Exploring the Potential of Aerated Concrete and Clay Bricks from Construction and Demolition Waste as Adsorbents for Pb(II) Removal from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Chemicals and Reagents
2.3. Material Characterization
2.4. Batch Experiments
2.5. Adsorption Isotherm Model
2.6. Statistical Analysis
3. Results and Discussion
3.1. Adsorbent Characterization
3.1.1. Pore Characteristics
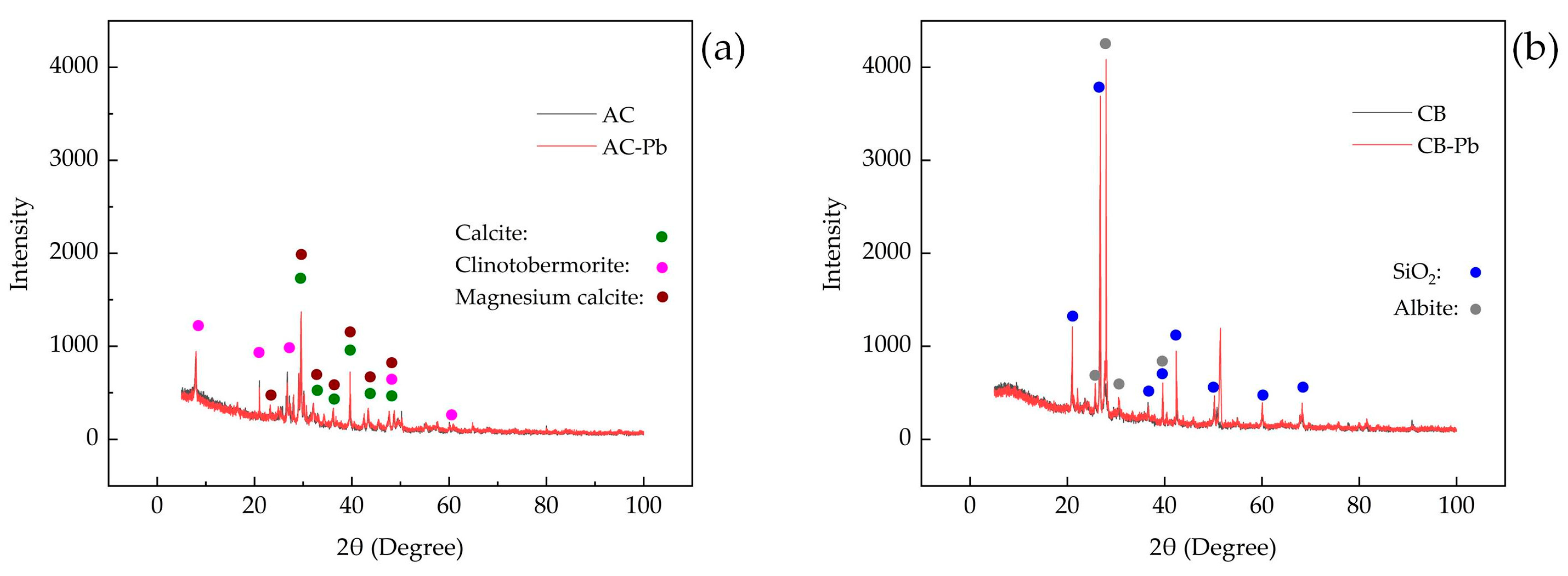
3.1.2. XRD Analysis
3.1.3. XRF Analysis
3.2. Batch Experiments for Lead Ions Removal
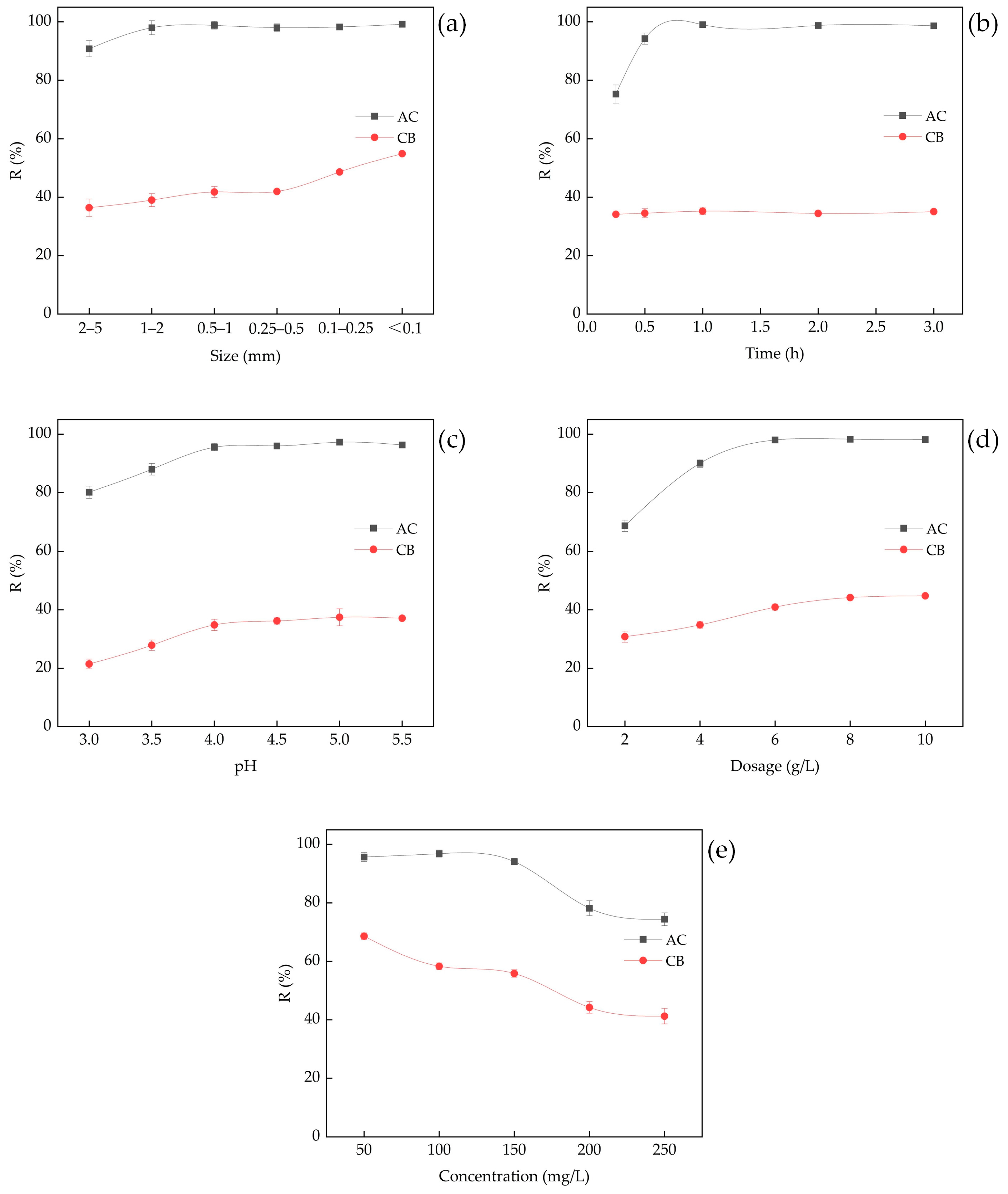
3.2.1. Effect of Particle Size
3.2.2. Effect of Contact Time
3.2.3. Effect of pH
3.2.4. Effect of Adsorbent Dosage
3.2.5. Effect of Initial Pb2+ Concentration and Adsorption Isotherms
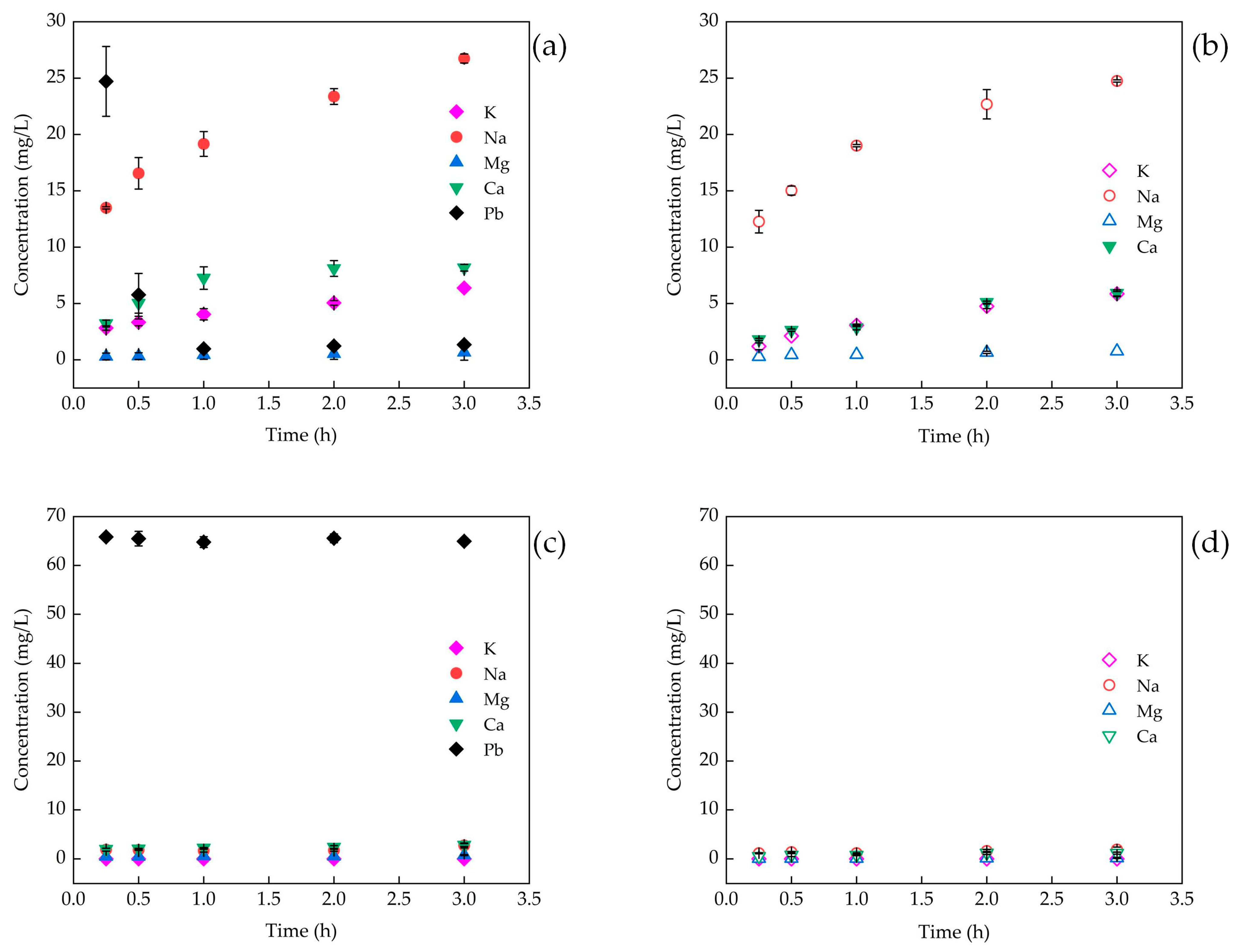
3.3. Mechanism of Pb(II) Removal by Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Kozera-Sucharda, B.; Gworek, B.; Kondzielski, I.; Chojnicki, J. The comparison of the efficacy of natural and synthetic aluminosilicates, including zeolites, in concurrent elimination of lead and copper from multi-component aqueous solutions. Processes 2021, 9, 812. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water contamination by heavy metals and their toxic effect on aquaculture and human health through food Chain. Lett. Appl. Nanobiosci. 2020, 10, 2148–2166. [Google Scholar]
- Collin, M.S.; Venkataraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Zhang, L.; Lin, X.; Wang, J.; Jiang, F.; Wei, L.; Chen, G.; Hao, X. Effects of lead and mercury on sulfate-reducing bacterial activity in a biological process for flue gas desulfurization wastewater treatment. Sci. Rep. 2016, 6, 30455. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.; Jaafar, J.; Ismail, A. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Mishra, P.C.; Patel, R.K. Removal of lead and zinc ions from water by low cost adsorbents. J. Hazard. Mater. 2009, 168, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.J.; Al-Ahmed, A. Removal of lead ions (Pb2+) from water and wastewater: A review on the low-cost adsorbents. Appl. Water Sci. 2022, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Hatiya, N.A.; Reshad, A.S.; Negie, Z.W. Chemical modification of neem (Azadirachta indica) biomass as bioadsorbent for removal of Pb2+ ion from aqueous waste water. Adsorpt. Sci. Technol. 2022, 2022, 7813513. [Google Scholar] [CrossRef]
- Ghorbani, M.; Seyedin, O.; Aghamohammadhassan, M. Adsorptive removal of lead (II) ion from water and wastewater media using carbon-based nanomaterials as unique sorbents: A review. J. Environ. Manag. 2020, 254, 109814. [Google Scholar] [CrossRef]
- Zahri, N.A.M.; Jamil, S.N.A.M.; Abdullah, L.C.; Huey, S.J.; Yaw, T.C.S.; Mobarekeh, M.N.; Rapeia, N.S.M. Equilibrium and kinetic behavior on cadmium and lead removal by using synthetic polymer. J. Water Process Eng. 2017, 17, 277–289. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; Jaafar, W.Z.; AlOmar, M.K.; Fayaed, S.S.; Mohd, N.S.; Hin, L.S.; El-Shafie, A. Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- Tahoon, M.A.; Siddeeg, S.M.; Alsaiari, N.S.; Mnif, W.; Rebah, F.B. Effective heavy metals removal from water using nanomaterials: A review. Processes 2020, 8, 645. [Google Scholar] [CrossRef]
- Kumara, G.M.P.; Kawamoto, K. Steel slag and autoclaved aerated concrete grains as low-cost adsorbents to remove Cd2+ and Pb2+ in wastewater: Effects of mixing proportions of grains and liquid-to-solid ratio. Sustainability 2021, 13, 10321. [Google Scholar] [CrossRef]
- Duan, H.; Miller, T.R.; Liu, G.; Tam, V.W. Construction debris becomes growing concern of growing cities. Waste Manag. 2019, 83, 1–5. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Li, B.; Zhou, N.; Xiao, X.; Li, M.; Zhu, C. Environmental behavior of construction and demolition waste as recycled aggregates for backfilling in mines: Leaching toxicity and surface subsidence studies. J. Hazard. Mater. 2020, 389, 121870. [Google Scholar] [CrossRef]
- Marzouk, M.; Azab, S. Environmental and economic impact assessment of construction and demolition waste disposal using system dynamics. Resour. Conserv. Recycl. 2014, 82, 41–49. [Google Scholar] [CrossRef]
- Liu, Q.; Li, B.; Xiao, J.; Singh, A. Utilization potential of aerated concrete block powder and clay brick powder from C&D waste. Constr. Build. Mater. 2020, 238, 117721. [Google Scholar]
- Bergmans, J.; Nielsen, P.; Snellings, R.; Broos, K. Recycling of autoclaved aerated concrete in floor screeds: Sulfate leaching reduction by ettringite formation. Constr. Build. Mater. 2016, 111, 9–14. [Google Scholar] [CrossRef]
- Arabyarmohammadi, H.; Salarirad, M.M.; Behnamfard, A. Characterization and utilization of clay-based construction and demolition wastes as adsorbents for zinc (II) removal from aqueous solutions: An equilibrium and kinetic study. Environ. Prog. Sustain. Energy 2014, 33, 777–789. [Google Scholar] [CrossRef]
- Djeribi, R.; Hamdaoui, O. Sorption of copper (II) from aqueous solutions by cedar sawdust and crushed brick. Desalination 2008, 225, 95–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, L.; Kang, Y.; Luo, J.; Li, W.; Zhang, Q. Sustainable use of autoclaved aerated concrete waste to remove low concentration of Cd (II) ions in wastewater. Desalin. Water Treat. 2017, 82, 170–178. [Google Scholar] [CrossRef]
- Pyrzynska, K. Removal of cadmium from wastewaters with low-cost adsorbents. J. Environ. Chem. Eng. 2019, 7, 102795. [Google Scholar] [CrossRef]
- Mokokwe, G.; Letshwenyo, M.W. Utilisation of cement brick waste as low cost adsorbent for the adsorptive removal of copper, nickel and iron from aqueous solution: Batch and column studies. Phys. Chem. Earth Parts A/B/C 2022, 126, 103156. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Darmayanti, L.; Kadja, G.T.; Notodarmojo, S.; Damanhuri, E.; Mukti, R.R. Structural alteration within fly ash-based geopolymers governing the adsorption of Cu2+ from aqueous environment: Effect of alkali activation. J. Hazard. Mater. 2019, 377, 305–314. [Google Scholar] [CrossRef]
- Van Ranst, E.; Utami, S.; Verdoodt, A.; Qafoku, N. Mineralogy of a perudic Andosol in central Java, Indonesia. Geoderma 2008, 144, 379–386. [Google Scholar] [CrossRef]
- Liu, X.; Bai, X.; Dong, L.; Liang, J.; Jin, Y.; Wei, Y.; Li, Y.; Huang, S.; Qu, J. Composting enhances the removal of lead ions in aqueous solution by spent mushroom substrate: Biosorption and precipitation. J. Clean. Prod. 2018, 200, 1–11. [Google Scholar] [CrossRef]
- Kikongi, P.; Salvas, J.; Gosselin, R. Curve-fitting regression: Improving light element quantification with XRF. X-ray Spectrom. 2017, 46, 347–355. [Google Scholar] [CrossRef]
- Chen, H.; Tan, X.; Xin, X.; Wang, Y.; Liu, F.; Cui, K. Preparation of activated charcoal from pyrolusite-added sewage sludge and adsorption of lead ion in wastewater. Chin. J. Environ. Eng. 2010, 4, 2473–2478. [Google Scholar]
- Bibi, S.; Farooqi, A.; Yasmin, A.; Kamran, M.A.; Niazi, N.K. Arsenic and fluoride removal by potato peel and rice husk (PPRH) ash in aqueous environments. Int. J. Phytorem. 2017, 19, 1029–1036. [Google Scholar] [CrossRef]
- Filote, C.; Volf, I.; Santos, S.C.; Botelho, C.M. Bioadsorptive removal of Pb (II) from aqueous solution by the biorefinery waste of Fucus spiralis. Sci. Total Environ. 2019, 648, 1201–1209. [Google Scholar] [CrossRef]
- Tao, B.; Li, W.; Xie, L.; Yu, J.; He, F. Performance analysis of demolition waste bricks for phosphorus removal from stormwater runoff. Urban Water J. 2020, 17, 144–153. [Google Scholar] [CrossRef]
- Ai, Y.; Yin, N.; Ouyang, Y.; Xu, Y.; Yang, P. Waste non-burn-free brick derived sulfhydryl functioned magnetic zeolites and their efficient removal of uranium (VI) ions. Appl. Surf. Sci. 2022, 571, 151241. [Google Scholar] [CrossRef]
- Mikyskova, E.; Dousova, B.; Mikysek, P.; Lhotka, M.; Kolousek, D. Equilibrium, kinetic and thermodynamic study of Pb2+ removal from aqueous solution by waste brick dust. Colloids Surf. A 2022, 634, 127939. [Google Scholar] [CrossRef]
- Ghazy, S.E.; Gad, A.H. Lead separation by sorption onto powdered marble waste. Arab. J. Chem. 2014, 7, 277–286. [Google Scholar] [CrossRef]
- Shawket, A.; Abderyim, S.; Ismayil, N. Analysis of the adsorption properties of lead ion onto natural sand particles by flame atomic absorption spectrometry. Spectrosc. Spectr. Anal. 2011, 31, 3126–3129. [Google Scholar]
- Sarı, A.; Tuzen, M.; Soylak, M. Adsorption of Pb (II) and Cr (III) from aqueous solution on Celtek clay. J. Hazard. Mater. 2007, 144, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Perić, J.; Trgo, M.; Medvidović, N.V. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jin, X.; Lu, X.; Chen, Z. Adsorption of Pb (II), Cd (II), Ni (II) and Cu (II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Abdellaoui, Y.; Olguín, M.T.; Abatal, M.; Ali, B.; Méndez, S.E.D.; Santiago, A.A. Comparison of the divalent heavy metals (Pb, Cu and Cd) adsorption behavior by montmorillonite-KSF and their calcium-and sodium-forms. Superlattices Microstruct. 2019, 127, 165–175. [Google Scholar] [CrossRef]
- Chantawong, V.; Harvey, N.; Bashkin, V. Comparison of heavy metal adsorptions by Thai kaolin and ballclay. Water Air Soil Pollut. 2003, 148, 111–125. [Google Scholar] [CrossRef]
- Ranđelović, M.; Purenović, M.; Zarubica, A.; Purenović, J.; Matović, B.; Momčilović, M. Synthesis of composite by application of mixed Fe, Mg (hydr) oxides coatings onto bentonite–a use for the removal of Pb (II) from water. J. Hazard. Mater. 2012, 199, 367–374. [Google Scholar] [CrossRef]
- Abdus-Salam, N.; Adekola, F. The influence of pH and adsorbent concentration on adsorption of lead and zinc on a natural goethite. Afr. J. Sci. Technol. 2005, 6, 55–66. [Google Scholar] [CrossRef]
- Ghahfarokhi, S.S.H.; Landi, A.; Khademi, H.; Hojati, S. Removal of Cd2+ and Pb2+ ions from aqueous solutions using iranian natural zeolite and sepiolite. J. Environ. Stud. 2014, 40, 43. [Google Scholar]
- Tiwari, D.; Kim, H.U.; Lee, S.M. Removal behavior of sericite for Cu (II) and Pb (II) from aqueous solutions: Batch and column studies. Sep. Purif. Technol. 2007, 57, 11–16. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Y.; Huang, S.; Li, Y.; Jin, Y.; Xu, W.; Qu, J. Interpretation of lead removal by two biomasses at different size via monitoring the solution environment. J. Clean. Prod. 2020, 244, 118756. [Google Scholar] [CrossRef]
- Pap, S.; Kirk, C.; Bremner, B.; Sekulic, M.T.; Shearer, L.; Gibb, S.W.; Taggart, M.A. Low-cost chitosan-calcite adsorbent development for potential phosphate removal and recovery from wastewater effluent. Water Res. 2020, 173, 115573. [Google Scholar] [CrossRef] [PubMed]



| Variable | AC | CB | Unit |
|---|---|---|---|
| BET surface area | 29.15 ± 0.81 | 2.82 ± 0.31 | m2/g |
| Langmuir surface area | 39.07 ± 0.83 | 3.78 ± 0.22 | m2/g |
| Total pore volume | 0.0070 ± 0.0004 | 0.0013 ± 0.0002 | cm3/g |
| Average pore width | 4.74 ± 0.14 | 6.56 ± 0.24 | nm |
| Samples | Pb | Ca | Mg | K | Fe | Cu | Zn | Mn | Na |
|---|---|---|---|---|---|---|---|---|---|
| AC | 0.0 | 33.4 ± 0.4 | 0.0 | 0.0 | 1.3 ± 0.2 | 0.4 ± 0.1 | 0.0 | 0.0 | 0.0 |
| AC-Pb | 1.1 ± 0.2 | 19.8 ± 1.0 | 0.0 | 0.0 | 0.8 ± 0.1 | 0.2 ± 0.1 | 0.0 | 0.0 | 0.0 |
| CB | 0.0 | 0.3 ± 0.1 | 0.0 | 0.0 | 4.0 ± 0.3 | 0.3 ± 0.1 | 0.0 | 0.1 ± 0.1 | 0.0 |
| CB-Pb | 0.5 ± 0.1 | 0.0 | 0.0 | 0.0 | 3.5 ± 0.4 | 0.3 ± 0.1 | 0.0 | 0.0 | 0.0 |
| Adsorbent | Langmuir Constant | Freundlich Constant | ||||
|---|---|---|---|---|---|---|
| KL | qmax | R2 | n | Kf | R2 | |
| AC | 0.001 | 184.9 | 0.994 | 1.189 | 0.378 | 0.981 |
| CB | 0.004 | 42.9 | 0.996 | 1.457 | 0.486 | 0.980 |
| Adsorbent | qmax (mg/g) | References |
|---|---|---|
| aerated concrete | 201.6 | This work |
| clay brick | 56.3 | This work |
| waste brick dust | 128.1 | [40] |
| powdered marble wastes | 101.6 | [41] |
| natural sand particles | 24.9 | [42] |
| celtek clay | 18.8 | [43] |
| zeolite | 64 | [44] |
| kaolinite | 2.35 | [45] |
| montmorillonite | 3.25 | [46] |
| illite | 4.29 | [47] |
| bentonite | 68.84 | [48] |
| goethite | 5 | [49] |
| sepiolite | 50 | [50] |
| sericite | 4.697 | [51] |
| CPb | pH | EC | Cie-K | Cie-Na | Cie-Ca | Csum | |
|---|---|---|---|---|---|---|---|
| CPb | 1 | −0.867 | −0.633 | 0.814 | 0.161 | −0.704 | −0.37 |
| pH | 1 | 0.921 * | −0.944 * | −0.046 | 0.51 | 0.046 | |
| EC | 1 | −0.886 * | 0.236 | 0.157 | −0.206 | ||
| Cie-K | 1 | 0.033 | −0.384 | 0.209 | |||
| Cie-Na | 1 | −0.759 | −0.167 | ||||
| Cie-Ca | 1 | 0.572 | |||||
| Csum | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Han, H.; Xie, R.; Zhu, L.; Ma, X.; Liu, X. Exploring the Potential of Aerated Concrete and Clay Bricks from Construction and Demolition Waste as Adsorbents for Pb(II) Removal from Aqueous Solutions. Processes 2023, 11, 1798. https://doi.org/10.3390/pr11061798
Yuan Y, Han H, Xie R, Zhu L, Ma X, Liu X. Exploring the Potential of Aerated Concrete and Clay Bricks from Construction and Demolition Waste as Adsorbents for Pb(II) Removal from Aqueous Solutions. Processes. 2023; 11(6):1798. https://doi.org/10.3390/pr11061798
Chicago/Turabian StyleYuan, Yaru, Hongpei Han, Ruifeng Xie, Lin Zhu, Xianfa Ma, and Xuesheng Liu. 2023. "Exploring the Potential of Aerated Concrete and Clay Bricks from Construction and Demolition Waste as Adsorbents for Pb(II) Removal from Aqueous Solutions" Processes 11, no. 6: 1798. https://doi.org/10.3390/pr11061798
APA StyleYuan, Y., Han, H., Xie, R., Zhu, L., Ma, X., & Liu, X. (2023). Exploring the Potential of Aerated Concrete and Clay Bricks from Construction and Demolition Waste as Adsorbents for Pb(II) Removal from Aqueous Solutions. Processes, 11(6), 1798. https://doi.org/10.3390/pr11061798





