Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particle System
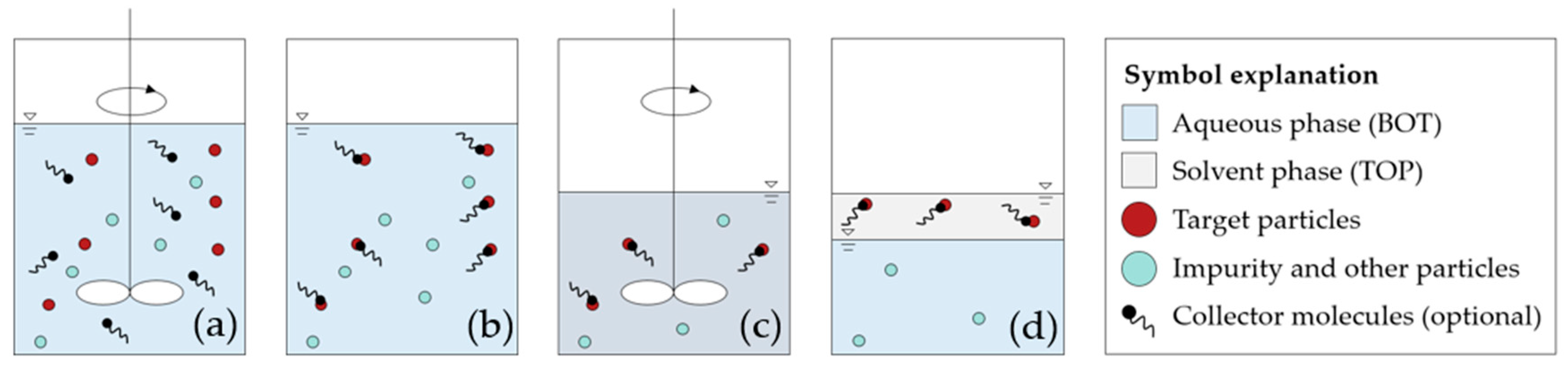
2.2. Liquid–Liquid Extraction
2.2.1. Experimental Procedure
2.2.2. Test Evaluation
2.3. Cake Washing
- (1)
- Removal of the collector molecules adsorbed on the particle surface;
- (2)
- Recovery of the applied solvent.
2.3.1. Experimental Procedure
2.3.2. Test Evaluation
3. Results and Discussion
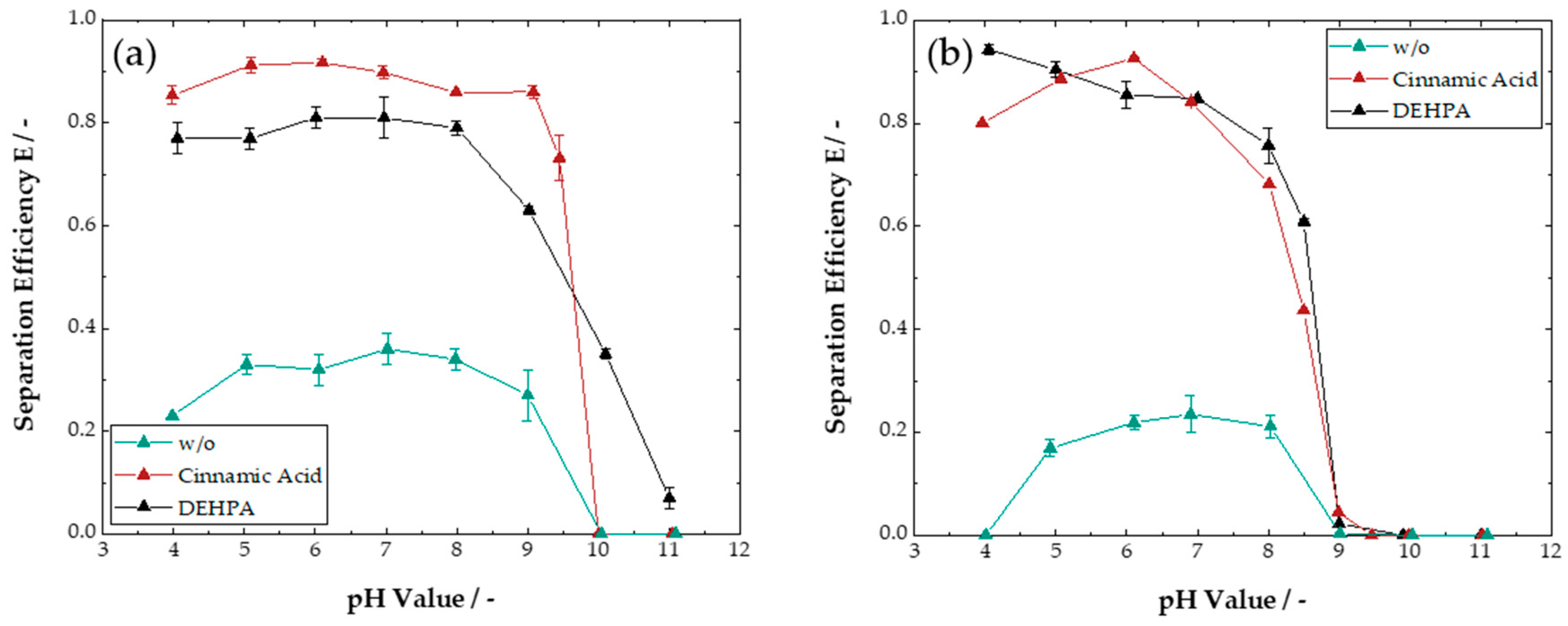
3.1. Liquid–Liquid Extraction
3.1.1. Single-System Extraction
3.1.2. Multi-System Extraction
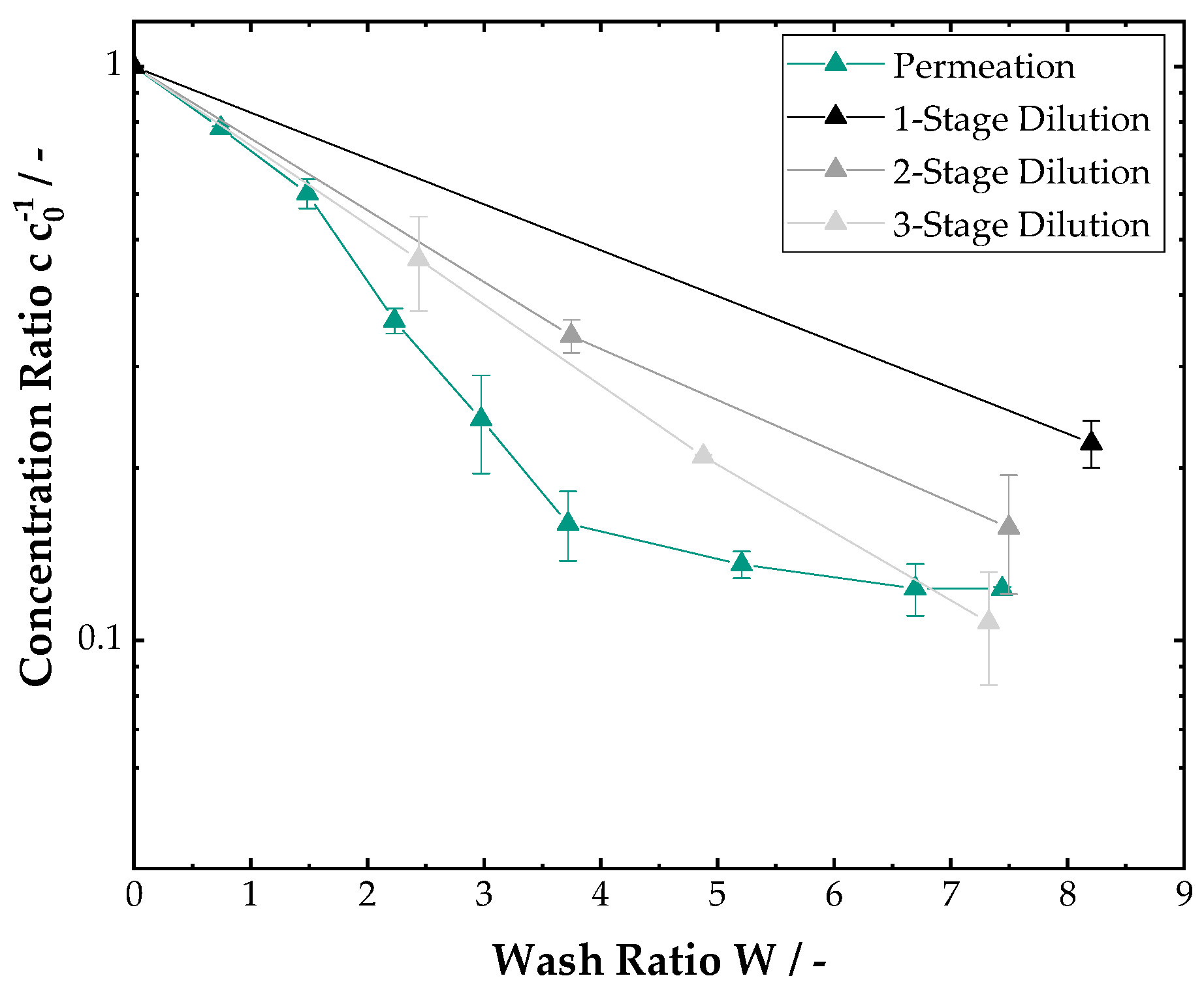
3.2. Filter Cake Washing
3.3. Continuous Process Chain
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andersson, M.; Ljunggren Söderman, M.; Sandén, B.A. Challenges of recycling multiple scarce metals: The case of Swedish ELV and WEEE recycling. Resour. Policy 2019, 63, 101403. [Google Scholar] [CrossRef]
- Sinclar, W.D. Electronic Metals (In, Ge and Ga): Present and Future Resources. Acta Geol. Sin.-Engl. Ed. 2014, 88, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, Y.; Liu, B.; Chang, C.-C. Supply and demand of some critical metals and present status of their recycling in WEEE. Waste Manag. 2017, 65, 113–127. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey. Mineral Commodity Summaries 2001. Available online: https://www.usgs.gov/publications/mineral-commodity-summaries-2001 (accessed on 15 May 2023).
- U.S. Geological Survey. Mineral Commodity Summaries 2023. Available online: https://www.usgs.gov/publications/mineral-commodity-summaries-2023 (accessed on 15 May 2023).
- Eggert, R.G. Minerals go critical. Nat. Chem. 2011, 3, 688–691. [Google Scholar] [CrossRef]
- Redlinger, M.; Eggert, R.; Woodhouse, M. Evaluating the availability of gallium, indium, and tellurium from recycled photovoltaic modules. Sol. Energy Mater. Sol. Cells 2015, 138, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Licht, C.; Peiró, L.T.; Villalba, G. Global Substance Flow Analysis of Gallium, Germanium, and Indium: Quantification of Extraction, Uses, and Dissipative Losses within their Anthropogenic Cycles. J. Ind. Ecol. 2015, 19, 890–903. [Google Scholar] [CrossRef]
- Nagy, S.; Bokányi, L.; Gombkötő, I.; Magyar, T. Recycling of Gallium from End-of-Life Light Emitting Diodes. Arch. Metall. Mater. 2017, 62, 1161–1166. [Google Scholar] [CrossRef] [Green Version]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.-S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef]
- Zhan, L.; Xia, F.; Ye, Q.; Xiang, X.; Xie, B. Novel recycle technology for recovering rare metals (Ga, In) from waste light-emitting diodes. J. Hazard. Mater. 2015, 299, 388–394. [Google Scholar] [CrossRef]
- Virolainen, S.; Ibana, D.; Paatero, E. Recovery of indium from indium tin oxide by solvent extraction. Hydrometallurgy 2011, 107, 56–61. [Google Scholar] [CrossRef]
- de La Torre, E.; Vargas, E.; Ron, C.; Gámez, S. Europium, Yttrium, and Indium Recovery from Electronic Wastes. Metals 2018, 8, 777. [Google Scholar] [CrossRef] [Green Version]
- Fontana, D.; Forte, F.; de Carolis, R.; Grosso, M. Materials recovery from waste liquid crystal displays: A focus on indium. Waste Manag. 2015, 45, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Retegan, T.; Steenari, B.-M.; Ekberg, C. Recovery of indium and yttrium from Flat Panel Display waste using solvent extraction. Sep. Purif. Technol. 2016, 166, 117–124. [Google Scholar] [CrossRef]
- Pereira, E.B.; Suliman, A.L.; Tanabe, E.H.; Bertuol, D.A. Recovery of indium from liquid crystal displays of discarded mobile phones using solvent extraction. Miner. Eng. 2018, 119, 67–72. [Google Scholar] [CrossRef]
- Yang, J.; Retegan, T.; Ekberg, C. Indium recovery from discarded LCD panel glass by solvent extraction. Hydrometallurgy 2013, 137, 68–77. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Ebin, B.; Isik, M.I. Pyrometallurgical Processes for the Recovery of Metals from WEEE. In WEEE Recycling; Elsevier: Amsterdam, The Netherlands, 2016; pp. 107–137. [Google Scholar]
- Sawistowski, H. Physical Aspects of Liquid-Liquid Extraction. In Mass Transfer with Chemical Reaction in Multiphase Systems. Alper, E., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1983; pp. 613–635. [Google Scholar]
- Kumar, A.; Negi, S.; Choudhury, T.; Mutreja, V.; Sunaina, S.; Sahoo, S.C.; Singh, A.; Mehta, S.K.; Kataria, R.; Saini, V. A highly sensitive and specific luminescent MOF determines nitric oxide production and quantifies hydrogen sulfide-mediated inhibition of nitric oxide in living cells. Mikrochim. Acta 2023, 190, 127. [Google Scholar] [CrossRef]
- Kirandeep; Kaur, J.; Sharma, I.; Zangrando, E.; Pal, K.; Mehta, S.K.; Kataria, R. Fabrication of novel copper MOF nanoparticles for nanozymatic detection of mercury ions. J. Mater. Res. Technol. 2023, 22, 278–291. [Google Scholar] [CrossRef]
- Kumar, A.; Mutreja, V.; Anand, V.; Mehta, S.K.; Zangrando, E.; Kataria, R. Solvent templated luminescent metal-organic frameworks for specific detection of vitamin C in aqueous media. J. Mol. Struct. 2023, 1284, 135365. [Google Scholar] [CrossRef]
- Buchheiser, S.; Deutschmann, M.P.; Rhein, F.; Allmang, A.; Fedoryk, M.; Stelzner, B.; Harth, S.; Trimis, D.; Nirschl, H. Particle and Phase Analysis of Combusted Iron Particles for Energy Storage and Release. Materials 2023, 16, 2009. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Snyder, R.L. RIR-Measurement and Use in Quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigma-Aldrich. Bis-(2-ethylhexyl)-phosphorsäure. Available online: https://www.sigmaaldrich.com/US/en/product/aldrich/237825 (accessed on 15 May 2023).
- Sigma-Aldrich. Cinnamic Acid. Available online: https://www.sigmaaldrich.com/US/en/substance/cinnamicacid14816140103 (accessed on 15 May 2023).
- Rhein, F.; Schmid, E.; Esquivel, F.B.; Nirschl, H. Opportunities and Challenges of Magnetic Seeded Filtration in Multidimensional Fractionation. Chem. Ing. Tech. 2020, 92, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Cerbelaud, M.; Videcoq, A.; Abélard, P.; Ferrando, R. Simulation of the heteroagglomeration between highly size-asymmetric ceramic particles. J. Colloid Interface Sci. 2009, 332, 360–365. [Google Scholar] [CrossRef]
- Anlauf, H. Wet Cake Filtration: Fundamentals, Equipment, Strategies; WILEY VCH: Weinheim, Germany, 2020. [Google Scholar]
- Wilkens, M.; Peuker, U.A. Grundlagen und aktuelle Entwicklungen der Filterkuchenwaschung. Chem. Ing. Tech. 2012, 84, 1873–1884. [Google Scholar] [CrossRef]
- Dobler, T.; Buchheiser, S.; Gleiß, M.; Nirschl, H. Development and Commissioning of a Small-Scale, Modular and Integrated Plant for the Quasi-Continuous Production of Crystalline Particles. Processes 2021, 9, 663. [Google Scholar] [CrossRef]

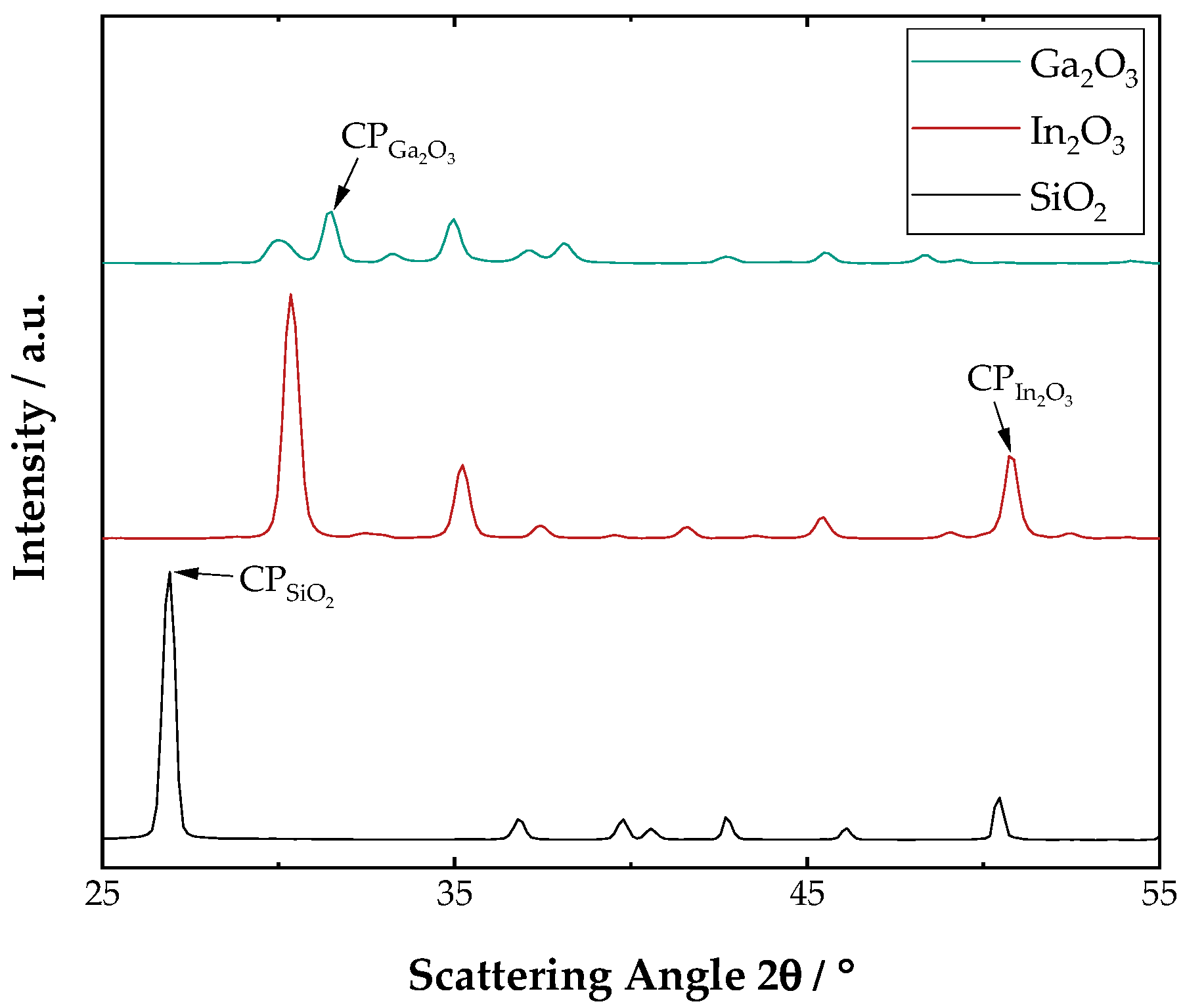




| Mixture () | Proportionality Constant /- | R-Square/- |
| In2O3/SiO2 | 0.333 | 0.995 |
| In2O3/Ga2O3 | 1.082 | 0.997 |
| Collector | Mixture | pH Value | Composition BOT (wt %) |
|---|---|---|---|
| Cinnamic Acid | In2O3SiO2 | 6 | 50|50 |
| In2O3|Ga2O3 | 9.5 | 50|50 | |
| In2O3|Ga2O3|SiO2 | 9.5 | 33.3|33.3|33.3 | |
| DEHPA | In2O3|SiO2 | 6 | 50|50 |
| In2O3|Ga2O3 | 10 | 50|50 | |
| In2O3|Ga2O3|SiO2 | 10 | 33.3|33.3|33.3 |
| Collector | Mixture | Composition TOP (wt %) | |
|---|---|---|---|
| Theoretical | Experimental | ||
| Cinnamic Acid | In2O3|SiO2 | 100|0 | 98.3|1.7 |
| In2O3|Ga2O3 | 100|0 | 76.7|23.3 | |
| In2O3|Ga2O3|SiO2 | 100|0|0 | 74.2|13.9|11.9 | |
| DEHPA | In2O3|SiO2 | 100|0 | 98.9|1.1 |
| In2O3|Ga2O3 | 100|0 | 93.1|6.9 | |
| In2O3|Ga2O3|SiO2 | 100|0|0 | 94.1|5.0|0.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobler, T.; Buchheiser, S.; Gaschler, T.; Platzk, S.; Kruggel-Emden, H.; Nirschl, H.; Gleiß, M. Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals. Processes 2023, 11, 1847. https://doi.org/10.3390/pr11061847
Dobler T, Buchheiser S, Gaschler T, Platzk S, Kruggel-Emden H, Nirschl H, Gleiß M. Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals. Processes. 2023; 11(6):1847. https://doi.org/10.3390/pr11061847
Chicago/Turabian StyleDobler, Timo, Simon Buchheiser, Thomas Gaschler, Stefan Platzk, Harald Kruggel-Emden, Hermann Nirschl, and Marco Gleiß. 2023. "Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals" Processes 11, no. 6: 1847. https://doi.org/10.3390/pr11061847
APA StyleDobler, T., Buchheiser, S., Gaschler, T., Platzk, S., Kruggel-Emden, H., Nirschl, H., & Gleiß, M. (2023). Development of a Continuous Process Chain for Selective Recovery and Purification of Rare Metals. Processes, 11(6), 1847. https://doi.org/10.3390/pr11061847







