Behavior of Carbothermal Dephosphorization of Phosphorus-Containing Converter Slag and Its Resource Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
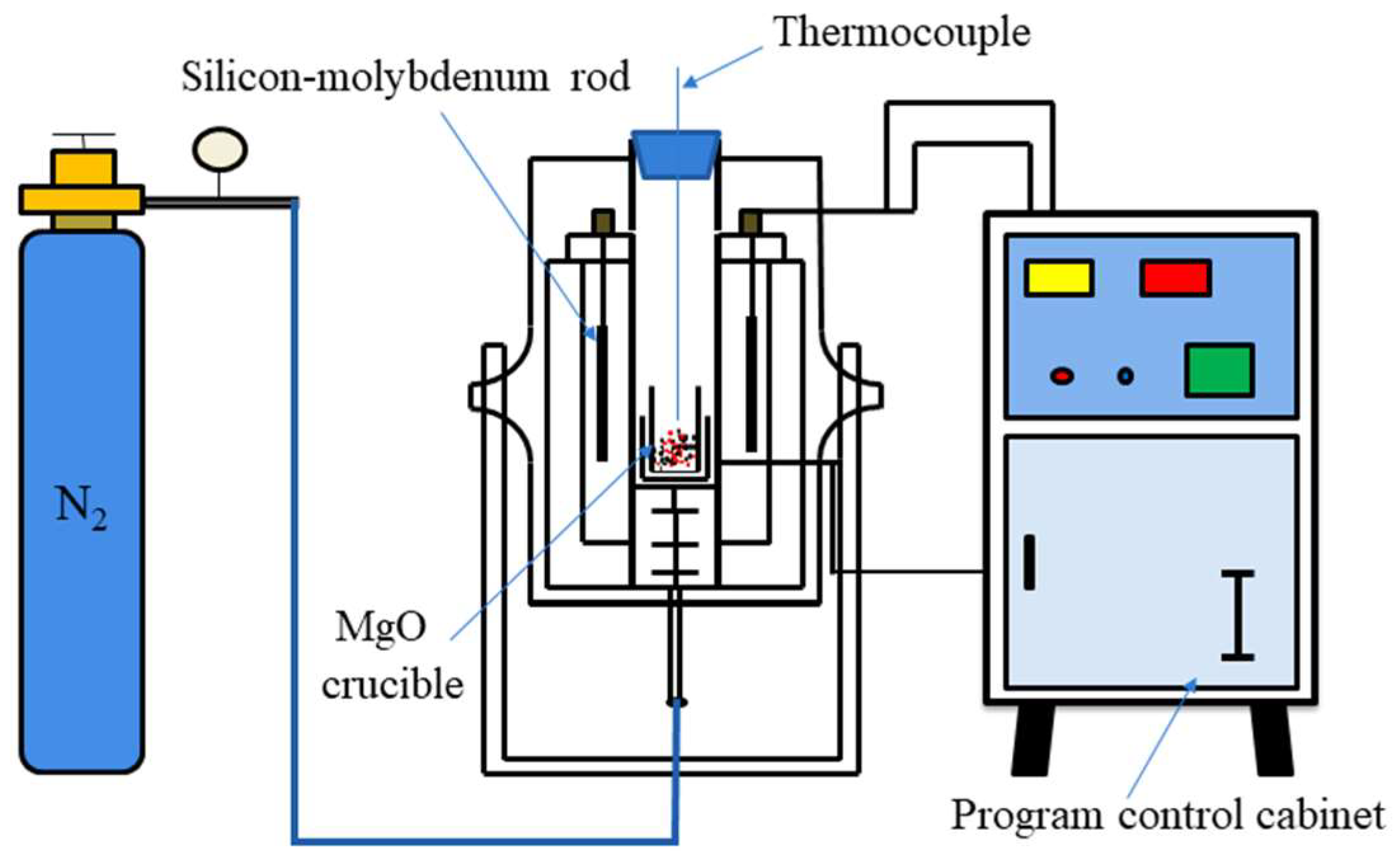
2.2. Methods
2.3. Characterization Methods
3. Experimental Results and Discussion
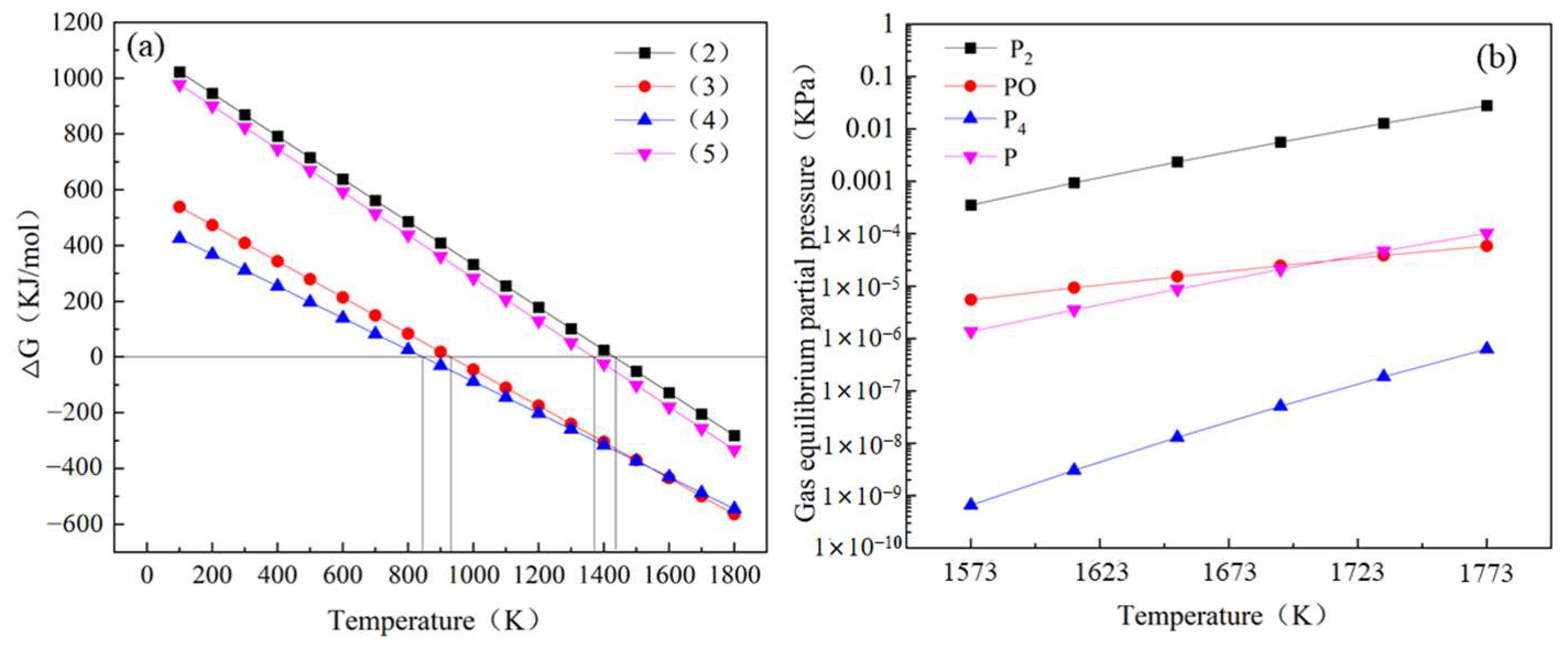
3.1. Thermodynamic Analysis
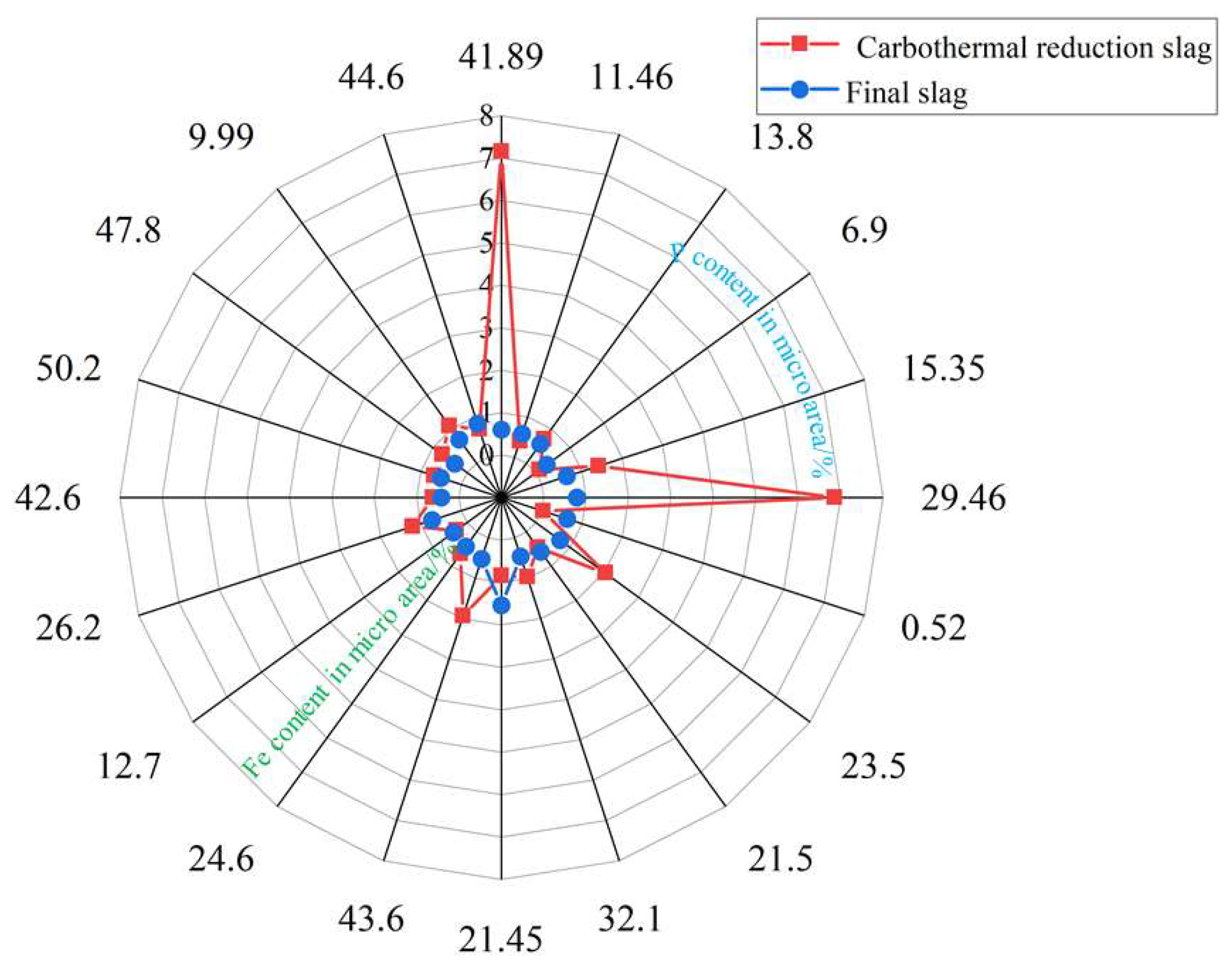
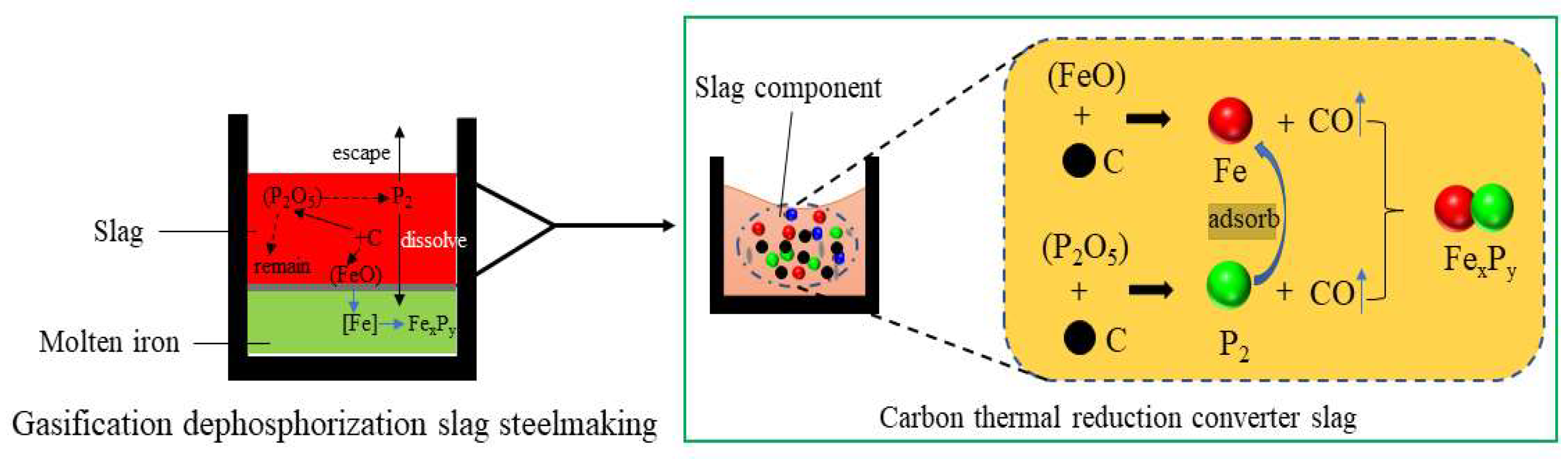
3.2. Dephosphorization Behavior
4. Resource Utilization of Converter Slag after Carbothermal Dephosphorization
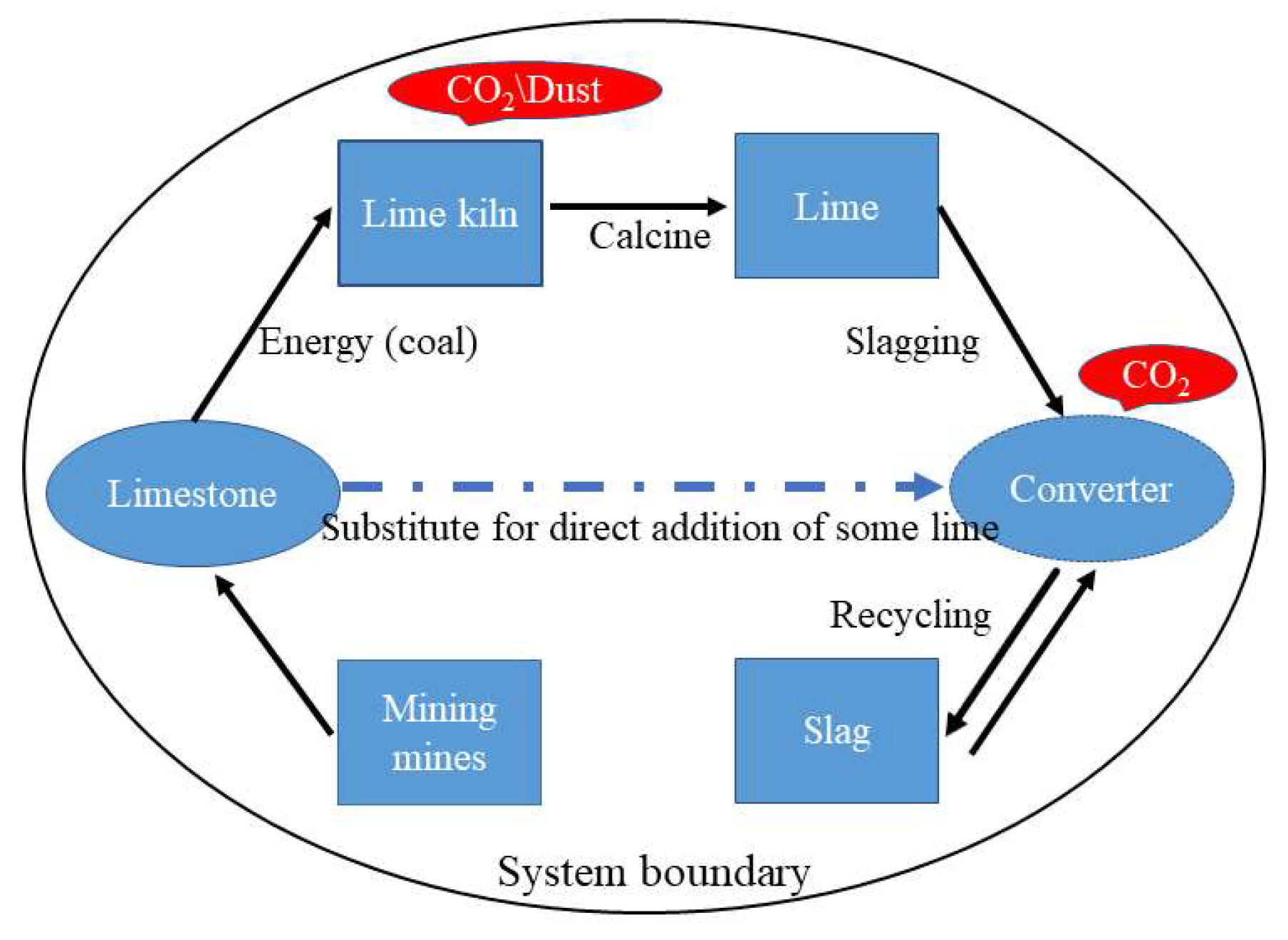
4.1. Application of Carbon Thermal Reduction Slag Circulation Steelmaking
4.1.1. Industrial Test Conditions
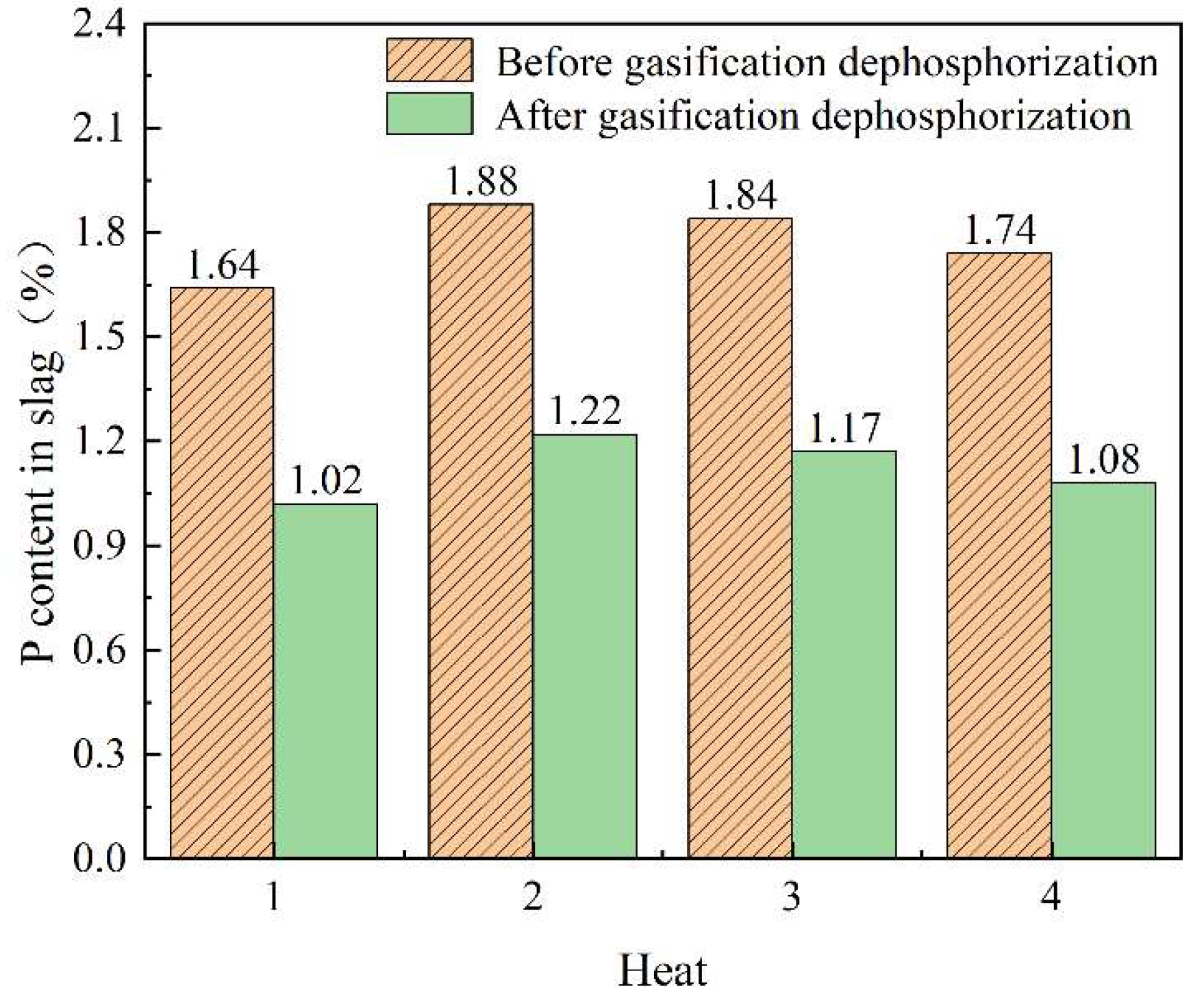
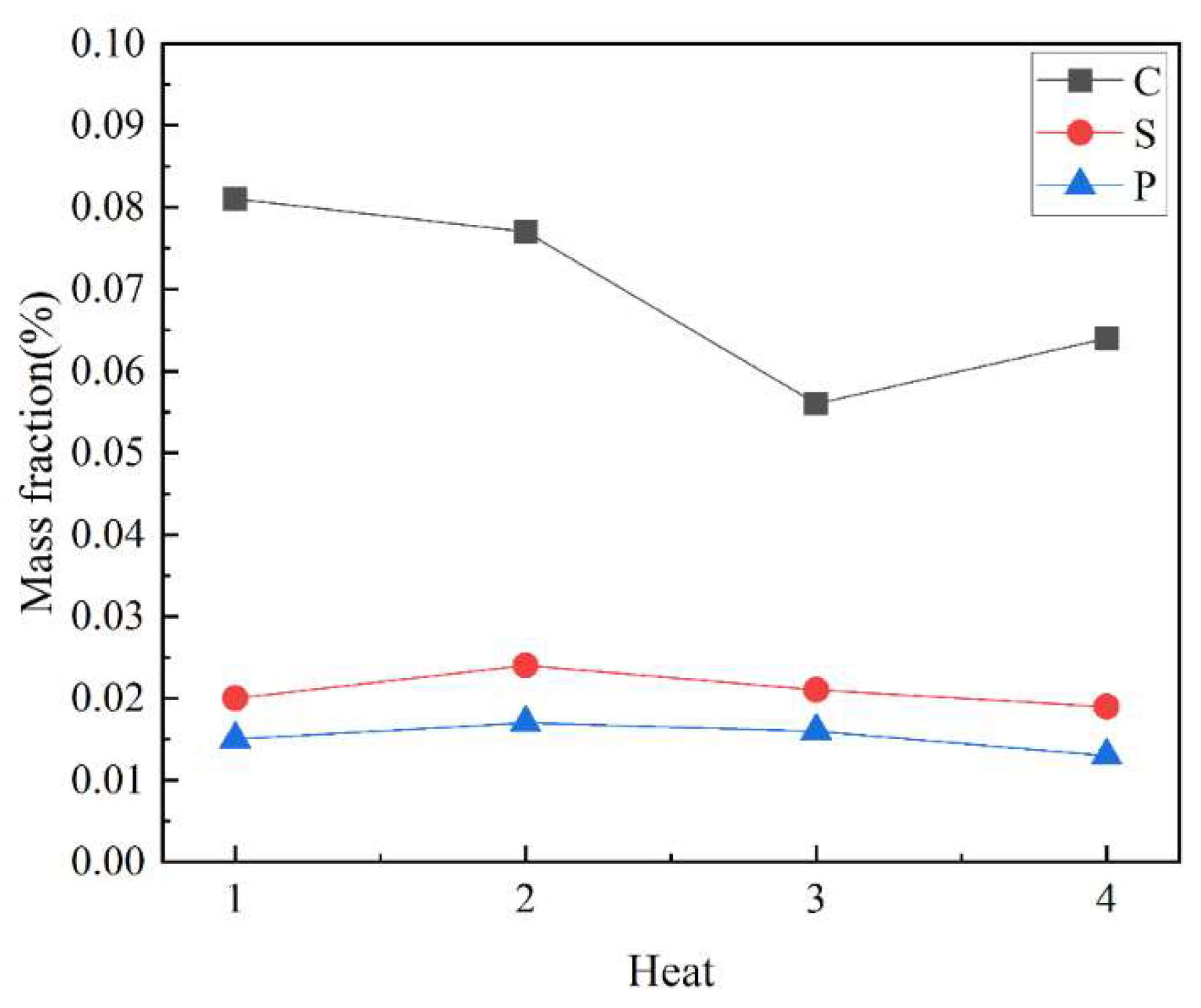
4.1.2. Changes of Phosphorus Content in Slag
4.1.3. Composition of Terminal Molten Steel
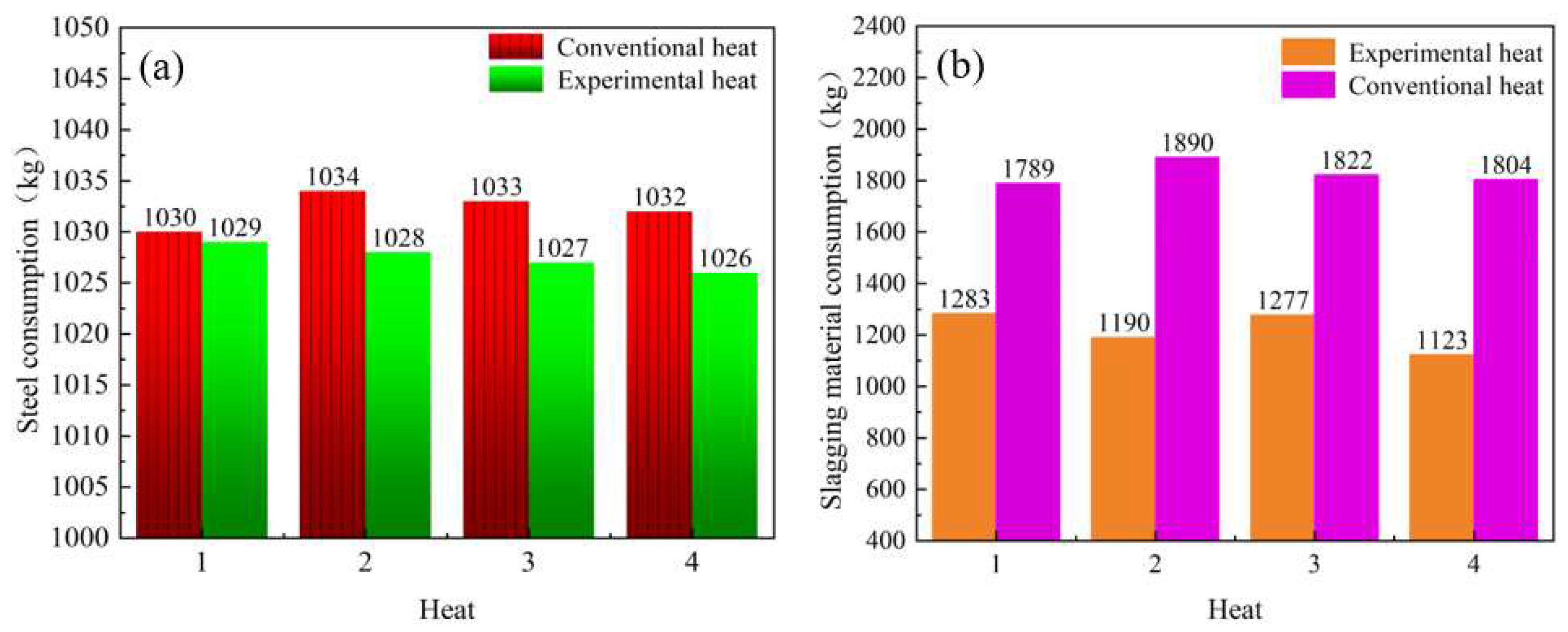
4.1.4. Comparison of Economic Indicators
4.2. Assessment and Analysis of Carbon Emission Reduction
4.3. Thoughts on Recovery and Utilization of Phosphorus Resources
- Making more P2 overflow and collecting it, and then carrying out an oxidation–hydration reaction to prepare the phosphoric acid for use as phosphate fertilizer, thereby making it less difficult for entry into the industry. This idea can provide a new direction for a steel chemical co-production project, which is an innovative development model of the iron and steel industry combined with the chemical industry. At present, there have been successful cases reported in China, such as the project of Shandong Shiheng Special Steel, in which formic acid was produced through the use of converter gas, and the production of sodium bicarbonate raw materials by Handan Xinxing Cast Pipe, and so on [32,33];
- After phosphorus-containing slag is processed by carbothermal reduction gasification dephosphorization, favorable conditions for dissolving the reduced phosphorus vapor into metallic iron phase are controlled, so that more phosphorus vapor can enter the molten iron to generate ferrophosphorus, which can be used as electrode raw materials or directly used as ferrophosphorus in steelmaking, with high added value [31,34].
5. Conclusions
- (1)
- On the basis of our experiments and thermodynamic calculations, the feasibility of phosphorus removal from converter slag by carbothermal reduction was demonstrated. When the temperature is higher than 1433 K, the carbothermal reduction reaction can take place, and the main reduction product is P2. The average dephosphorization rate was 32.57% under the experimental conditions;
- (2)
- The production practice of Cheng steel shows that the dephosphorization effect of the subsequent furnace procedure will not be affected by the carbothermic reduction of converter slag. The composition of the terminal molten steel was stable, and the average consumption of iron and steel per heat was reduced by 4.74 kg, the slagging material was reduced by 608 kg, and CO2 emissions were reduced by 4.86 kg, which will result in emissions reductions and energy savings;
- (3)
- Part of the P2 produced by the carbothermal reduction enters the Fe phase, and the other part is discharged with the furnace gas. With an increase in Fe content, the amount of P2 also increases. In order to efficiently recycle phosphorus resources, appropriate carbothermal reduction conditions and optimal gasification-dephosphorization parameters should be fully considered so that more P2 can enter the molten iron to generate ferrophosphorus.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.G.; Yang, H.Z.; Ai, L.Q. Research status and prospect of recycling technology of converter slag containing phosphorus. Iron Steel 2021, 56, 22–39. [Google Scholar]
- Zhang, J.; Yan, D.L.; Qi, Y.H. Difficulty analysis on treatment and utilization of iron and steel smelting slag. Steel 2020, 55, 1–5. [Google Scholar]
- Wang, Z.L.; Bao, Y.P.; Wang, D.Z.; Wang, M. Effective removal of phosphorus from high phosphorus steel slag using carbonized rice husk. J. Environ. Sci. 2023, 124, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Bao, Y.P.; Wang, D.Z. Leaching rule of phosphorus element in high phosphorus converter slag with different acids. Iron Steel 2021, 56, 103–110. [Google Scholar]
- Wang, Y.C.; Li, H.Y.; Luo, G.P. Microkinetic of gasification dephosphorization of converter slag by microwave carbon thermal reduction. J. Iron Steel Res. 2017, 29, 93–97. [Google Scholar]
- Zhang, W.; Liu, W.X.; Li, J. Main factors of gasification dephosphorization of high phosphorus steel slag. Iron Steel 2015, 50, 11–14. [Google Scholar]
- Ai, L.Q.; Zhang, Y.L.; Zhu, W.H. Research on carbothermic reduction for dephosphorization from converter slag by microwave heating. Iron Steel Vanadium Titan. 2015, 36, 63–67. [Google Scholar]
- Zhao, C.L.; Zhang, N.; Kang, L. Experiment on dephosphorization of converter slag by carbothermal reduction. Iron Steel 2016, 51, 41–44. [Google Scholar]
- Lin, L.L.; Zhi, Y.Q.; He, J.G. Influence of slag temperature on phosphorus enrichment in P-bearing steelmaking slag. Ironmak. Steelmak. 2021, 48, 334. [Google Scholar] [CrossRef]
- Kubo, H.; Matsubae, Y.K.; Nagasaka, T. Magnetic separation of phosphorus enriched phase from multiphase dephosphorization slag. Trans. Iron Steel Inst. Jpn. 2010, 50, 59–64. [Google Scholar] [CrossRef]
- Wu, M. Low carbon steel production with BOF slag-remaining practice. Steelmaking 2009, 25, 16–19. [Google Scholar]
- Wu, X.R.; An, J.N.; Chen, R.H. Distribution and concentration of phosphorus in factory converter slags and growth of P-concentrating phase. J. Anhui Univ. Technol. Nat. Sci. 2010, 3, 233–237. [Google Scholar]
- Wang, N.; Liang, Z.G.; Chen, M. Enrichment behavior of phosphorus in CaO-SiO2-FetO-P2O5 Slag. J. Northeast. Univ. Nat. Sci. 2011, 32, 814–817. [Google Scholar]
- Li, C.X.; Li, H.; Zhou, B. Experimental study on steelmaking using limestone instead of lime as the slagging material in 100t converter. China Metall. 2015, 25, 22–26. [Google Scholar]
- Liang, Y.J.; Che, C.Y. Handbook for Inorganic Thermodynamic Calculation; Northeast University Press: Shenyang, China, 2001. [Google Scholar]
- Li, G.Q.; Zhang, F.Q.; Zhang, L. Recycle of converter thermal reducction. J. Mater. Metall. 2003, 2, 167. [Google Scholar]
- Jia, J.R.; Ai, L.Q. Reduction dephosphorization behavior of steelmaking slag in microwave heating field. Iron Steel 2012, 47, 70. [Google Scholar]
- Dong, J.S.; Xu, G.; Shigeru, U. Separation of phosphorus and manganese from steelmaking slag by selective reduction. Metall. Mater. Trans. B 2019, 50, 1248–1259. [Google Scholar]
- Xue, Y.K.; Tian, P.; Li, C.X. Reduction mechanism of P2O5 insteel slag. Trans. Indian Inst. Met. 2020, 73, 251–258. [Google Scholar] [CrossRef]
- Tong, S.; Li, C.X.; Wang, S.H. Effect of temperature on the reuse of dephosphorization slag from converter gasification. Iron Steel Vanadium Titan. 2021, 42, 109–114. [Google Scholar]
- Li, C.X.; Wang, S.H.; Xue, Y.K. Experiment on gasification dephosphorization of converter slag with coke powder reduction. Iron Steel 2018, 53, 20–24. [Google Scholar]
- Zhou, C.G.; Ai, L.Q.; Wang, S.H. Study on the mechanism of gasification dephosphorization and its influence on the next smelting proces. Vanadium Titan. Iron Steel 2018, 39, 129–136. [Google Scholar]
- Guo, R.H.; Wang, S.H.; Li, C.X. Gasification dephosphorization of converter slag by coke reduction. Iron Steel 2020, 55, 118–124. [Google Scholar]
- Wang, S.H.; Tong, S.; Li, C.X. Phosphorus migration behaviorin the process of converter slag gasification dephosphorization. Metalurgija 2022, 61, 149–152. [Google Scholar]
- Li, C.X.; Zhao, D.G.; Xue, Y.K. Experimental study on gasification and dephosphorization of semi-steel slag in Chenggang. Steelmaking 2019, 35, 19–22. [Google Scholar]
- Yang, Y.; Ma, L.; Yu, J. Life cycle assessment introduced by using nanorefrigerant of organic rankine cycle system for waste heat recovery. J. Renew. Mater. 2023, 11, 27. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, W.; Li, W. A carbon flow tracing and carbon accounting method for exploring CO2 emissions of the iron and steel industry: An integrated material–energy–carbon hub. Appl. Energy 2022, 309, 118485. [Google Scholar] [CrossRef]
- Paustian, K.; Ravindranath, N.; Amstel, H. 2006 IPCC Guidelines for National Greenhouse gas Inventories; International Panel on Climate Change: Geneva, Switzerland, 2006. [Google Scholar]
- Gomez, D.R.; Wattersson, J.; Americano, B. Chapter 2, Energy, 2006 IPCC Guidelines for National Greenhouse Gas Emissions Inventories; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2008. [Google Scholar]
- Li, C.X.; Li, H.; Zhu, S.N. Estimation of energy saving and emission reduction of limestone replacing lime as raw material for steelmaking and slagging. China Metall. 2015, 25, 66–69+72. [Google Scholar]
- Liu, Y.Q.; Lin, L.; He, S. Research status of dephosphorization and resource utilization of phosphorus-containing steel slag by carbothermal reduction. J. Iron Steel Res. 2022, 34, 1034–1046. [Google Scholar]
- Beijing Peking University Pioneer Technology Co., Ltd. The World’s First Set of Sulphuric Acid Plant for Purifying CO from Converter Gas [EB/OL]. 2021. Available online: https://www.pioneer-pku.com/show-312.html (accessed on 15 June 2021).
- News Center. Beijing’s First Central Enterprise Moved Abroad Settled in Handan. [EB/OL]. 2014. Available online: http://news.sina.com.cn/o/2014-05-16/015930145049.shtml (accessed on 16 May 2014).
- Guo, H.Y.; Wang, G.X.; Jia, L.C. Progress of the synthesis of energy materials using ferrophosphorus. Phosphate Compd. Fertil. 2012. [Google Scholar]

| FeO | CaO | SiO2 | MgO | MnO | P2O5 | Basicity (R) |
|---|---|---|---|---|---|---|
| 15.82 | 42.85 | 12.55 | 7.15 | 3.35 | 3.08 | 3.41 |
| Fixed Carbon | CaO | SiO2 | MgO | P2O5 | S |
|---|---|---|---|---|---|
| 86.55 | 1.37 | 6.42 | 0.33 | 0.36 | 0.98 |
| Reduction Condition | TFe | CaO | SiO2 | MgO | MnO | P2O5 | Dephosphorization Rate/% |
|---|---|---|---|---|---|---|---|
| Final slag | 19.69 | 44.22 | 16.32 | 8.30 | 3.09 | 2.22 | 27.92 |
| Final slag of R = 2.81 | 20.43 | 42.05 | 19.03 | 7.38 | 3.47 | 1.90 | 38.31 |
| Final slag of w(FeO) = 24% | 23.66 | 42.01 | 15.96 | 7.17 | 3.27 | 2.11 | 31.49 |
| Type | C | P | S | V |
|---|---|---|---|---|
| Semi-molten steel | 3.5~4.0 | 0.17~0.20 | 0.025~0.06 | <0.04 |
| Terminal molten steel | 0.04~0.11 | 0.017~0.032 | 0.022~0.045 | vestige |
| Energy Type | Ton of Lime Consumes Energy | CO2 Emission Factor |
|---|---|---|
| Anthracite | 90 kg | 2.68 kg/kg |
| Converter gas | 770 m3 | 0.79 kg/m3 |
| Electric power | 60 kW·h | 1.03 (kg/kW·h) |
| Emission Link | Standard Coal Quantity Converted into Energy Consumption per Ton of Lime/Kg | CO2 Emission/Kg |
|---|---|---|
| Electricity consumption (production and anthracite supply) | 24.88 | 62.2 |
| Limestone decomposition and lime calcination | 339.8 | 849.5 |
| Lime slagging | 48.75 | 121.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, S.; Li, C.; Ai, L.; Wang, S.; Zhang, S. Behavior of Carbothermal Dephosphorization of Phosphorus-Containing Converter Slag and Its Resource Utilization. Processes 2023, 11, 1943. https://doi.org/10.3390/pr11071943
Tong S, Li C, Ai L, Wang S, Zhang S. Behavior of Carbothermal Dephosphorization of Phosphorus-Containing Converter Slag and Its Resource Utilization. Processes. 2023; 11(7):1943. https://doi.org/10.3390/pr11071943
Chicago/Turabian StyleTong, Shuai, Chenxiao Li, Liqun Ai, Shuhuan Wang, and Shuai Zhang. 2023. "Behavior of Carbothermal Dephosphorization of Phosphorus-Containing Converter Slag and Its Resource Utilization" Processes 11, no. 7: 1943. https://doi.org/10.3390/pr11071943
APA StyleTong, S., Li, C., Ai, L., Wang, S., & Zhang, S. (2023). Behavior of Carbothermal Dephosphorization of Phosphorus-Containing Converter Slag and Its Resource Utilization. Processes, 11(7), 1943. https://doi.org/10.3390/pr11071943





