Design of Nano-Catalyst for Removal of Phenolic Compounds from Wastewater by Oxidation Using Modified Digital Basket Baffle Batch Reactor: Experiments and Modeling
Abstract
1. Introduction
2. Materials Used and Experimental Design
2.1. Material Application
2.1.1. Feedstock
2.1.2. Ferric Nitrate Nonahydrate
2.1.3. Activated Carbon (AC)
2.1.4. Hydrogen Peroxide (30% H2O2)
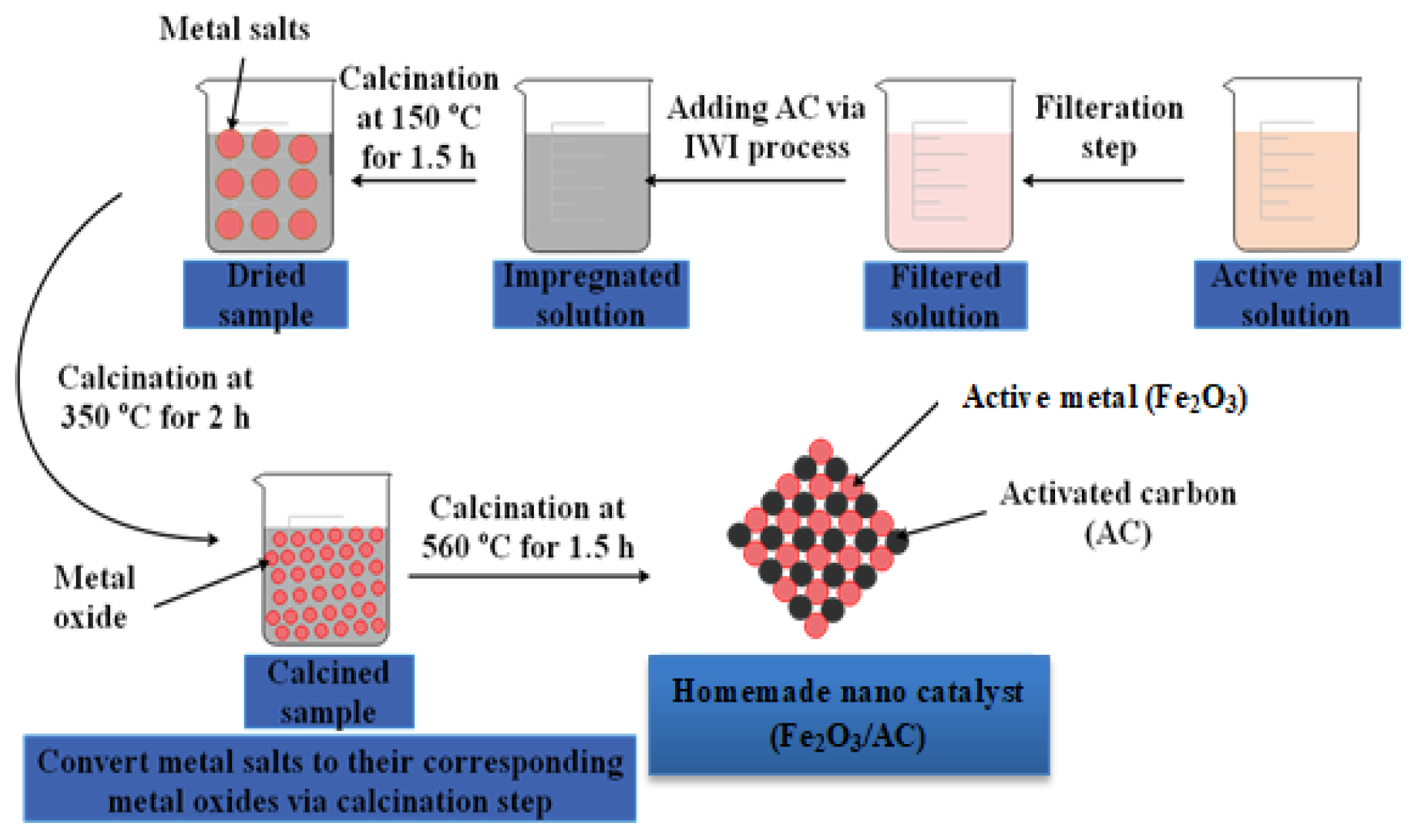
2.2. Incipient Wetness Impregnation (IWI) Process
2.3. Experimental Procedure
2.3.1. Digital Basket Baffled Batch Reactor (DBBBR)
2.3.2. Catalytic Oxidation of Phenol Process
2.3.3. UV Spectrophotometer Analysis
2.3.4. Characterization of the Prepared Nano-Catalyst (8% Fe2O3/AC)
Brunauer–Emmett–Teller (BET)
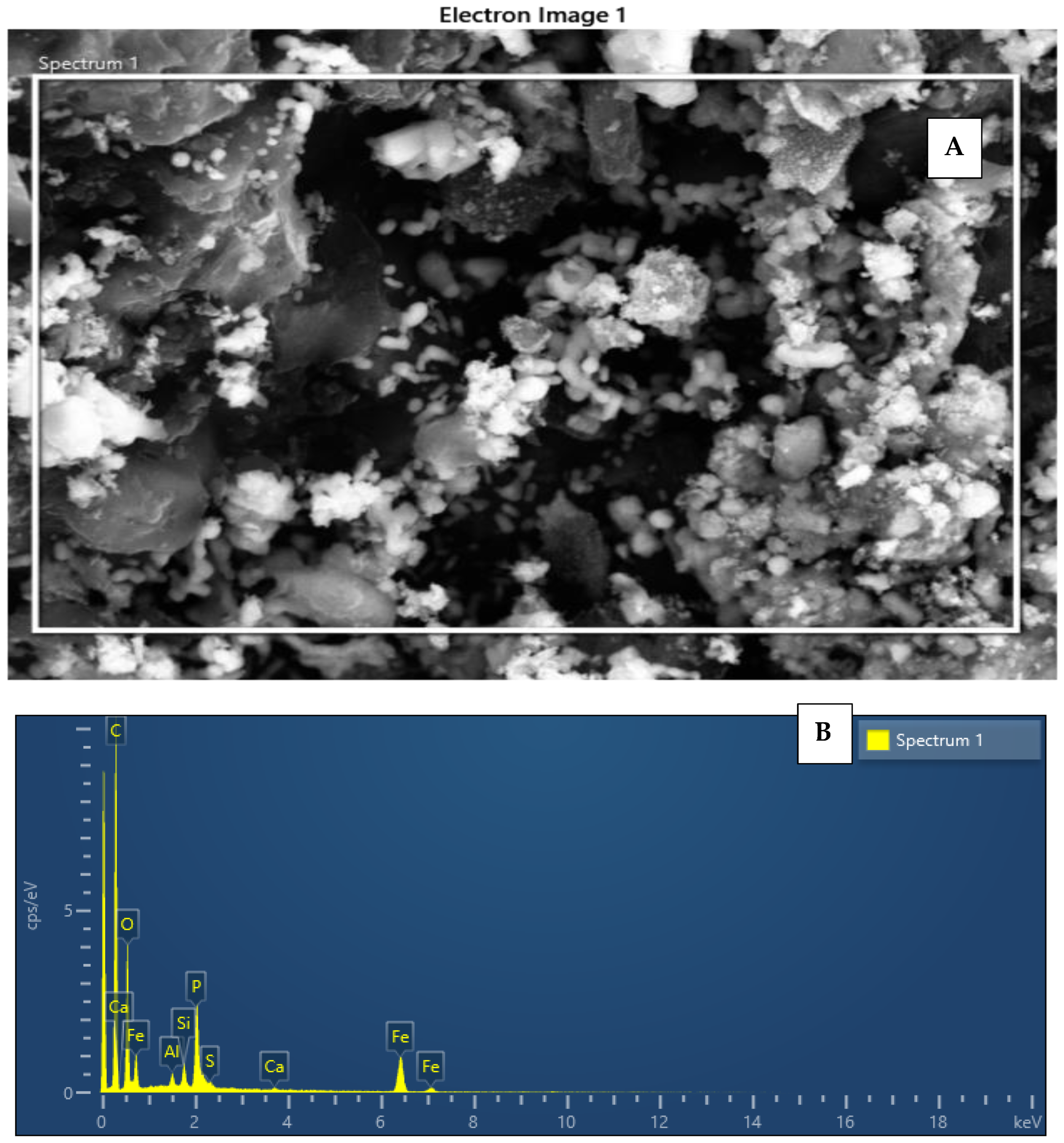
Scanning Electron Microscope with Energy Dispersive X-ray (SEM-EDX)
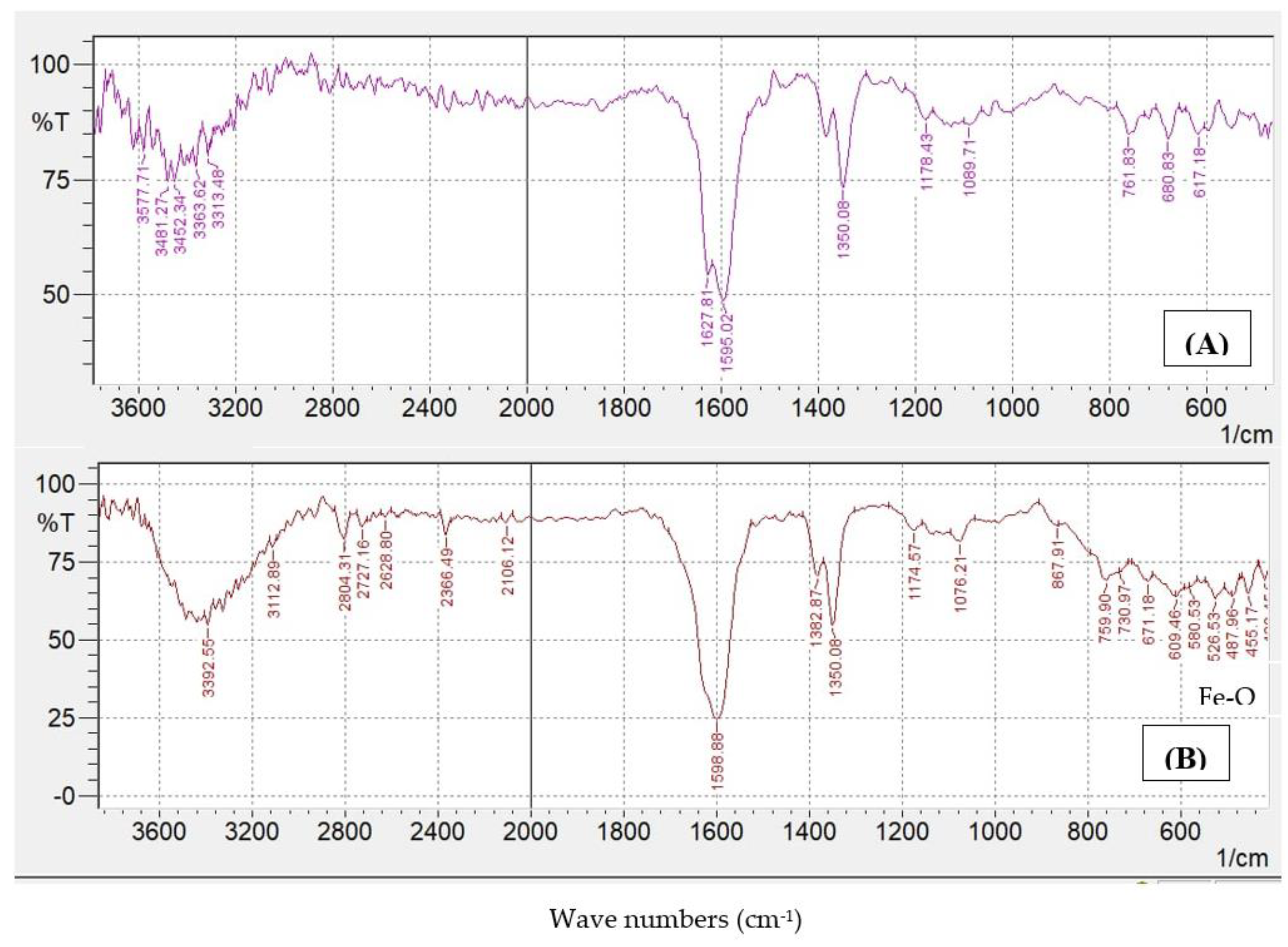
Fourier Transform Infrared (FTIR) Spectroscopic Analysis
3. Results and Discussion
3.1. Effect of Operating Conditions on the Catalytic Oxidation of Phenol
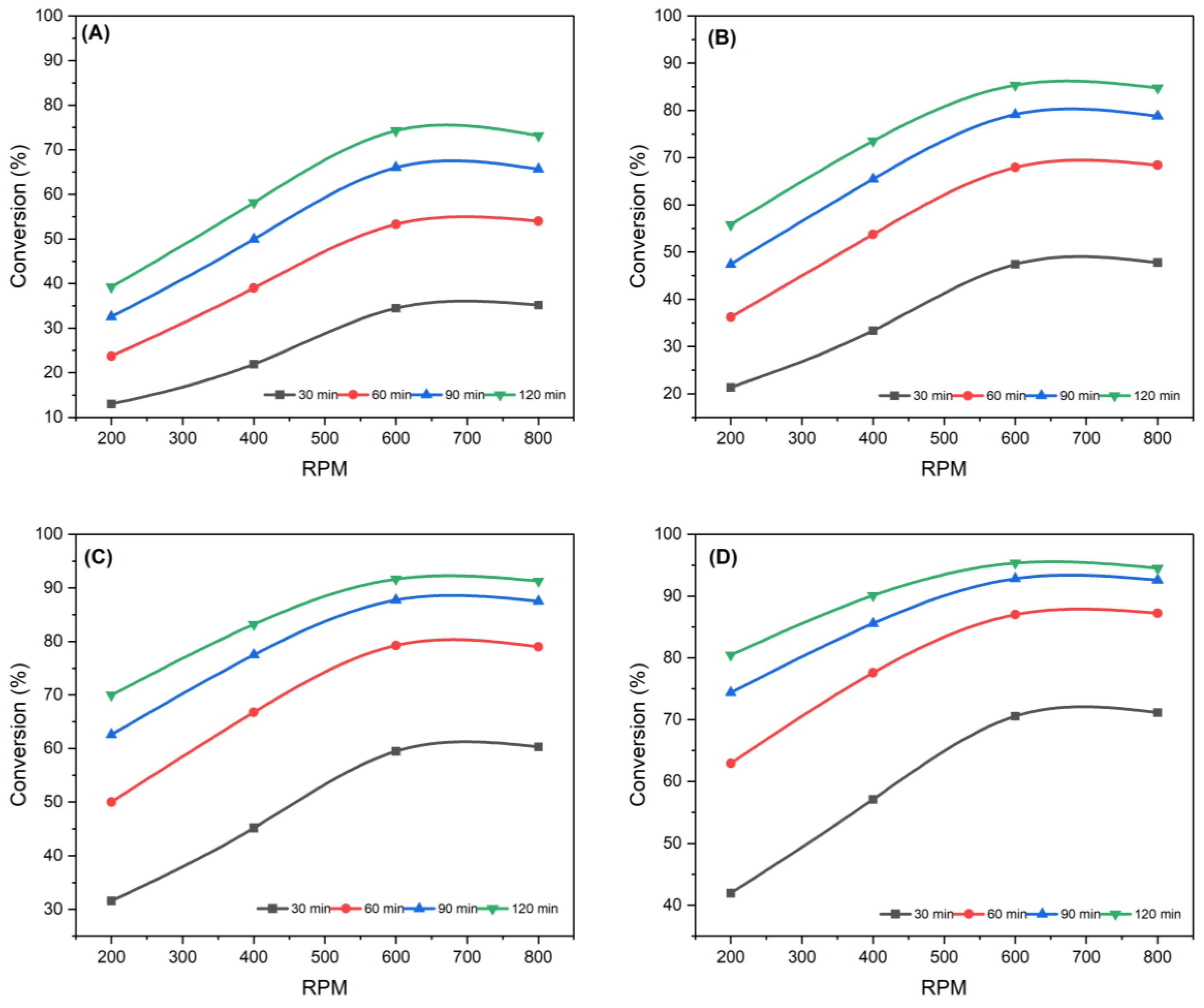
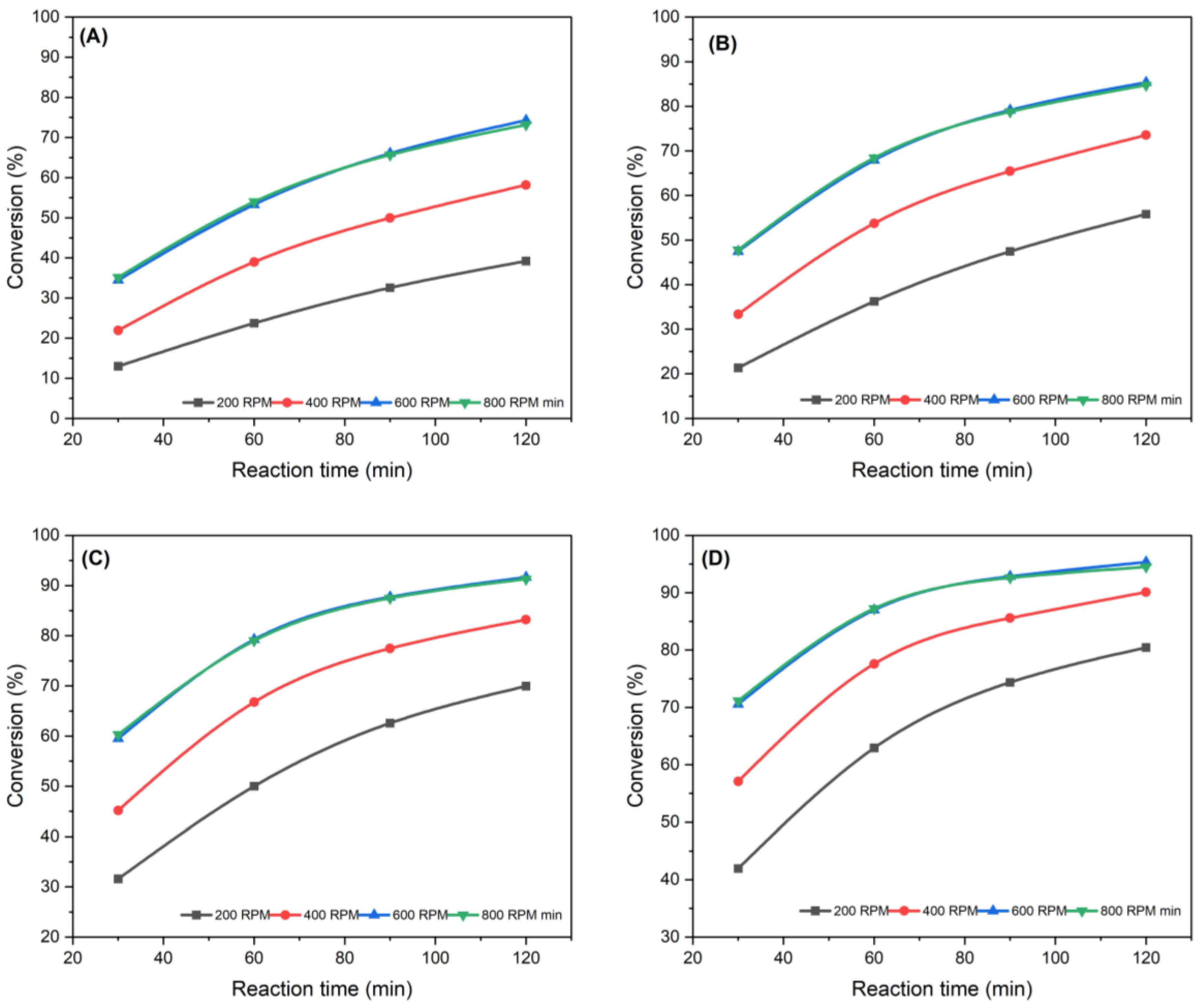
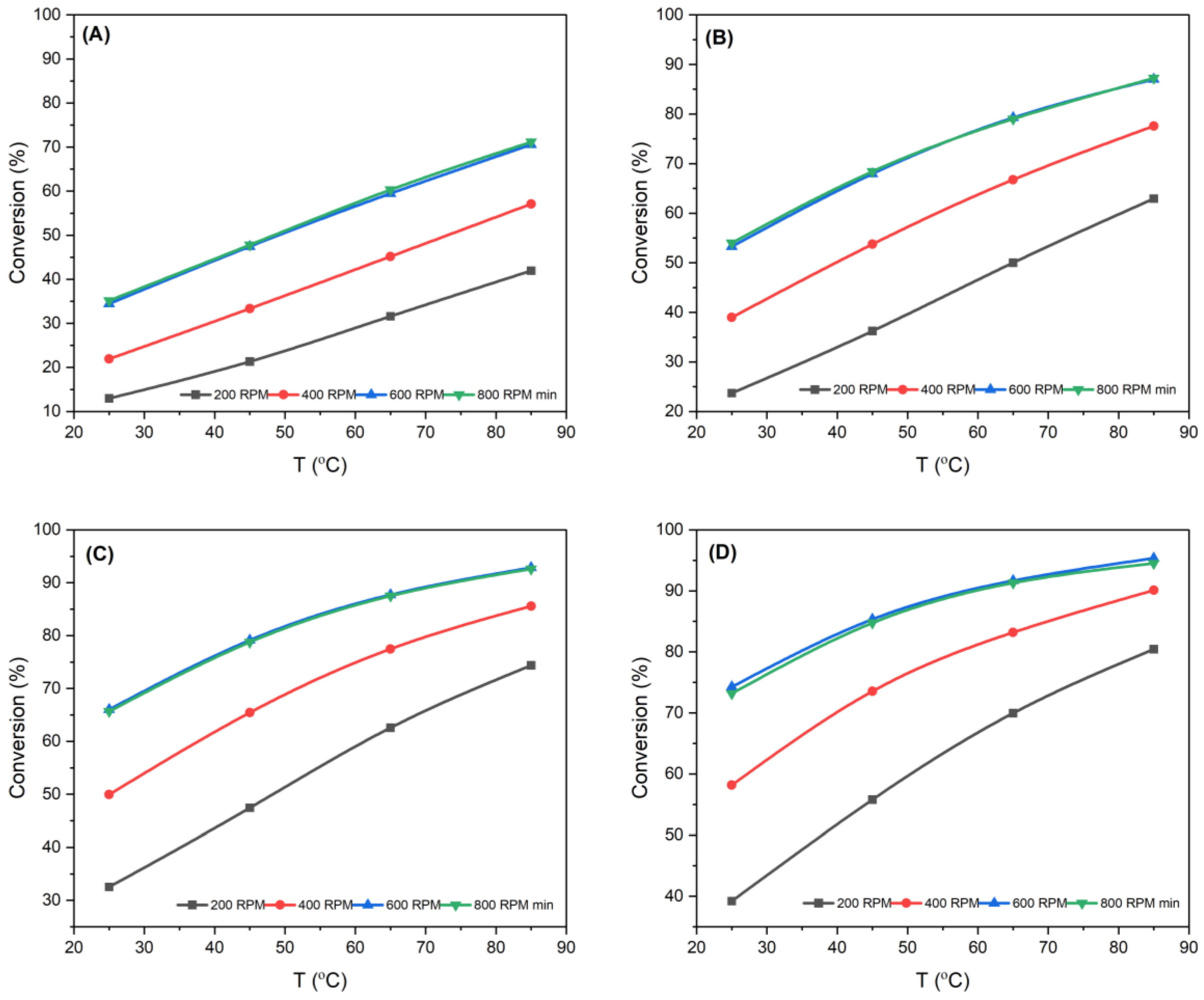
3.1.1. Effect of Stirrer Speed
3.1.2. Effect of Reaction Time
3.1.3. Influence of Reaction Temperature
4. Mathematical Model of DBBBR for Catalytic Phenol Oxidation Process
5. Kinetic Parameters Estimation Technique
6. Optimization Problem Formulation for Evaluation of Kinetic Parameter
7. Description of Mathematical Modeling
7.1. Determination of Kinetic Parameters
7.2. Results of Experiment and Simulation
8. Maximizing Phenol Removal Based on Optimal Operating Conditions
8.1. Formulation of Optimization Problem
8.2. Optimal Values of Operating Conditions for Minimum Phenolic Compounds
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Phenol concentration (mol/cm3) | |
| Conversion of phenol (-) | |
| Final concentration of phenol (mol/cm3) | |
| Initial concentration of phenol present in wastewater (mol/cm3) | |
| k | Reaction rate constant |
| kApp | Apparent rate constant |
| Effective diffusivity (cm2/s) | |
| Knudsen diffusivity | |
| Molecular diffusivity (cm2/s) | |
| EA | Activation energy (J/mol K) |
| Molecular weight of phenol (gm/gmol) | |
| Tc | Critical temperature of phenol (°R) |
| Tb | Boiling point temperature of phenol (°C) |
| Tbr | Reduced boiling point temperature |
| Tr | Reduced temperature |
| R | Gas constant (J/mol K) |
| Order of reaction | |
| Reaction rate of phenol | |
| Pore radius (nm) | |
| Particle radius (nm) | |
| External surface area of catalyst particle (cm2/gm) | |
| Specific surface area of particle (cm2) | |
| External volume of catalyst particle (cm3) | |
| Pore volume (cm3/gm) | |
| Liquid molar volume | |
| Critical molar volume of liquid (cm3/gmole) | |
| Molar volume of phenol | |
| Critical volume of phenol (cm3/gmole) | |
| Greek Symbols | |
| Effectiveness factor | |
| Constant factor | |
| Volume fraction of molecule | |
| Porosity | |
| Tortuosity | |
| Bulk density (gm/cm3) | |
| Particle density (gm/cm3) | |
| Viscosity of phenol (mPa s) | |
| Viscosity of phenol at boiling point (mPa s) | |
| Initial (at time = 0) | |
References
- Zhang, S.; Zhou, L.; Li, Z.; Esmailpour, A.A.; Li, K.; Wang, S.; Liu, R.; Li, X.; Yun, J. Efficient Treatment of Phenol Wastewater by Catalytic Ozonation over Micron-Sized Hollow MgO Rods. ACS Omega 2021, 6, 25506–25517. [Google Scholar] [CrossRef] [PubMed]
- Iurascu, B.; Siminiceanu, I.; Vione, D.; Vicente, M.; Gil, A. Phenol degradation in water through a heterogeneous photo-Fenton process catalyzed by Fe-treated laponite. Water Res. 2009, 43, 1313–1322. [Google Scholar] [CrossRef]
- Hamad, K.I.; Humadi, J.I.; Issa, Y.S.; Gheni, S.A.; Ahmed, M.A.; Hassan, A.A. Enhancement of activity and lifetime of nano-iron oxide catalyst for environmentally friendly catalytic phenol oxidation process. Clean. Eng. Technol. 2022, 11, 100570. [Google Scholar] [CrossRef]
- Turhan, K.; Uzman, S. Removal of phenol from water using ozone. Desalination 2008, 229, 257–263. [Google Scholar] [CrossRef]
- Hamin, J.M.; Aysar, T.J.; Ban, A.A.; Hala, M.H. Preparation of synthetic composite nano-catalyst for oxidative desulfu-rization of kerosene. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 1672–1685. [Google Scholar]
- Jarullah, A.T.; Ahmed, A.N.; Ahmed, B.A.; Ahmed, A.M. Design of New Composites Nano-Catalysts for Naphtha Reforming Process: Experiments and Process Modeling. Tikrit J. Eng. Sci. 2023, 30, 46–59. [Google Scholar] [CrossRef]
- Pan, W.; Fang, B.X.; Xin, L.Y. Catalytic oxidation of phenol in wastewater-A new application of the amorphous Fe78Si9B13 alloy. Chin. Sci. Bull. 2012, 57, 33–40. [Google Scholar]
- Hamoud, S.; Sayari, A.; Belkacemi, K.; Bonneviot, L.; Larachi, F. Catalytic wet oxidation of phenol over Ptx-Ag1−xMnO2/CeO2 catalysts. Catal. Today 2000, 62, 379–388. [Google Scholar] [CrossRef]
- Barrault, J.; Bouchoule, C.; Tatibouët, J.M.; Abdellaoui, M.; Majesté, A.; Louloudi, I.; Papayannakos, N.; Yan, Y.; Wu, X.; Zhang, H. Catalytic wet peroxide oxidation of phenol over Fe2O3/MCM-41 in a fixed bed reactor. Sep. Purif. Technol. 2016, 171, 52–61. [Google Scholar]
- Jangid, P.; Inbaraj, M.P. Applications of nanomaterials in wastewater treatment. Mater. Today Proc. 2021, 43, 2877–2881. [Google Scholar] [CrossRef]
- Humadi, J.I.; Nawaf, A.T.; Jarullah, A.T.; Ahmed, M.A.; Hameed, S.A.; Mujtaba, I.M. Design of new nano-catalysts and digital basket reactor for oxidative desulfurization of fuel: Experiments and modelling. Chem. Eng. Res. Des. 2023, 190, 634–650. [Google Scholar] [CrossRef]
- Pinho, M.T.; Ribeiro, R.S.; Gomes, H.T.; Faria, J.L.; Silva, A.M.T. Screening of Activated Carbons for the Treatment of Highly Concentrated Phenol Solutions Using Catalytic Wet Peroxide Oxidation: The Effect of Iron Impurities on the Catalytic Activity. Catalysts 2020, 10, 1318. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon na-noparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Nawaf, A.T.; Jarullah, A.T.; Gheni, S.A.; Mujtaba, I.M. Development of Kinetic and Process Models for the Oxidative Desulfurization of Light Fuel, Using Experiments and the Parameter Estimation Technique. Ind. Eng. Chem. Res. 2015, 54, 12503–12515. [Google Scholar] [CrossRef]
- Jarullah, A.T.; Ahmed, M.A.; Al-Tabbakh, B.A.; Mujtaba, I.M. Design of a new synthetic nanocatalyst resulting high fuel quality based on multiple supports: Experimental investigation and modeling. Chem. Prod. Process. Model. 2022, 18, 265–293. [Google Scholar] [CrossRef]
- Aabid, A.A.; Humadi, J.I.; Ahmed, G.S.; Jarullah, A.T.; Ahmed, M.A.; Abdullah, W.S. Enhancement of Desulfurization Process for Light Gas Oil Using New Zinc Oxide Loaded Over Alumina Nanocatalyst. Appl. Sci. Eng. Prog. 2023, 16, 6756. [Google Scholar] [CrossRef]
- Humadi, J.I.; Gheni, S.A.; Ahmed, S.M.R.; Abdullah, G.H.; Phan, A.N.; Harvey, A.P. Fast, non-extractive, and ultradeep desulfurization of diesel in anoscillatory baffled reactor. Process Saf. Environ. Prot. 2021, 152, 178–187. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanotechnology in Oil and Gas Industries: Principles and Applications; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Saka, C. BET, TG–DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. J. Anal. Appl. Pyrolysis 2012, 95, 21–24. [Google Scholar] [CrossRef]
- Bakti, A.I.; Gareso, P.L. Characterization of Active Carbon Prepared from Coconuts Shells using FTIR, XRD and SEM Techniques. J. Ilm. Pendidik. Fis. Al Biruni 2018, 7, 33–39. [Google Scholar] [CrossRef]
- Ramzannezhad, A.; Bahari, A. Characteristics of Fe3O4, α -Fe2O3, and γ -Fe2O3 Nanoparticles as Suitable Candidates in the Field of Nanomedicine. J. Supercond. Nov. Magn. 2017, 30, 2165–2174. [Google Scholar]
- Khoshnam, M.; Farahbakhsh, J.; Zargar, M.; Mohammad, A.W.; Benamor, A.; Ang, W.L.; Mahmoudi, E. α-Fe2O3/graphene oxide powder and thin film nanocomposites as peculiar photocatalysts for dye removal from wastewater. Sci. Rep. 2021, 11, 20378. [Google Scholar] [CrossRef] [PubMed]
- Kuśmierek, K.; Świątkowski, A. The influence of different agitation techniques on the adsorption kinetics of 4-chlorophenol on granular activated carbon. React. Kinet. Catal. Lett. 2015, 116, 261–271. [Google Scholar] [CrossRef]
- Humadi, J.I.; Gheni, S.A.; Ahmed, S.M.R.; Harvey, A. Dimensionless evaluation and kinetics of rapid and ultradeep desulfurization of diesel fuel in an oscillatory baffled reactor. RSC Adv. 2022, 12, 14385–14396. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.S.; Humadi, J.I.; Aabid, A.A. Mathematical Model, Simulation and Scale up of Batch Reactor Used in Oxidative Desulfurization of Kerosene. Iraqi J. Chem. Pet. Eng. 2021, 22, 11–17. [Google Scholar] [CrossRef]
- Zazo, J.A.; Pliego, G.; Blasco, S.; Casas, J.A.; Rodriguez, J.J. Intensification of the Fenton process by increasing the tem-perature. Ind. Eng. Chem. Res. 2011, 50, 866–870. [Google Scholar] [CrossRef]
- Lann, L.M.V.; Cabassud, M.; Casamatta, G. Modeling, Optimization and Control of Batch Chemical Reactors in Fine Chemical Production. IFAC Proc. Vol. 1998, 31, 715–724. [Google Scholar] [CrossRef]
- Froment, G.F.; Bischoff, K.B.; Wilde, J.D. Chemical Reactor Analysis and Design; Wiley: New York, NY, USA, 1990. [Google Scholar]
- Marroquín, G.; Ancheyta, J.; Esteban, C. A batch reactor study to determine effectiveness factors of commercial HDS catalyst. Catal. Today 2005, 104, 70–75. [Google Scholar] [CrossRef]
- Jarullah, A.T.; Ahmed, G.S.; Al-Tabbakh, B.A.; Mujtaba, I.M. Enhancement of light naphtha quality and environment using new synthetic nano-catalyst for oxidative desulfurization: Experiments and process modeling. Comput. Chem. Eng. 2020, 140, 106869. [Google Scholar] [CrossRef]
- Humadi, J.I.; Razzaq, G.H.A.; Khamees, L.A.; Ahmed, M.A.; Saeed, L.I. Improved Kerosene Quality with the Use of a Gamma Alumina Nanoparticles Supported Zinc Oxide Catalyst in a Digital Batch Baffled Reactor: Experiments and Process Modelling. Korean Chem. Eng. Res. 2023, 61, 226–233. [Google Scholar]
- Mohammed, A.E.; Jarullah, A.T.; Ghenia, S.A.; Mujtaba, I.M. Optimal design and operation of an industrial three phase reactor for the oxidation of phenol. Comput. Chem. Eng. 2016, 94, 257–271. [Google Scholar] [CrossRef]
- Duduković, M.P.; Larachi, F.; Mills, P.L. Multiphase catalytic reactors: A perspective on current knowledge and future trends. Catal. Rev. 2002, 44, 123–246. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Jarullaha, A.T.; Ghenia, S.A.; Mujtaba, I.M. Significant Cost and energy Savings Opportunities in industrial three phase reactor for phenol oxidation. Comput. Chem. Eng. 2017, 104, 201–210. [Google Scholar] [CrossRef]
- Nawaf, A.T. Optimal Kinetic Parameters of Trickle bed Reactor for Oxidation of 2-Proplymercaptan in Naphtha. Diyala J. Eng. Sci. 2019, 12, 83–100. [Google Scholar] [CrossRef]
- Al-Obaidi, M.A.; Kara-Zaïtri, C.; Mujtaba, I.M. Removal of phenol from wastewater using spiral-wound reverse osmosis process: Model development based on experiment and simulation. J. Water Process Eng. 2017, 18, 20–28. [Google Scholar] [CrossRef]
- Al-Obaidi, M.A.; Jarullah, A.T.; Kara-Zaïtri, C.; Mujtaba, I.M. Simulation of hybrid trickle bed reactor–reverse osmosis process for the removal of phenol from wastewater. Comput. Chem. Eng. 2018, 113, 264–273. [Google Scholar] [CrossRef]
- Jarullah, A.T.; Ahmed, A.M.; Hussein, H.M.; Ahmed, A.N.; Mohammed, H.J. Evaluation of synthesized Pt/HY-H- Mor-denite composite catalyst for Isomerization of Light Naphtha. Tikrit J. Eng. Sci. 2023, 30, 94–103. [Google Scholar] [CrossRef]
- Nawaf, A.T.; Gheni, S.A.; Jarullah, A.T.; Mujtaba, I.M. Optimal Design of a Trickle Bed Reactor for Light Fuel Oxidative Desulfurization Based on Experiments and Modeling. Energy Fuels 2015, 29, 3366–3376. [Google Scholar] [CrossRef]
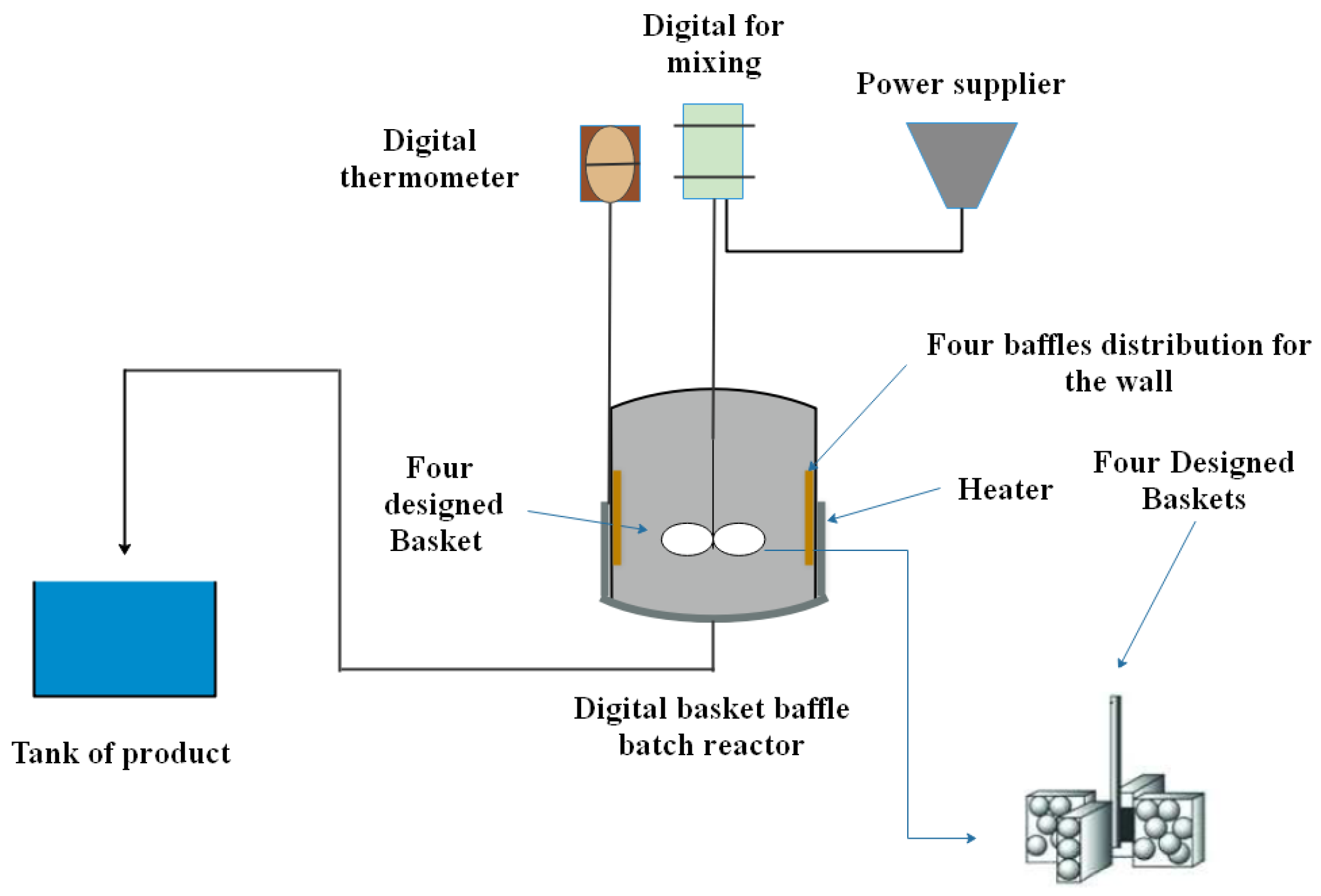
| Description | Specification |
|---|---|
| Dimension of reactor | Height = 12 cm, Diameter = 10 m |
| Length of the rod | 37 cm |
| Type of impeller (Stainless steel) | Four basket impeller |
| Height of basket | 1.2 cm |
| Length of basket | 1.2 cm |
| Width of basket | 1.2 cm |
| Impeller diameter | 94 mm |
| Height of baffle (Stainless steel) | 10 cm |
| Width of baffle | 1.5–1.8 cm |
| Property | Support (AC) | Nano-Catalyst (8% Fe2O3/AC) |
|---|---|---|
| Surface area, m2g−1 | 908.880 | 778.107 |
| Total pore volume, cm3g−1 | 0.512 | 0.4416 |
| Mean pore diameter, nm | 2.255 | 2.3824 |
| Element | Line Type | Weight % | Weight % Sigma | Atomic % |
|---|---|---|---|---|
| C | K series | 57.77 | 0.35 | 70.37 |
| O | K series | 26.80 | 0.33 | 24.51 |
| Fe | K series | 10.46 | 0.19 | 2.74 |
| Parameter (Symbol) | Equations/Values | Equation No. |
|---|---|---|
| Rate of reaction () | (1) | |
| Arrhenius equation (k) | k = k0 | (2) |
| The final phenol content () | (3) | |
| The effectiveness factor () | (4) | |
| Thiele modulus () | (5) | |
| The catalyst effective diffusivity () | (6) | |
| The porosity of catalyst () | (7) | |
| Particle density () | (8) | |
| The tortuosity factor () | Tortuosity factor () of the pore network (2 to 7) | ---- |
| The Knudsen diffusivity () | (9) | |
| Mean pore radius () | (10) | |
| The molecular diffusivity () | (11) | |
| The molar volume of the liquid () | (12) | |
| The molar volume of the phenol compound () | (13) | |
| The external volume of the catalyst (For sphere particle) () | (14) | |
| The external surface of the catalyst (For sphere particle) (Sp) | (15) | |
| The phenol viscosity () | (16) | |
| Constant factor ( | 0.1175 for alcohols and 0.248 for other materials. | ---- |
| Molecule volume fraction () | (17) |
| Parameter, Unit | Value |
|---|---|
| Initial content of phenol (Ct), ppm | 839 |
| Reaction time 1, 2, 3, 4, min | 30, 60, 90, 120 |
| Reaction temperature (T1, 2, 3, 4), °C | 25, 45, 65, 85 |
| R, J/mole.°K | 8.314 |
| Vg, cm3/gm | 0.4536 |
| Sg, cm2/gm | 7,073,700 |
| Vp, cm3 | 0.0114137 |
| Sp, cm2 | 0.28274 |
| ρB. gm/cm3 | 1.79377 |
| M.Wt of phenol | 184 |
| rg, nm | 1.28249 |
| , cm3/gmole | 55.95 |
| , cm3/gmole | 229 |
| Tc, °R | 1250 |
| Parameter | Value |
|---|---|
| n (-) | 1.9997 |
| EA (KJ/mol) | 28.8292 |
| ko ( | 9989.6200 |
| SSE (-) | 1.13996 × 10−5 |
| Parameter | Value |
|---|---|
| n (-) | 1.9869 |
| EA (KJ/mol) | 28.1398 |
| ko ( | 15,712.4700 |
| SSE (-) | 7.81139 × 10−6 |
| Parameter | Value |
|---|---|
| n (-) | 1.9265 |
| EA (KJ/mol) | 25.6211 |
| ko ( | 11,854.0200 |
| SSE (-) | 7.3293 × 10−6 |
| Parameter | Value |
|---|---|
| n (-) | 1.9629 |
| EA (KJ/mol) | 25.9162 |
| ko ( | 14,265.0800 |
| SSE (-) | 7.0075 × 10−6 |
| Temperature (°C) | Time (min) | Phenol Concentration (ppm) | Conversion % | Error % | ||
|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | |||
| 25 | 30 | 730 | 725.39 | 12.99 | 13.54 | 0.63 |
| 25 | 60 | 640 | 633.26 | 23.72 | 24.52 | 1.05 |
| 25 | 90 | 566 | 558.64 | 32.54 | 33.42 | 1.301 |
| 25 | 120 | 510 | 497.62 | 39.21 | 40.69 | 2.43 |
| 45 | 30 | 660 | 654.52 | 21.34 | 21.99 | 0.831 |
| 45 | 60 | 535 | 523.04 | 36.23 | 37.66 | 2.24 |
| 45 | 90 | 441 | 428.62 | 47.44 | 48.91 | 2.81 |
| 45 | 120 | 371 | 359.36 | 55.79 | 57.17 | 3.14 |
| 65 | 30 | 574 | 570.53 | 31.59 | 31.10 | 0.60 |
| 65 | 60 | 419 | 408.45 | 50.01 | 51.32 | 2.52 |
| 65 | 90 | 314 | 308.15 | 62.57 | 63.27 | 1.86 |
| 65 | 120 | 252 | 243.12 | 69.96 | 71.02 | 3.52 |
| 85 | 30 | 487 | 479.45 | 41.95 | 42.86 | 1.56 |
| 85 | 60 | 311 | 303.12 | 62.93 | 63.88 | 2.53 |
| 85 | 90 | 215 | 211.06 | 74.37 | 74.84 | 1.83 |
| 85 | 120 | 164 | 158.26 | 80.45 | 81.14 | 3.50 |
| Temperature (°C) | Time (min) | Phenol Concentration (ppm) | Conversion % | Error % | ||
|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | |||
| 25 | 30 | 655 | 655.10 | 21.93 | 21.81 | 0.15 |
| 25 | 60 | 512 | 524.26 | 38.98 | 37.51 | 2.40 |
| 25 | 90 | 420 | 428.99 | 49.94 | 48.87 | 2.14 |
| 25 | 120 | 351 | 358.80 | 58.16 | 57.24 | 2.22 |
| 45 | 30 | 559 | 564.31 | 33.37 | 32.74 | 0.95 |
| 45 | 60 | 388 | 398.98 | 53.75 | 52.45 | 2.84 |
| 45 | 90 | 290 | 297.35 | 65.44 | 64.56 | 2.53 |
| 45 | 120 | 222 | 232.05 | 73.54 | 72.34 | 4.53 |
| 65 | 30 | 460 | 464.45 | 45.17 | 44.64 | 0.97 |
| 65 | 60 | 279 | 285.33 | 66.75 | 65.10 | 2.27 |
| 65 | 90 | 189 | 194.17 | 77.47 | 76.86 | 2.73 |
| 65 | 120 | 141 | 143.06 | 83.19 | 82.95 | 1.46 |
| 85 | 30 | 360 | 365.53 | 57.09 | 56.43 | 1.54 |
| 85 | 60 | 188 | 194.56 | 77.59 | 76.81 | 3.49 |
| 85 | 90 | 121 | 122.90 | 85.58 | 85.35 | 1.57 |
| 85 | 120 | 83 | 87.07 | 90.11 | 89.62 | 4.90 |
| Temperature (°C) | Time (min) | Phenol Concentration (ppm) | Conversion % | Error % | ||
|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | |||
| 25 | 30 | 550 | 547.30 | 34.45 | 34.77 | 0.50 |
| 25 | 60 | 392 | 375.16 | 53.28 | 55.29 | 4.30 |
| 25 | 90 | 285 | 271.57 | 66.03 | 67.63 | 4.71 |
| 25 | 120 | 216 | 206.50 | 74.26 | 75.39 | 4.40 |
| 45 | 30 | 441 | 422.03 | 47.44 | 49.70 | 4.30 |
| 45 | 60 | 269 | 259.08 | 67.94 | 69.12 | 3.69 |
| 45 | 90 | 175 | 169.28 | 79.14 | 79.82 | 3.27 |
| 45 | 120 | 123 | 120.62 | 85.34 | 85.62 | 1.94 |
| 65 | 30 | 340 | 339.86 | 59.48 | 59.49 | 0.04 |
| 65 | 60 | 174 | 169.83 | 79.26 | 79.76 | 2.40 |
| 65 | 90 | 103 | 102.10 | 87.72 | 87.83 | 0.87 |
| 65 | 120 | 70 | 69.65 | 91.66 | 91.70 | 0.50 |
| 85 | 30 | 247 | 249.93 | 70.56 | 70.21 | 1.19 |
| 85 | 60 | 109 | 108.22 | 87.01 | 87.10 | 0.72 |
| 85 | 90 | 60 | 61.44 | 92.85 | 92.68 | 2.40 |
| 85 | 120 | 39 | 40.91 | 95.35 | 95.12 | 4.90 |
| Temperature (°C) | Time (min) | Phenol Concentration (ppm) | Conversion % | Error % | ||
|---|---|---|---|---|---|---|
| Experimental | Predicted | Experimental | Predicted | |||
| 25 | 30 | 544 | 522.02 | 35.16 | 37.78 | 4.04 |
| 25 | 60 | 386 | 381.91 | 53.99 | 54.48 | 1.06 |
| 25 | 90 | 288 | 278.99 | 65.67 | 66.75 | 3.13 |
| 25 | 120 | 225 | 213.98 | 73.18 | 74.50 | 4.90 |
| 45 | 30 | 438 | 446.77 | 47.80 | 46.75 | 2.00 |
| 45 | 60 | 265 | 265.04 | 68.42 | 68.41 | 0.02 |
| 45 | 90 | 178 | 175.31 | 78.78 | 79.11 | 1.51 |
| 45 | 120 | 128 | 126.32 | 84.74 | 84.94 | 1.31 |
| 65 | 30 | 333 | 344.20 | 60.31 | 58.98 | 3.36 |
| 65 | 60 | 172 | 174.80 | 79.50 | 79.17 | 1.63 |
| 65 | 90 | 105 | 106.76 | 87.49 | 87.28 | 1.68 |
| 65 | 120 | 73 | 73.83 | 91.30 | 91.20 | 1.13 |
| 85 | 30 | 242 | 253.76 | 71.16 | 69.76 | 4.86 |
| 85 | 60 | 107 | 112.24 | 87.250 | 86.62 | 4.90 |
| 85 | 90 | 62 | 64.96 | 92.61 | 92.26 | 4.77 |
| 85 | 120 | 46 | 43.93 | 94.52 | 94.77 | 4.52 |
| Parameter, Unit | Values | |||
|---|---|---|---|---|
| RPM-1 | RPM-2 | RPM-3 | RPM-4 | |
| , ppm | 865 | 864 | 1104 | 956 |
| T, °C | 77 | 80 | 87 | 81 |
| Time, min | 200 | 193 | 190 | 180 |
| Conversion, % | 86.99 | 93.49 | 98.10 | 96.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaf, A.T.; Humadi, J.I.; Jarullah, A.T.; Ahmed, M.A.; Hameed, S.A.; Mujtaba, I.M. Design of Nano-Catalyst for Removal of Phenolic Compounds from Wastewater by Oxidation Using Modified Digital Basket Baffle Batch Reactor: Experiments and Modeling. Processes 2023, 11, 1990. https://doi.org/10.3390/pr11071990
Nawaf AT, Humadi JI, Jarullah AT, Ahmed MA, Hameed SA, Mujtaba IM. Design of Nano-Catalyst for Removal of Phenolic Compounds from Wastewater by Oxidation Using Modified Digital Basket Baffle Batch Reactor: Experiments and Modeling. Processes. 2023; 11(7):1990. https://doi.org/10.3390/pr11071990
Chicago/Turabian StyleNawaf, Amer T., Jasim I. Humadi, Aysar T. Jarullah, Mustafa A. Ahmed, Shymaa Ali Hameed, and Iqbal M. Mujtaba. 2023. "Design of Nano-Catalyst for Removal of Phenolic Compounds from Wastewater by Oxidation Using Modified Digital Basket Baffle Batch Reactor: Experiments and Modeling" Processes 11, no. 7: 1990. https://doi.org/10.3390/pr11071990
APA StyleNawaf, A. T., Humadi, J. I., Jarullah, A. T., Ahmed, M. A., Hameed, S. A., & Mujtaba, I. M. (2023). Design of Nano-Catalyst for Removal of Phenolic Compounds from Wastewater by Oxidation Using Modified Digital Basket Baffle Batch Reactor: Experiments and Modeling. Processes, 11(7), 1990. https://doi.org/10.3390/pr11071990







