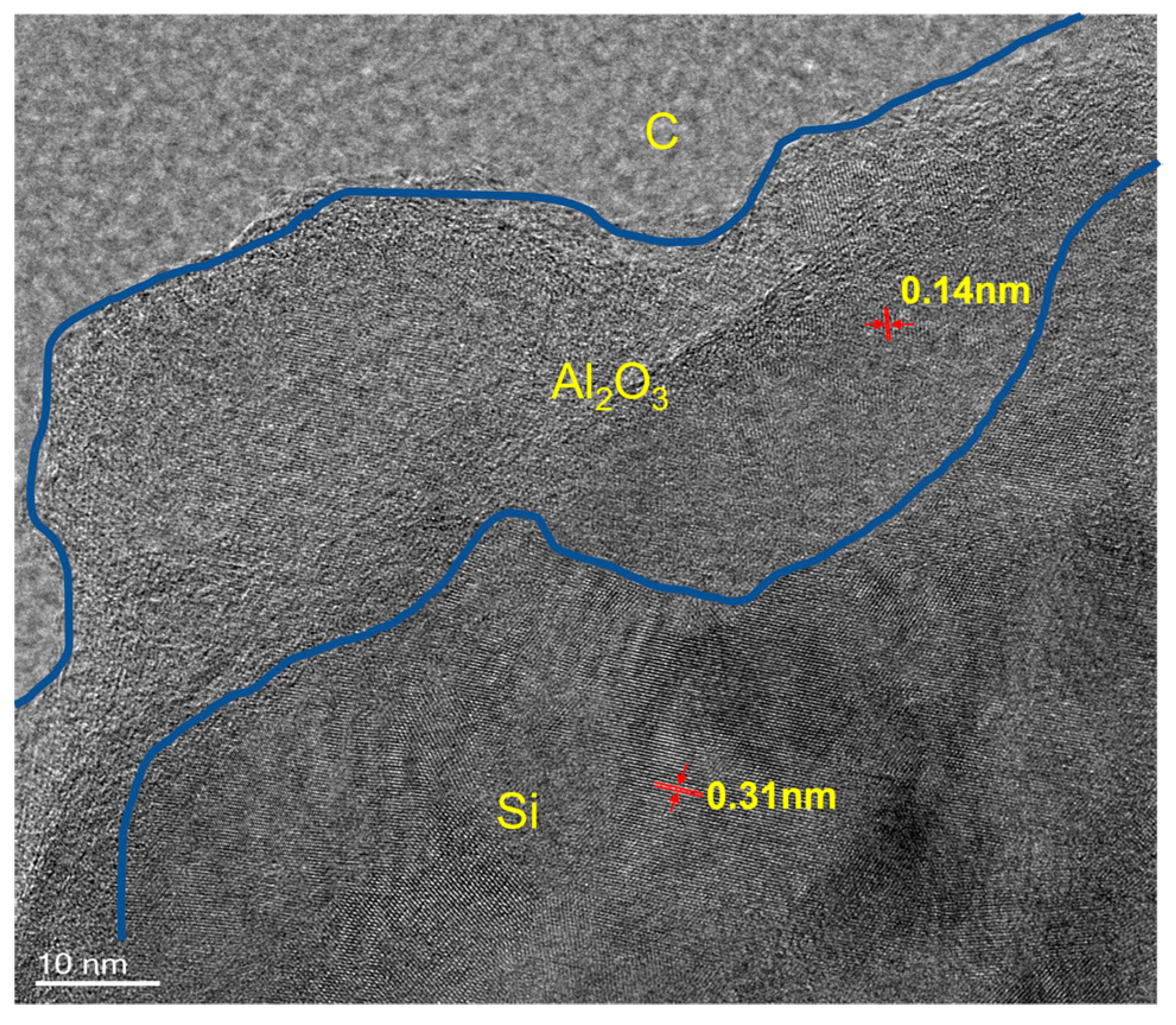
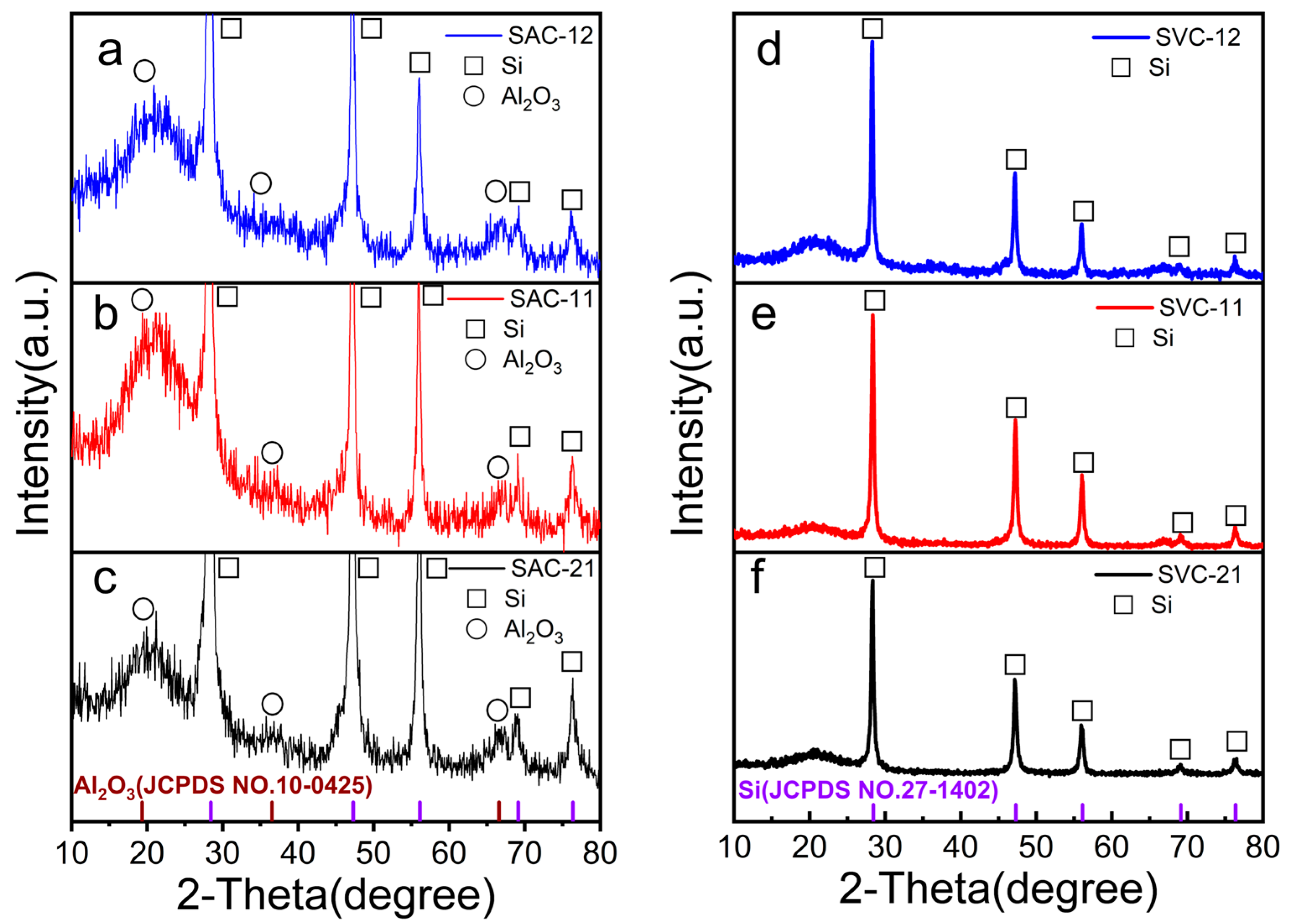
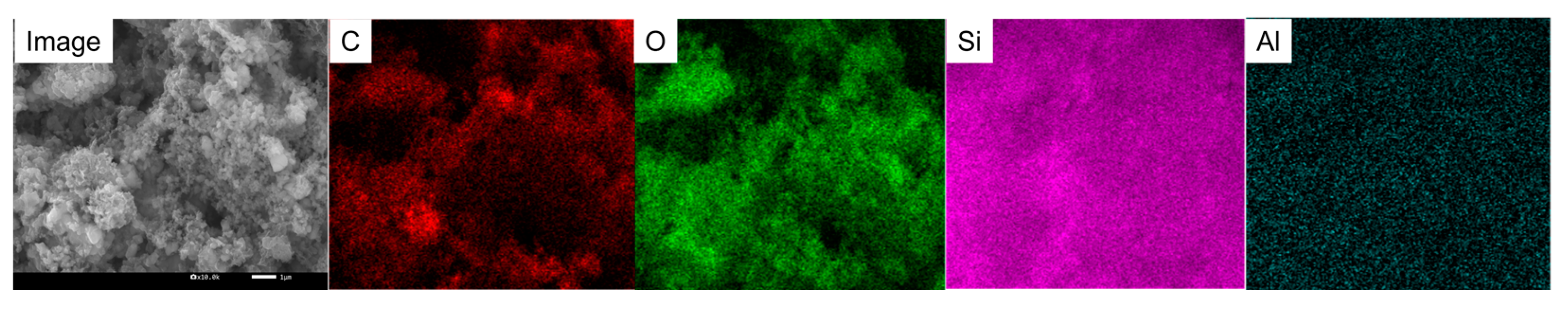
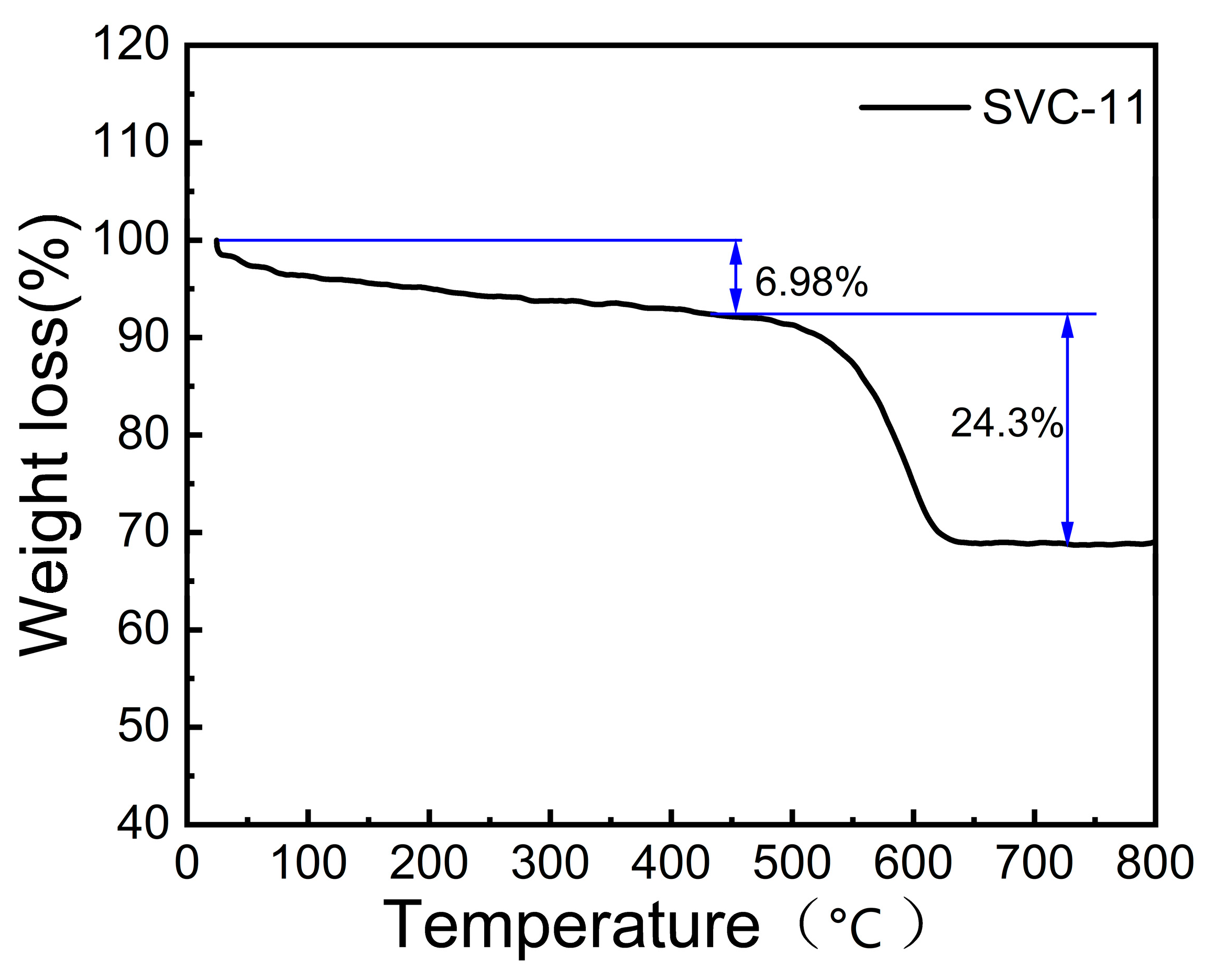
3.1. Material Morphology, Structure, and Chemical Composition
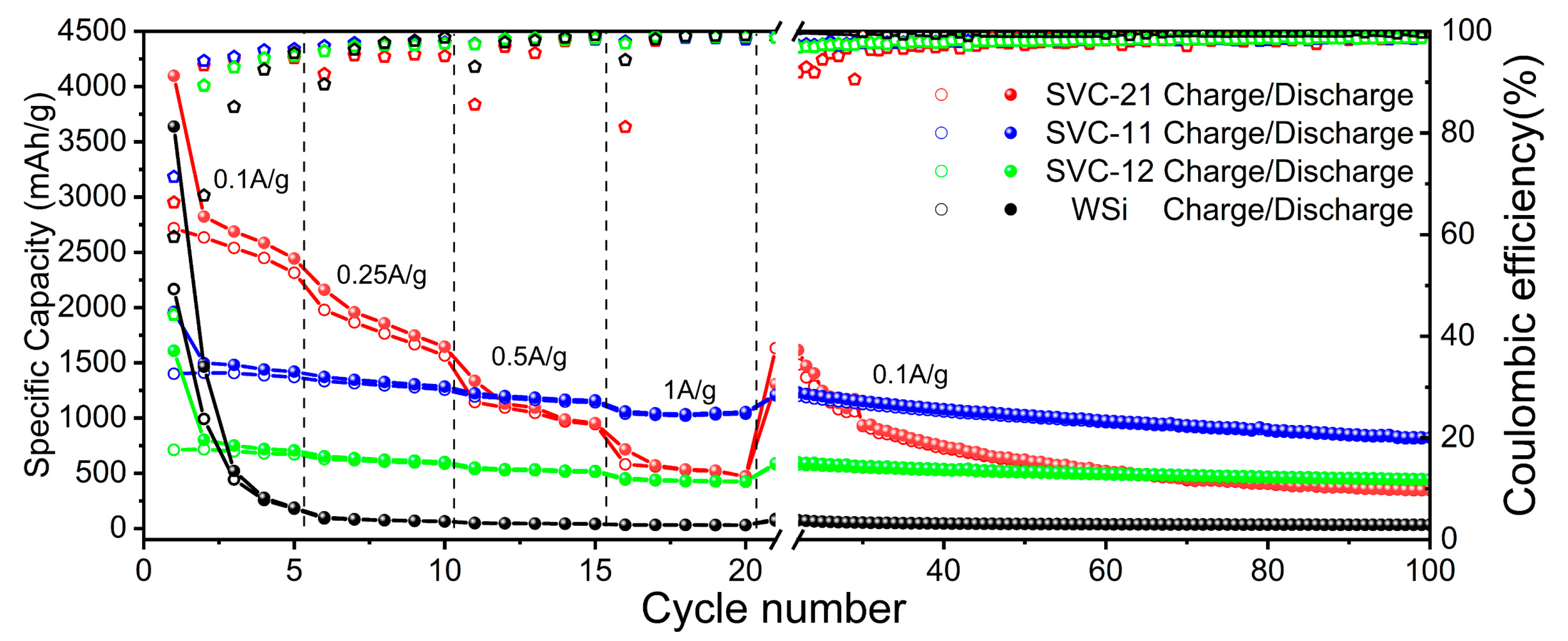
3.2. Electrochemical Performance of Si@void@C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Swaans, E.; Chen, S.; Basak, S.; Harks, P.P.R.; Peng, B.; Zandbergen, H.W.; Borsa, D.M.; Mulder, F.M. A high–performance Li–ion anode from direct deposition of Si nanoparticles. Nano Energy 2017, 38, 477–485. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, P.; Ge, S.; Wu, Y.; Shi, B.; Shao, G.; Gu, C.; Su, Z.; Liu, A. An egg holders–inspired structure design for large–volume–change anodes with long cycle life. J. Alloys Compd. 2020, 816, 152497. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.; Mi, H.; Li, Y.; Zhang, P.; Zhang, H. A carob–inspired nanoscale design of yolk–shell Si@void@TiO2–CNF composite as anode material for high–performance lithium–ion batteries. Dalton Trans. 2019, 48, 6846–6852. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Luo, T.; Dai, L.; He, Z.; Wang, Q. Topology design of cold plates for pouch battery thermal management considering heat distribution characteristics. Appl. Therm. Eng. 2023, 224, 119940. [Google Scholar] [CrossRef]
- Gu, Y.; Jiao, Z.; Wu, M.; Luo, B.; Lei, Y.; Wang, Y.; Wang, L.; Zhang, H. Construction of point–line–plane (0–1–2 dimensional) Fe2O3–SnO2/graphene hybrids as the anodes with excellent lithium storage capability. Nano Res. 2017, 10, 121–133. [Google Scholar] [CrossRef]
- Lee, J.I.; Song, G.; Cho, S.; Han, D.Y.; Park, S. Lithium metal interface modification for high–energy batteries: Approaches and characterization. Batter. Supercaps 2020, 3, 828–859. [Google Scholar] [CrossRef]
- Ji, H.; Li, J.; Li, S.; Cui, Y.; Liu, Z.; Huang, M.; Xu, C.; Li, G.; Zhao, Y.; Li, H. High–Value Utilization of Silicon Cutting Waste and Excrementum Bombycis to Synthesize Silicon–Carbon Composites as Anode Materials for Li–Ion Batteries. Nanomaterials 2022, 12, 2875. [Google Scholar] [CrossRef]
- Ji, H.; Luo, T.; Dai, L.; He, Z.; Wang, Q. Numerical investigation on the polarization and thermal characteristics of LiFePO4-based batteries during charging process. Appl. Therm. Eng. 2022, 214, 118709. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gao, D.; Chen, S.; Tan, L.; Li, L. Facile synthesis of yolk–shell structured Si–C nanocomposites as anodes for lithium–ion batteries. Chem. Commun. 2014, 50, 5878–5880. [Google Scholar] [CrossRef] [Green Version]
- Ru, Y.; Evans, D.G.; Zhu, H.; Yang, W. Facile fabrication of yolk–shell structured porous Si–C microspheres as effective anode materials for Li–ion batteries. RSC Adv. 2014, 4, 71–75. [Google Scholar] [CrossRef]
- Sun, Z.; Song, X.; Zhang, P.; Gao, L. Controlled synthesis of yolk–mesoporous shell Si@SiO2 nanohybrid designed for high performance Li ion battery. RSC Adv. 2014, 4, 20814–20820. [Google Scholar] [CrossRef]
- Sun, Z.; Tao, S.; Song, X.; Zhang, P.; Gao, L. A silicon/double–shelled carbon yolk–like nanostructure as high–performance anode materials for lithium–ion battery. J. Electrochem. Soc. 2015, 162, A1530. [Google Scholar] [CrossRef]
- Li, J.; Lin, Y.; Shi, J.; Ban, B.; Sun, J.; Ma, Y.; Wang, F.; Lv, W.; Chen, J. Recovery of high purity Si from kerf–loss Si slurry waste by flotation method using PEA collector. Waste Manag. 2020, 115, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, I.; Liu, C.; Hsu, H.; Lan, C. Recycling and reuse of kerf–loss silicon from diamond wire sawing for photovoltaic industry. Waste Manag. 2019, 84, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, Y.; Wang, F.; Shi, J.; Sun, J.; Ban, B.; Liu, G.; Chen, J. Progress in recovery and recycling of kerf loss silicon waste in photovoltaic industry. Sep. Purif. Technol. 2021, 254, 117581. [Google Scholar] [CrossRef]
- Drouiche, N.; Cuellar, P.; Kerkar, F.; Medjahed, S.; Boutouchent-Guerfi, N.; Hamou, M.O. Recovery of solar grade silicon from kerf loss slurry waste. Renew. Sustain. Energy Rev. 2014, 32, 936–943. [Google Scholar] [CrossRef]
- Xiang, K.; Wang, X.; Chen, M.; Shen, Y.; Shu, H.; Yang, X. Industrial waste silica preparation of silicon carbide composites and their applications in lithium–ion battery anode. J. Alloys Compd. 2017, 695, 100–105. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Zhu, M.; Wang, W.; Wang, L.; Xie, S.; Wang, L.; Yang, X.; He, X.; Sun, Y. Recycling of lignin and Si waste for advanced Si/C battery anodes. ACS Appl. Mater. Interfaces 2020, 12, 57055–57063. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wu, X.; Sun, H.; Yang, F.; Wang, K.; Zhang, L.; Fan, C.; Zhang, J. A novel approach to prepare Si/C nanocomposites with yolk–shell structures for lithium ion batteries. RSC Adv. 2014, 4, 36218–36225. [Google Scholar] [CrossRef]
- Zhang, L.; Rajagopalan, R.; Guo, H.; Hu, X.; Dou, S.; Liu, H. A Green and Facile way to prepare Granadilla–like silicon-based anode materials for Li–ion batteries. Adv. Funct. Mater. 2016, 26, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Gao, Y.; Tang, Y.; Zhang, Y.; Li, X.; Liu, L. Porous silicon from industrial waste engineered for superior stability lithium–ion battery anodes. J. Nanopart. Res. 2021, 23, 209. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Xi, F.; Wan, X.; Ding, Z.; Chen, Z.; Ma, W.; Deng, R. Si@SiOx/Ag composite anodes with high initial coulombic efficiency derive from recyclable silicon cutting waste. Chem. Eng. J. 2022, 447, 137563. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, X.; Wang, X.; Gu, A.; Chen, X.; Yu, Z. Facile preparation of nitrogen–doped yolk–shell Si@void@C/CNTs microspheres as high–performance anode in lithium–ion batteries. Mater. Today Commun. 2020, 25, 101589. [Google Scholar] [CrossRef]
- Guan, H.; Wang, Q.; Wu, X.; Pang, J.; Jiang, Z.; Chen, G.; Dong, C.; Wang, L.; Gong, C. Biomass derived porous carbon (BPC) and their composites as lightweight and efficient microwave absorption materials. Compos. Part B Eng. 2021, 207, 108562. [Google Scholar] [CrossRef]
- Yang, H.; Wang, G.; Ding, N.; Yin, C.; Chen, Y. Size–controllable synthesis of carbon spheres with assistance of metal ions. Synth. Met. 2016, 214, 1–4. [Google Scholar] [CrossRef]
- Hu, L.; Luo, B.; Wu, C.; Hu, P.; Wang, L.; Zhang, H. Yolk–shell Si/C composites with multiple Si nanoparticles encapsulated into double carbon shells as lithium–ion battery anodes. J. Energy Chem. 2019, 32, 124–130. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Z.; Su, W.; Wei, Z.; Zhong, G.; Wang, C.; Huang, X. Alumina coated nano silicon synthesized by aluminothermic reduction as anodes for lithium ion batteries. Funct. Mater. Lett. 2017, 10, 1650073. [Google Scholar] [CrossRef]
- Lu, Y.; Chang, P.; Wang, L.; Nzabahimana, J.; Hu, X. Yolk–shell Si/SiOx@Void@C composites as anode materials for lithium–ion batteries. Funct. Mater. Lett. 2019, 12, 1850094. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Han, T.; Zhou, T.; Qiao, X.; Chen, X.; Zhou, P.; Liu, J. A novel silicon nanoparticles–infilled capsule prepared by an oil–in–water emulsion strategy for high–performance Li–ion battery anodes. Nanotechnology 2020, 31, 335403. [Google Scholar] [CrossRef]
- Mi, H.; Yang, X.; Li, Y.; Zhang, P.; Sun, L. A self–sacrifice template strategy to fabricate yolk–shell structured silicon@void@ carbon composites for high–performance lithium–ion batteries. Chem. Eng. J. 2018, 351, 103–109. [Google Scholar] [CrossRef]
- Shao, T.; Liu, J.; Gan, L.; Gong, Z.; Long, M. Yolk–shell Si@void@C composite with Chito–oligosaccharide as a C–N precursor for high capacity anode in lithium–ion batteries. J. Phys. Chem. Solids 2021, 152, 109965. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, L.; Liu, Y.; Zhu, X.; Xie, X. From sand to fast and stable silicon anode: Synthesis of hollow Si@void@C yolk–shell microspheres by aluminothermic reduction for lithium storage. Chin. Chem. Lett. 2019, 30, 610–617. [Google Scholar] [CrossRef]
- Yoon, D.; Kim, D.H.; Chung, K.Y.; Chang, W.; Kim, S.M.; Kim, J. Hydrogen–enriched porous carbon nanosheets with high sodium storage capacity. Carbon 2016, 98, 213–220. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, X.; Xu, R.; Wu, X.; Feng, Y.; Zhang, H.; Peng, S.; Yu, Y. Boosting the potassium storage performance of carbon anode via integration of adsorption–intercalation hybrid mechanisms. Nano Energy 2020, 73, 104807. [Google Scholar] [CrossRef]
- Ji, H.; Chen, Q.; Hua, K.; Ma, Q.; Wang, R.; Zhang, L.; Zhang, C. Si nanoparticles embedded in porous N-doped carbon fibers as a binder–free and flexible anode for high–performance lithium–ion batteries. J. Alloys Compd. 2023, 936, 168256. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Liu, X.; Teng, Y.; Li, Y.; Guo, Y.; Zhu, Y.; Wang, X.; Chao, Y. Enabling the ability of Li storage at high rate as anodes by utilizing natural rice husks–based hierarchically porous SiO2/N–doped carbon composites. Electrochim. Acta 2020, 359, 136933. [Google Scholar] [CrossRef]
- Domi, Y.; Usui, H.; Shimizu, M.; Kakimoto, Y.; Sakaguchi, H. Effect of phosphorus–doping on electrochemical performance of silicon negative electrodes in lithium–ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 7125–7132. [Google Scholar] [CrossRef]
- Liu, M.; Fan, H.; Zhuo, O.; Chen, J.; Hu, Z. A general strategy to construct yolk–shelled metal oxides inside carbon nanocages for high–stable lithium–ion battery anodes. Nano Energy 2019, 68, 104368. [Google Scholar] [CrossRef]
- Zhang, Y.C.; You, Y.; Xin, S.; Yin, Y.X.; Guo, Y.G. Rice Husk–Derived Hierarchical Silicon/Nitrogen–Doped Carbon/Carbon Nanotube Spheres as Low–Cost and High–Capacity Anodes for Lithium–Ion Batteries. Nano Energy 2016, 25, 120–127. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Peng, H.; Yang, Z.; Martin, D.J.; Bund, A.; Nanjundan, A.K.; Yamauchi, Y. Electrochemical Characteristics of Cobaltosic Oxide in Organic Electrolyte According to Bode Plots: Double–Layer Capacitance and Pseudocapacitance. Chemelectrochem 2019, 6, 2456–2463. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Tao, L.; Hu, B.; Xu, J.; Ding, J. Enhanced rate and low–temperature performance of LiFePO4 cathode with 2D Ti3C2 MXene as conductive network. Electroanal. Chem. 2023, 928, 117047. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, T.Y.; Duh, J.G. Constructing cellulose nanofiber/Si composite 3D–Net structure from waste rice straw via freeze drying process for lithium–ion battery anode materials. Mater. Chem. Phys. 2022, 285, 126107. [Google Scholar] [CrossRef]
- Gao, J.; Zuo, S.; Liu, H.; Jiang, Q.; Wang, C.; Yin, H.; Wang, Z.; Wang, J. An interconnected and scalable hollow Si–C nanospheres/graphite composite for high–performance lithium–ion batteries. J. Colloid Interface Sci. 2022, 624, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Guan, P.; Lv, C.; Yang, C.; Han, N.; Wang, X.; Song, G.; Peng, Z. One–pot sol–gel synthesis of Si/C yolk–shell anodes for high performance lithium–ion batteries. J. Alloys Compd. 2020, 835, 155135. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, Y.; Zeng, Y.; Zhang, Y.; Cheng, W.; Wang, W.; Jia, D.; Tang, X.; Wang, L. Designed Formation of Yolk–Shell–Like N–Doped Carbon–Coated Si Nanoparticles by a Facile Method for Lithium–Ion Battery Anodes. ACS Appl. Energy Mater. 2022, 5, 1471–1477. [Google Scholar] [CrossRef]





| Element | Si | Fe | Cr | Zr | Ni | Ca | Mg | Cu |
|---|---|---|---|---|---|---|---|---|
| KL Si | 89.94 | 5.33 | 1.28 | 1.21 | 0.66 | 0.048 | 0.042 | 0.032 |
| pretreated Si | 98.56 | 0.0096 | / | 0.024 | 0.020 | 0.0090 | / | / |
| No. | Material | Method | Specific Capacitance | Reference |
|---|---|---|---|---|
| 1 | Si–CNF2 | freeze drying | 640 mAh g−1 at 0.5 A g−1 after 100 cycles | [42] |
| 2 | Si/C | magnesiothermic reduction reaction | 662 mAh g−1 at 0.5 A g−1 after 200 cycles | [43] |
| 3 | Si@HC | one–pot sol–gel approach | 999.5 mAh g−1 at 0.1 A g−1 after 100 cycles | [44] |
| 4 | Si@void@C | hydrothermal method | 1038.5 mAh g−1 at 0.42 A g−1 after 200 cycles | [31] |
| 5 | Si@void@NC | solution coating and calcination process | 697.7 mAh g−1 at 0.2 A g−1 after 200 cycles | [45] |
| 6 | SVC−11 | metathesis reaction | 836 mAh g−1 at 0.1 A g−1 after 100 cycles | present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Liu, Z.; Li, X.; Li, J.; Yan, Z.; Tang, K. Recycling Silicon Waste from the Photovoltaic Industry to Prepare Yolk–Shell Si@void@C Anode Materials for Lithium–Ion Batteries. Processes 2023, 11, 1764. https://doi.org/10.3390/pr11061764
Ji H, Liu Z, Li X, Li J, Yan Z, Tang K. Recycling Silicon Waste from the Photovoltaic Industry to Prepare Yolk–Shell Si@void@C Anode Materials for Lithium–Ion Batteries. Processes. 2023; 11(6):1764. https://doi.org/10.3390/pr11061764
Chicago/Turabian StyleJi, Hengsong, Zhijin Liu, Xiang Li, Jun Li, Zexuan Yan, and Kai Tang. 2023. "Recycling Silicon Waste from the Photovoltaic Industry to Prepare Yolk–Shell Si@void@C Anode Materials for Lithium–Ion Batteries" Processes 11, no. 6: 1764. https://doi.org/10.3390/pr11061764
APA StyleJi, H., Liu, Z., Li, X., Li, J., Yan, Z., & Tang, K. (2023). Recycling Silicon Waste from the Photovoltaic Industry to Prepare Yolk–Shell Si@void@C Anode Materials for Lithium–Ion Batteries. Processes, 11(6), 1764. https://doi.org/10.3390/pr11061764






