D-Limonene as a Promising Green Solvent for the Detachment of End-of-Life Photovoltaic Solar Panels under Sonication
Abstract
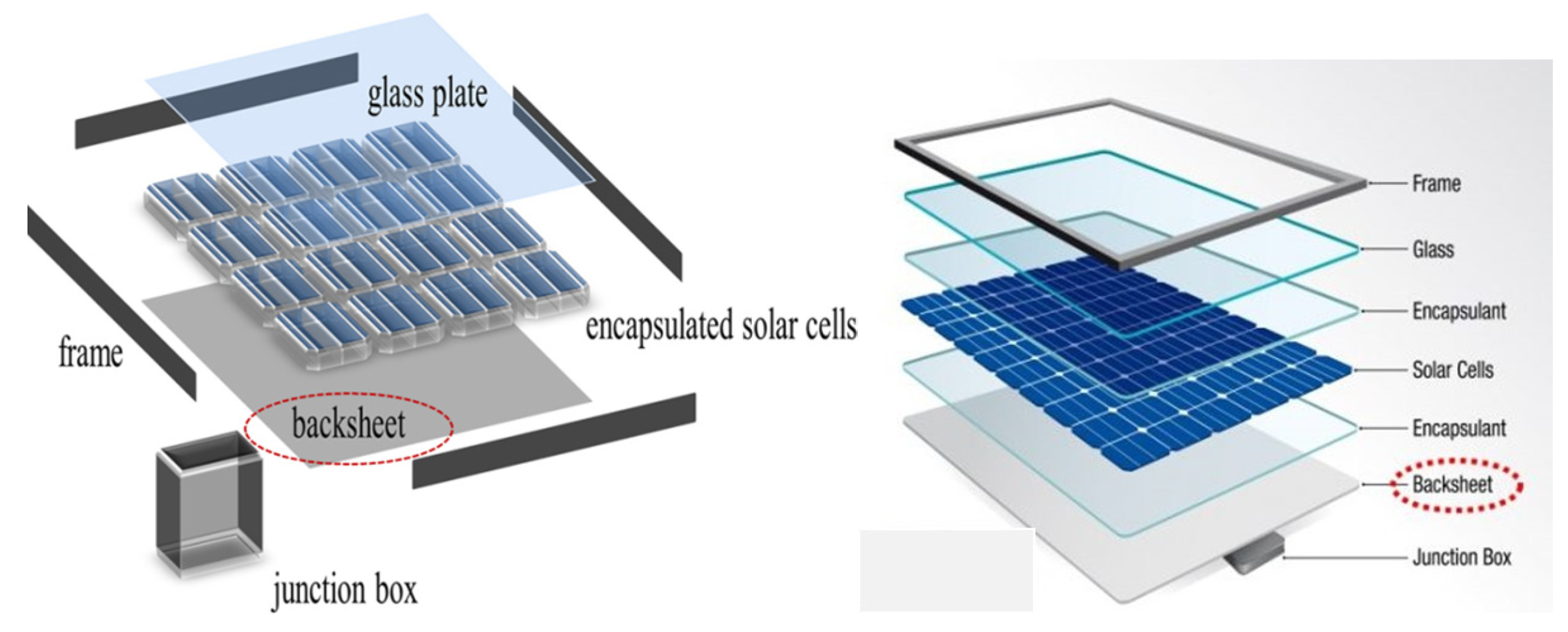
:1. Introduction
- To provide a structural support and positioning in the module-design layout;
- To achieve and maintain optical coupling between the solar cell and the glass, keeping the incidence of solar radiation at least 90% during the years of operation;
- To maintain the physical isolation of solar cells and components and to protect the circuit under operative conditions;
- To achieve and protect the electrical insulation between the solar cells and circuit elements during the operating life of the photovoltaic modules;
- To maintain the integrity of the electric circuit, which generates the required current and voltage under light exposure; moreover, the transparency of the encapsulant must also be maintained throughout the operative lifetime of the photovoltaic modules.
2. Materials and Methods
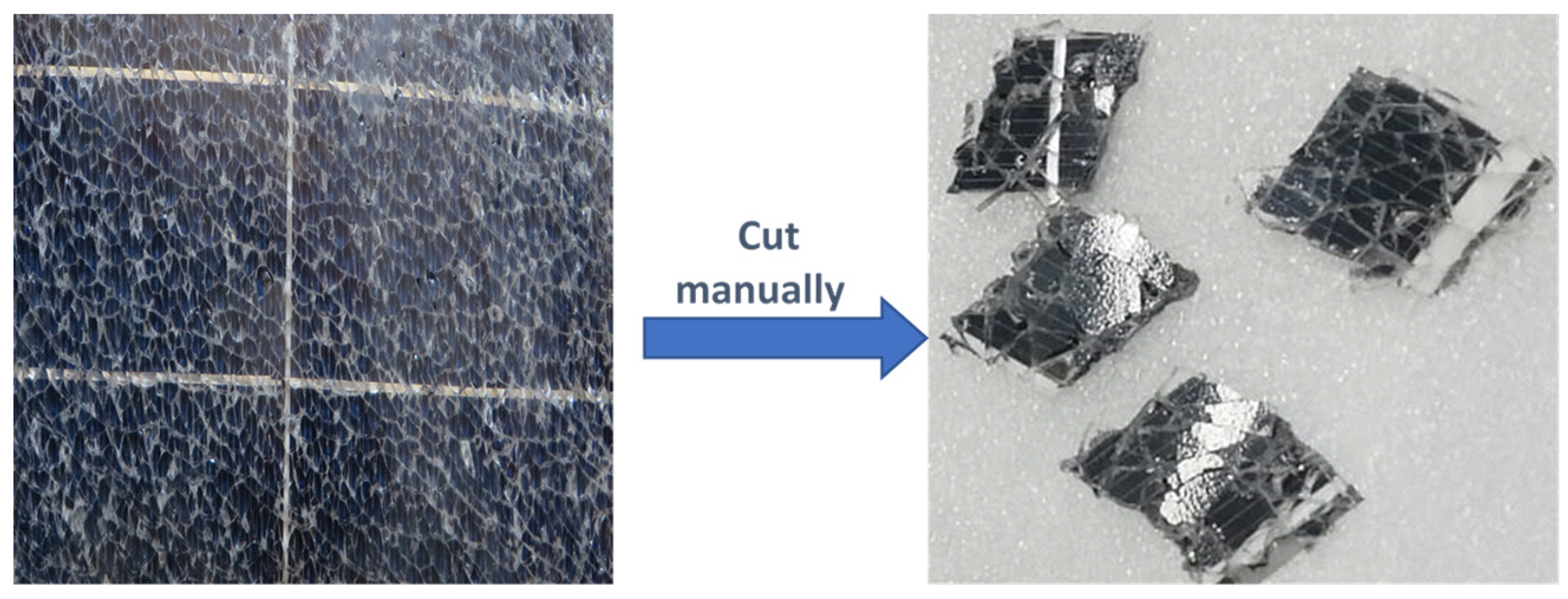
2.1. Sample Preparation
2.2. Detachment Procedures
2.3. Calculation
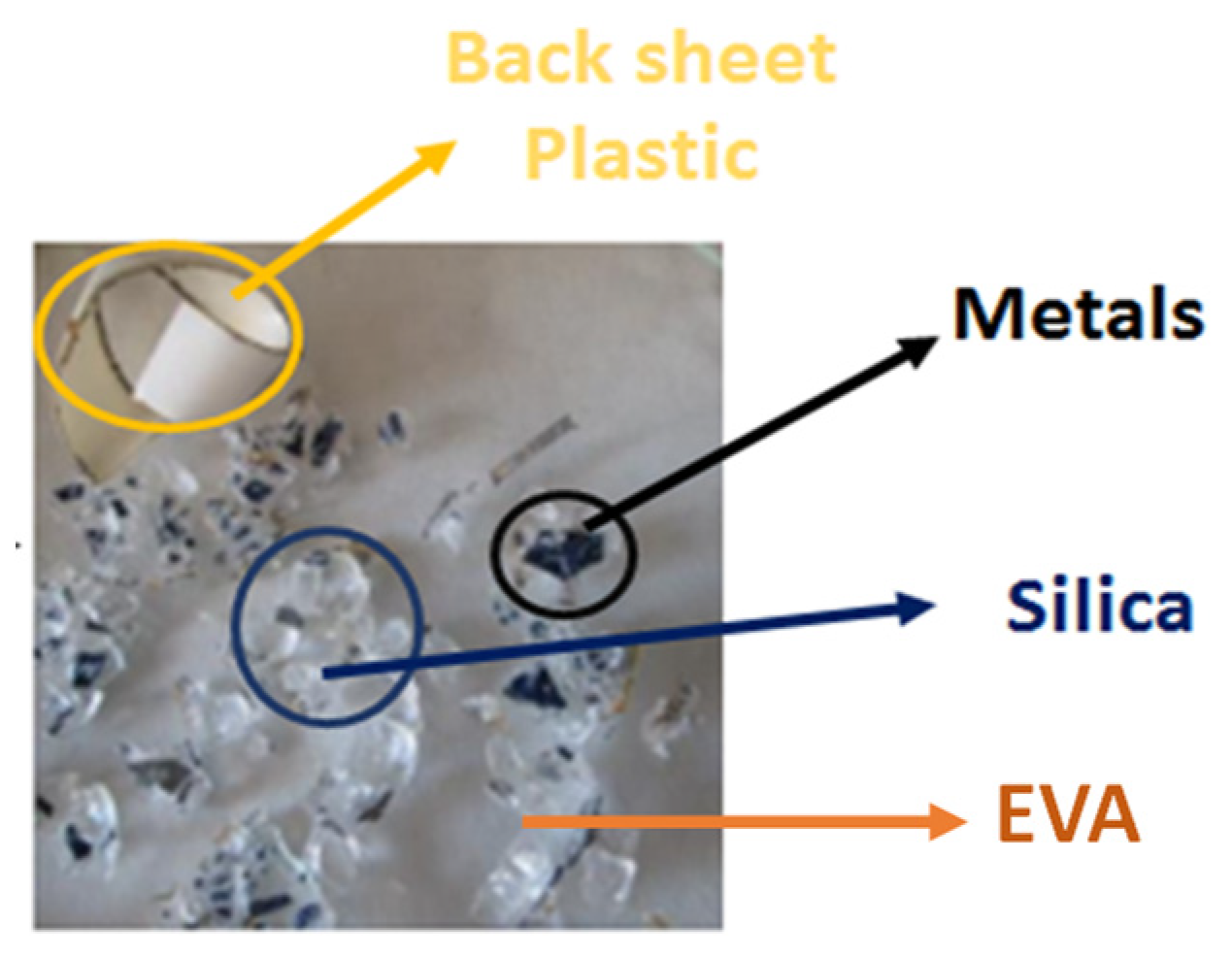
3. Results and Discussion
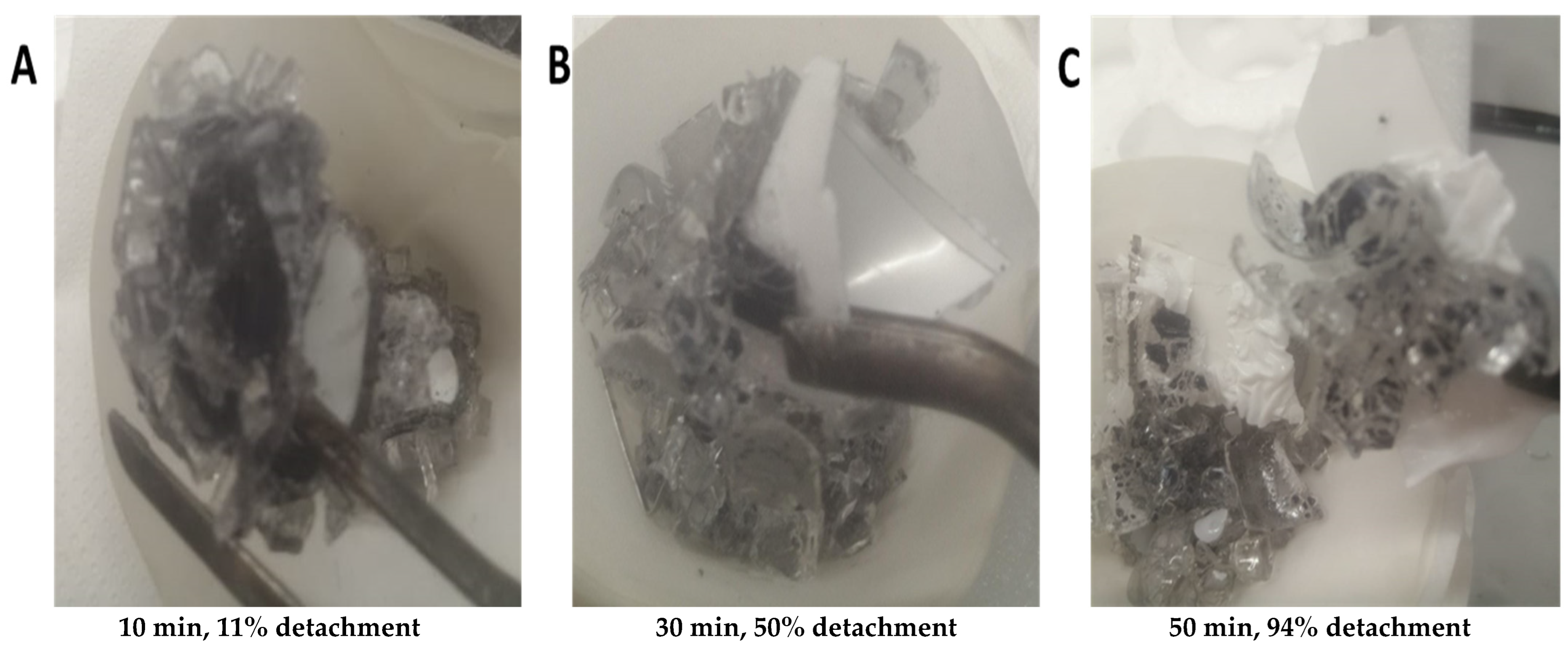
Detachment Condition
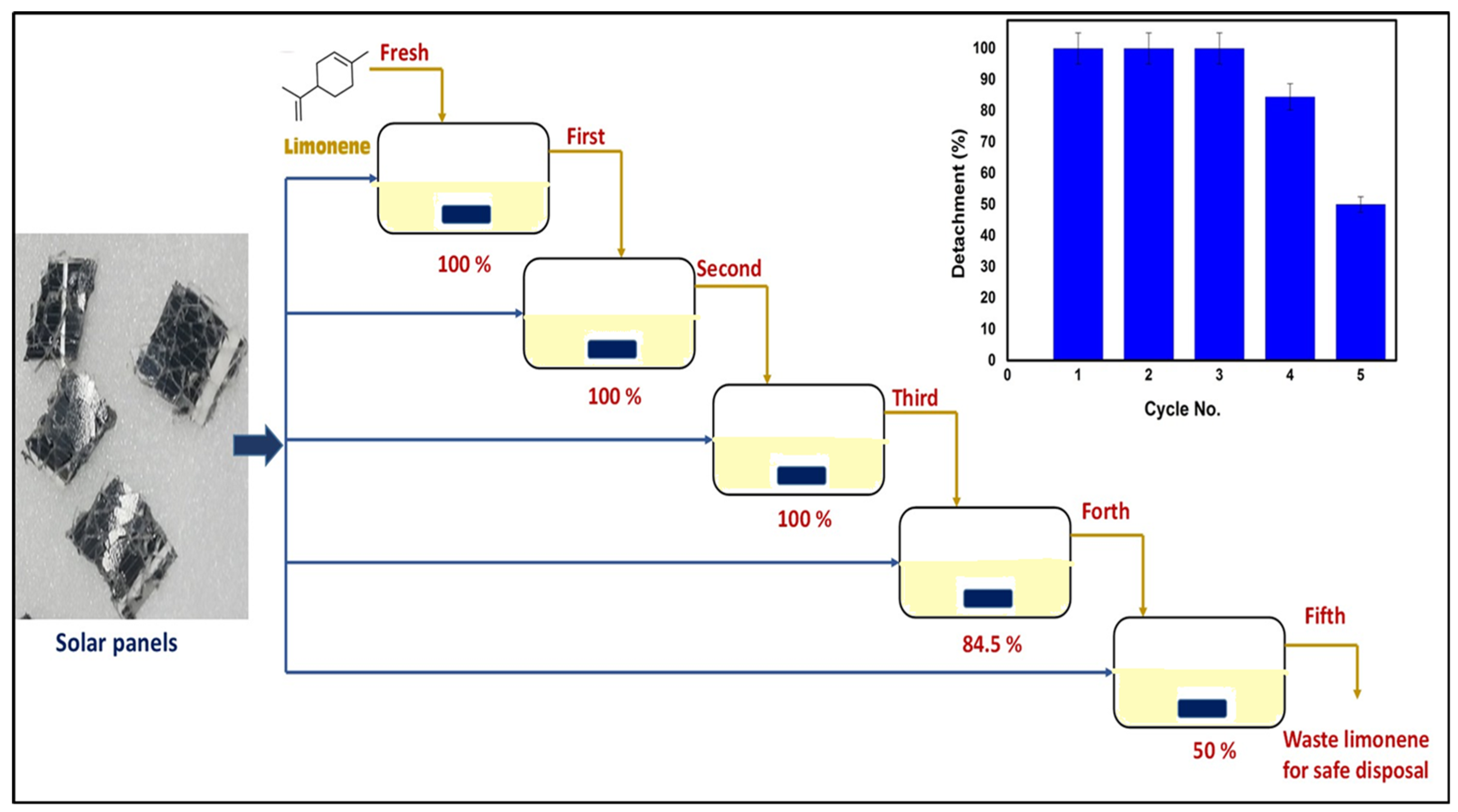
4. Solvent Recyclability
5. Conclusions
- (1)
- The effect of temperature is strongly effective above 25 °C, and in fact, at this value of temperature (25 °C) and for a contact time lower than 50 min, the reaction yields are lower than 40%;
- (2)
- At low sonication power (200 W), the thermal pre-treatment has no effect on the EVA dissolution, and the temperature is the main parameter influencing the process;
- (3)
- At an average sonication power of 450 W, the pre-treatment has no effect on EVA dissolution, and time is the main parameter influencing the process;
- (4)
- A temperature of 60 °C and 120 min of contact time are required to obtain total detachment regardless of the sonification power;
- (5)
- With a high sonification power of 700 W, the pre-treatment does not have a positive effect, and the residence time is the main parameter influencing the process. Furthermore, separations of more than 80% can be obtained in 50 min of contact time both at 60 °C and at 80 °C.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Pang, J.; Teng, F.; Gong, R.; Springer, C. The environmental co-benefit and economic impact of China’s low-carbon pathways: Evidence from linking bottom-up and top-down models. Renew. Sustain. Energy Rev. 2021, 136, 110438. [Google Scholar] [CrossRef]
- Curtin, J.; McInerney, C.; Gallachóir, B.Ó.; Hickey, C.; Deane, P.; Deeney, P. Quantifying stranding risk for fossil fuel assets and implications for renewable energy investment: A review of the literature. Renew. Sustain. Energy Rev. 2019, 116, 109402. [Google Scholar] [CrossRef]
- Khan, R.; Go, Y.I. Assessment of Malaysia’s Large-Scale Solar Projects: Power System Analysis for Solar PV Grid Integration. Glob. Chall. 2020, 4, 1900060. [Google Scholar] [CrossRef] [Green Version]
- IRENA; IEA-PVPS. End-of-Life Management: Solar Photovoltaic Panels. International Renewable Energy Agency and International Energy Agency Photovoltaic Power Systems. 2016. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2016/IRENA_IEAPVPS_End-of-Life_Solar_PV_Panels_2016.pdf (accessed on 1 June 2023).
- Wang, X.; Tian, X.; Chen, X.; Ren, L.; Geng, C. A review of end-of-life crystalline silicon solar photovoltaic panel recycling technology. Sol. Energy Mater. Sol. Cells 2022, 248, 111976. [Google Scholar] [CrossRef]
- Sinha, P.; Heath, G.; Wade, A.; Komoto, K. Human Health Risk Assessment Methods for PV, Part 3: Module Disposal Risks, International Energy Agency (IEA) PVPS Task 12; Report T12-16:2020; IEA: Paris, France, 2019; ISBN 978-3-906042-96-1. Available online: https://iea-pvps.org/research-tasks/pv-sustainability/ (accessed on 3 June 2023).
- Tawalbeh, M.; Al-Othman, A.; Kafiah, F.; Abdelsalam, E.; Almomani, F.; Alkasrawi, M. Environmental impacts of solar photovoltaic systems: A critical review of recent progress and future outlook. Sci. Total Environ. 2021, 759, 143528. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; El-Sheltawy, C.T.; Abdo, D.M. Green Processes for Electronic Waste Recycling: A Review. J. Sustain. Metall. 2018, 4, 295–311. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Tan, Q.; Peters, A.L.; Yang, C. Global status of recycling waste solar panels: A review. Waste Manag. 2018, 75, 450–458. [Google Scholar] [CrossRef]
- Komoto, K.; Lee, J.-S.; Zhang, J.; Ravikumar, D.; Sinha, P.; Wade, A.; Heath, G.A. End-of-Life Management of Photovoltaic Panels: Trends in PV Module Recycling Technologies; OSTI.GOV Technical Report; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2018. [CrossRef]
- Chowdhury, M.S.; Rahman, K.S.; Chowdhury, T.; Nuthammachot, N.; Techato, K.; Akhtaruzzaman, M.; Tiong, S.K.; Sopian, K.; Amin, N. An overview of solar photovoltaic panels’ end-of-life material recycling. Energy Strategy Rev. 2020, 27, 100431. [Google Scholar] [CrossRef]
- Niekurzak, M.; Lewicki, W.; Coban, H.H.; Brelik, A. Conceptual Design of a Semi-Automatic Process Line for Recycling Photovoltaic Panels as a Way to Ecological Sustainable Production. Sustainability 2023, 15, 2822. [Google Scholar] [CrossRef]
- Frisson, L.; Lieten, K.; Bruton, T.; Declercq, K.; Szlufcik, J.; de Moor, H.; Gorts, M.; Benali, A.; Aceves, O. Recent Improvements in Industrial PV Module Recycling. In Sixteenth European Photovoltaic Solar Energy Conference; Routledge: Oxford, UK, 2020; pp. 2160–2163. [Google Scholar] [CrossRef]
- Tammaro, M.; Rimauro, J.; Fiandra, V.; Salluzzo, A. Thermal treatment of waste photovoltaic module for recovery and recycling: Experimental assessment of the presence of metals in the gas emissions and in the ashes. Renew. Energy 2015, 81, 103–112. [Google Scholar] [CrossRef]
- Azeumo, M.F.; Conte, G.; Ippolito, N.M.; Medici, F.; Piga, L.; Santilli, S. Photovoltaic module recycling, a physical and a chemical recovery process. Sol. Energy Mater. Sol. Cells 2019, 193, 314–319. [Google Scholar] [CrossRef]
- Granata, G.; Pagnanelli, F.; Moscardini, E.; Havlik, T.; Toro, L. Recycling of photovoltaic panels by physical operations. Sol. Energy Mater. Sol. Cells 2014, 123, 239–248. [Google Scholar] [CrossRef]
- Akimoto, Y.; Iizuka, A.; Shibata, E. High-voltage pulse crushing and physical separation of polycrystalline silicon photovoltaic panels. Miner. Eng. 2018, 125, 1–9. [Google Scholar] [CrossRef]
- Klugmann-Radziemska, E.; Ostrowski, P. Chemical treatment of crystalline silicon solar cells as a method of recovering pure silicon from photovoltaic modules. Renew. Energy 2010, 35, 1751–1759. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Abo Atia, T.; Toro, L. Photovoltaic panel recycling: From type-selective processes to flexible apparatus for simultaneous treatment of different types. Miner. Process. Extr. Met. 2016, 125, 221–227. [Google Scholar] [CrossRef]
- Theocharis, M.; Tsakiridis, P.E.; Kousi, P.; Hatzikioseyian, A.; Zarkadas, I.; Remoundaki, E.; Lyberatos, G. Hydrometallurgical Treatment for the Extraction and Separation of Indium and Gallium from End-of-Life CIGS Photovoltaic Panels. Mater. Proc. 2021, 5, 51. [Google Scholar] [CrossRef]
- Geretschläger, K.J.; Wallner, G.M.; Fischer, J. Structure and basic properties of photovoltaic module backsheet films. Sol. Energy Mater. Sol. Cells 2016, 144, 451–456. [Google Scholar] [CrossRef]
- Sherwood, J. Closed-loop recycling of polymers using solvents. Johns. Matthey Technol. Rev. 2020, 64, 4–15. [Google Scholar] [CrossRef]
- Xu, J.; Peng, L.; Chu, J.; Shi, J.; Cui, Q.; Shi, Q. D-limonene as an alternative for the extraction and purification of nuciferine from lotus leaf via multi-stage vortex assisted two-phase solvent extraction integrated with solid phase extraction using mesoporous material SBA-15 as adsorbent. Sustain. Chem. Pharm. 2023, 32, 100997. [Google Scholar] [CrossRef]
- Mollehuara, M.A.; Cuadrado, A.R.; Vidal, V.L.; Camargo, S.D. Systematic review: Analysis of the use of D-limonene to reduce the environmental impact of discarded expanded polystyrene (EPS). IOP Conf. Ser. Earth Environ. Sci. 2022, 1048, 012003. [Google Scholar] [CrossRef]
- Li, J.; Shao, J.; Yao, X.; Li, J. Life cicle analysis of the economic cost and environmental benefits of photovoltaic modules waste recycling in China. Resour. Conserv. Recycl. 2023, 196, 107027. [Google Scholar] [CrossRef]
- Prasad, D.S.; Sanjana, B.; Kiran, D.S.; Srinivasa Kumar, P.P.; Ratheesh, R. Process optimization studies of essential parameters in the organic solvent method for the recycling of waste crystalline silicon photovoltaic modules. Sol. Energy Mater. Sol. Cells 2022, 245, 111850. [Google Scholar] [CrossRef]
- Moradi, N.; Rahimi, M. Effect of ultrasound- and pulsed electric field-assisted enzymatic treatment on the recovery and quality of sunflower oil. Sep. Sci. Technol. 2019, 54, 1043–1054. [Google Scholar] [CrossRef]
- Ripin, A.; Mudalip, S.K.A.; Sukaimi, Z.; Yunus, R.M.; Manan, Z.A. Effects of ultrasonic waves on vapor-liquid equilibrium of an azeotropic mixture. Sep. Sci. Technol. 2009, 44, 2707–2719. [Google Scholar] [CrossRef]
- Tembo, P.M.; Heninger, M.; Subramanian, V. An Investigation of the Recovery of Silicon Photovoltaic Cells by Application of an Organic Solvent Method. ECS J. Solid State Sci. Technol. 2021, 10, 025001. [Google Scholar] [CrossRef]
- Doi, T.; Tsuda, I.; Unagida, H.; Murata, A.; Sakuta, K.; Kurokawa, K. Experimental study on PV module recycling with organic solvent method. Sol. Energy Mater. Sol. Cells 2001, 67, 397–403. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Yuan, X.; Liu, C.; Shen, C.Y.; Dai, G.C. Separation of backsheets from waste photovoltaic(PV) modules by ultrasonic irradiation. IOP Conf. Ser. Earth Environ. Sci. 2019, 242, 032046. [Google Scholar] [CrossRef]
- Chitra; Sah, D.; Saini, P.; Kumar, S. Recovery and analysis of polymeric layers from waste solar modules by chemical route. Sol. Energy 2022, 244, 31–39. [Google Scholar] [CrossRef]
- Magdy, D.A.; El-Shazly, A.N.; Medici, F. Recovery of Valuable Materials from End-of-Life Photovoltaic Solar Panels. Materials 2023, 16, 2840. [Google Scholar]
- D’adamo, I.; Ferella, F.; Gastaldi, M.; Ippolito, N.M.; Rosa, P. Circular solar: Evaluating the profitability of a photovoltaic panel recycling plant. Waste Manag. Res. 2023, 41, 1144–1154. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Huda, N.; Behnia, M. Environmental impacts and economic feasibility of end of life photovoltaic panels in Australia: A comprehensive assessment. J. Clean. Prod. 2020, 260, 120996. [Google Scholar] [CrossRef]
- Choi, J.K.; Fthenakis, V. Crystalline silicon photovoltaic recycling planning: Macro and micro perspectives. J. Clean. Prod. 2014, 66, 443–449. [Google Scholar] [CrossRef]
- Deng, R.; Chang, N.L.; Ouyang, Z.; Chong, C.M. A techno-economic review of silicon photovoltaic module recycling. Renew. Sustain. Energy Rev. 2019, 109, 532–550. [Google Scholar] [CrossRef]


| Method | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Thermal treatment |
|
| [13,14] |
| Physical treatment |
|
| [15,16,17] |
| Chemical treatment |
|
| [18,19,20] |
| Sonication Power | 100% Detachment Condition | Pre-Treatment | Main Affecting Parameter |
|---|---|---|---|
| Low (200 W) | Temperature 80 °C for 120 min | No effect | Temperature |
| Medium (450 W) | Temperature 60 °C for 120 min | No effect | Time |
| High (700 W) | Temperature 80 °C for 120 min | No effect | Time |
| Solvent Used | Applied Conditions | Detachment | Ref. |
|---|---|---|---|
| D-limonene | T = 60 °C, no pre-treatment, 120 min, and power 450 W | 100% | Current study |
| Hexane | T = 70 °C, ultrasonication for 15 min, for 24 h | 92.4% | [29] |
| Trichloroethylene | T = 70 °C, with a module to solvent ratio of 1:7.44, and in a horizontal position for 8 h | 100% | [26] |
| o-Dichlorobenzene | T = 120 °C for one week and with pre-treatment at 155 °C for 15 min | 100% | [30] |
| Butanone | T = 70 °C for 210 min and high sonication power with 50% concentration | 100% | [31] |
| Toluene | T = room temperature for 3 days | 100% | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, D.M.; Mangialardi, T.; Medici, F.; Piga, L. D-Limonene as a Promising Green Solvent for the Detachment of End-of-Life Photovoltaic Solar Panels under Sonication. Processes 2023, 11, 1848. https://doi.org/10.3390/pr11061848
Abdo DM, Mangialardi T, Medici F, Piga L. D-Limonene as a Promising Green Solvent for the Detachment of End-of-Life Photovoltaic Solar Panels under Sonication. Processes. 2023; 11(6):1848. https://doi.org/10.3390/pr11061848
Chicago/Turabian StyleAbdo, Dina Magdy, Teresa Mangialardi, Franco Medici, and Luigi Piga. 2023. "D-Limonene as a Promising Green Solvent for the Detachment of End-of-Life Photovoltaic Solar Panels under Sonication" Processes 11, no. 6: 1848. https://doi.org/10.3390/pr11061848
APA StyleAbdo, D. M., Mangialardi, T., Medici, F., & Piga, L. (2023). D-Limonene as a Promising Green Solvent for the Detachment of End-of-Life Photovoltaic Solar Panels under Sonication. Processes, 11(6), 1848. https://doi.org/10.3390/pr11061848








