Study on the Preparation of ZnFeO4 by Roasting Zinc-Containing Gossan Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Principles and Characterization Methods
2.2.1. Test Methods and Principles
2.2.2. Characterization Methods
3. Results and Discussion
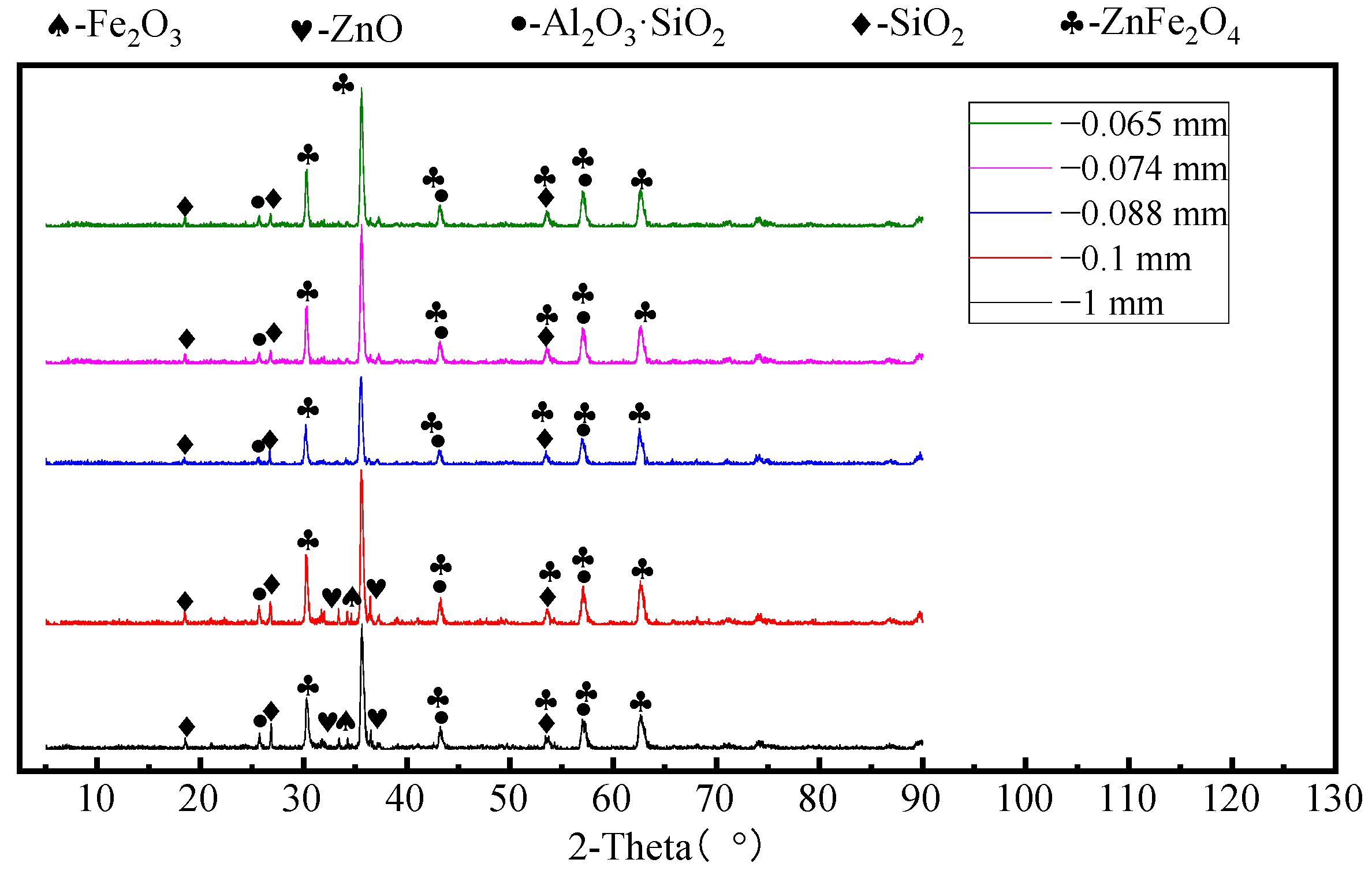
3.1. Effect of Ore Particle Size
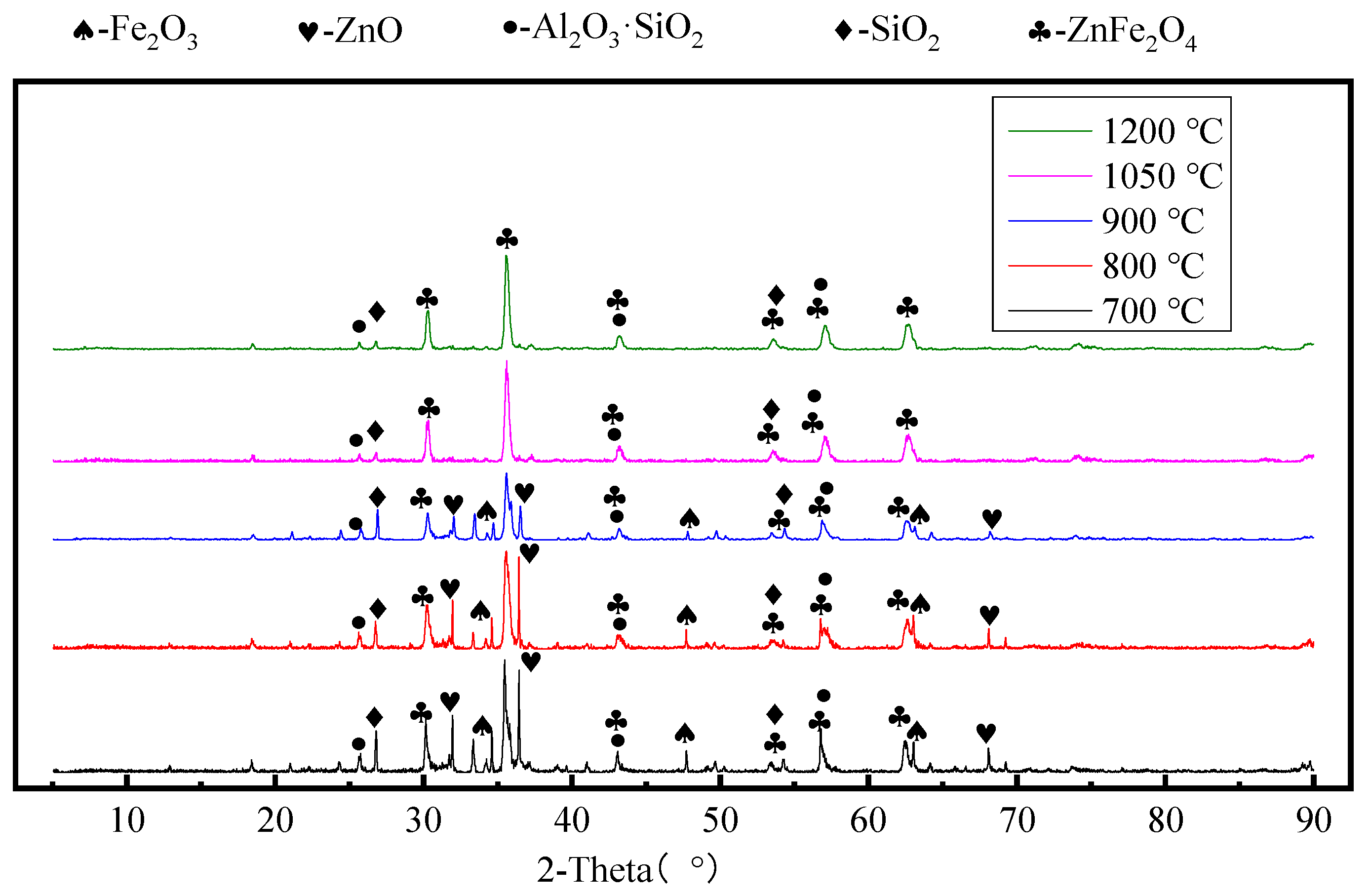
3.2. Effect of Roasting Temperature
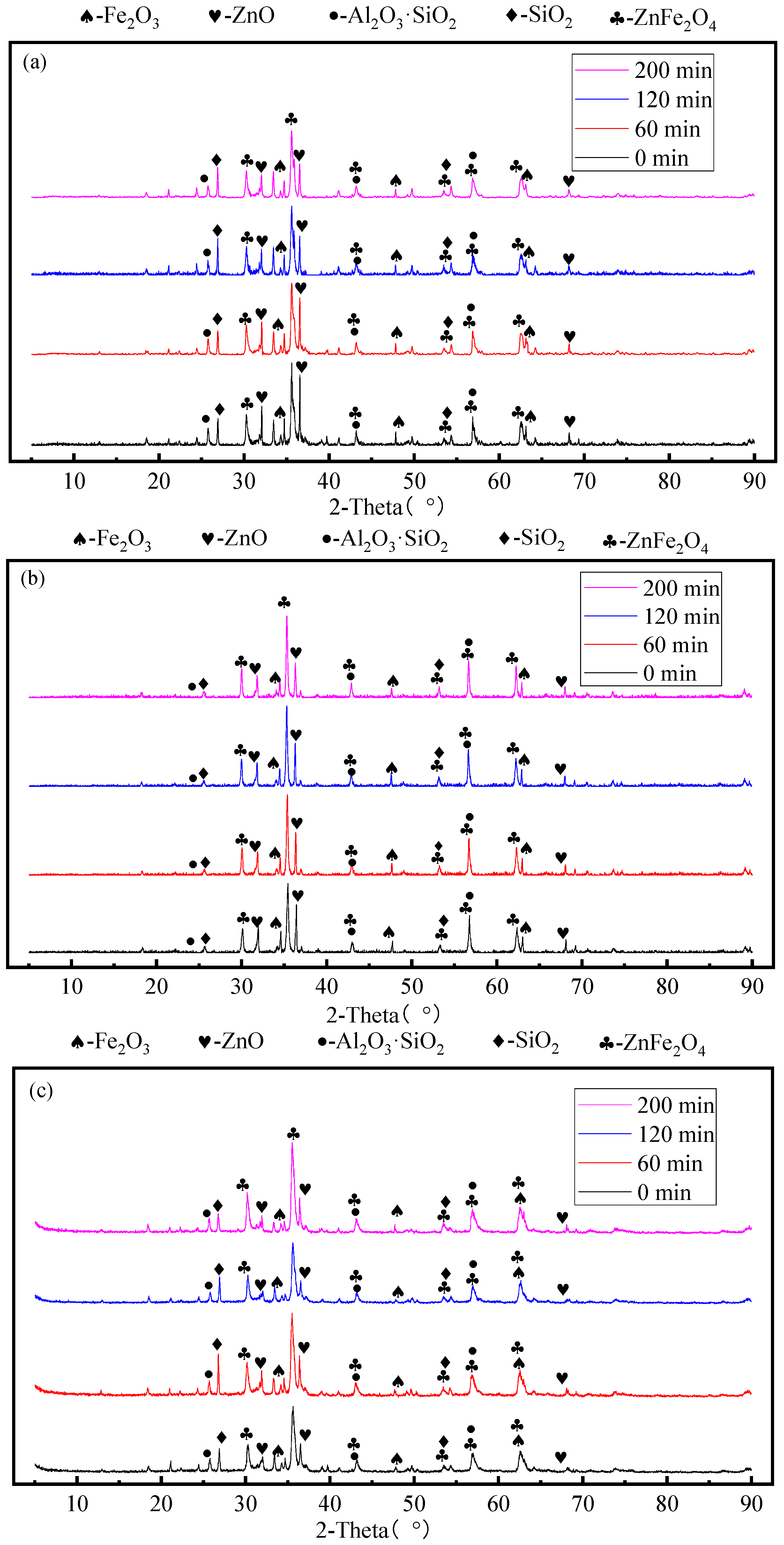
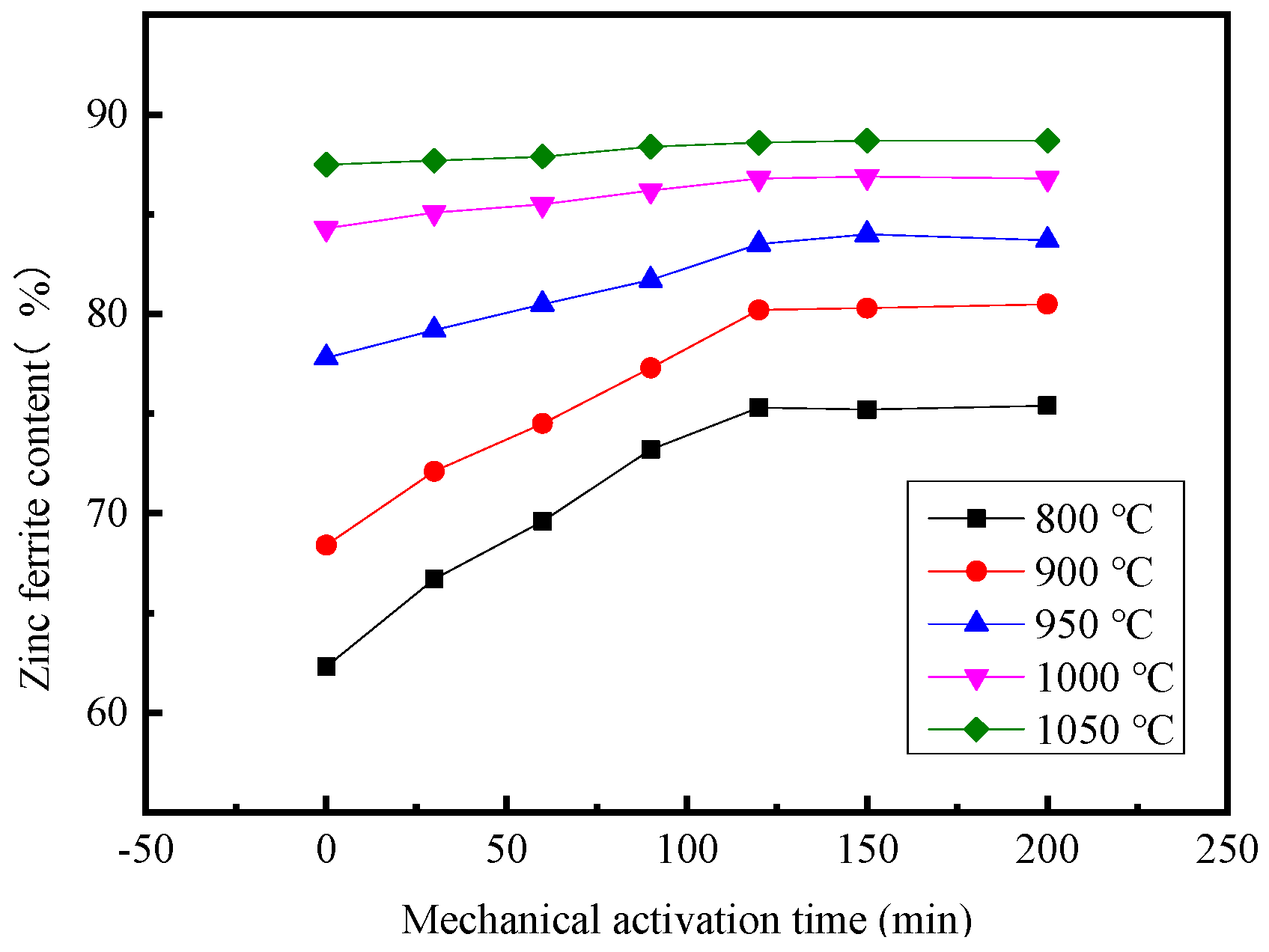
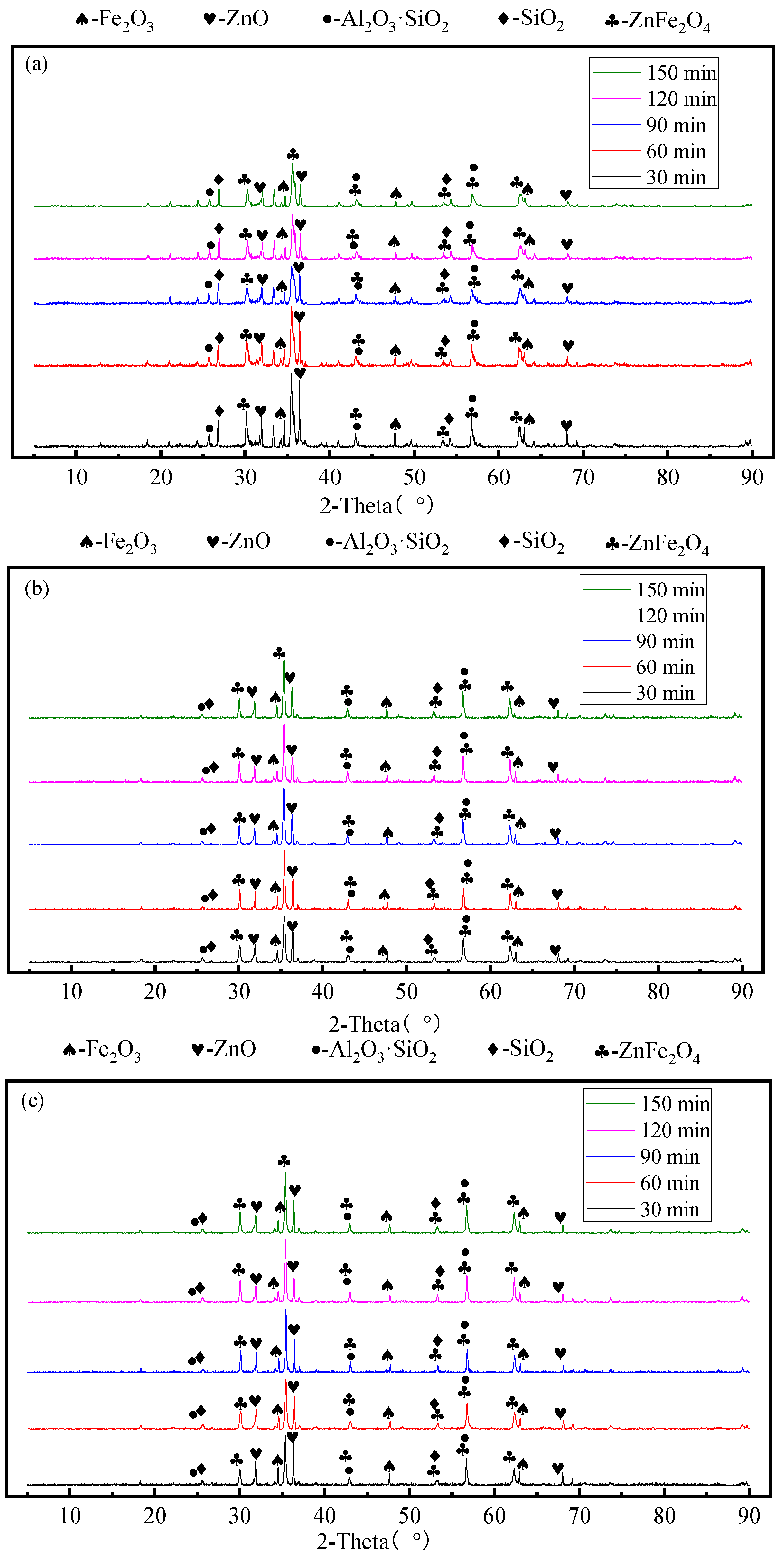
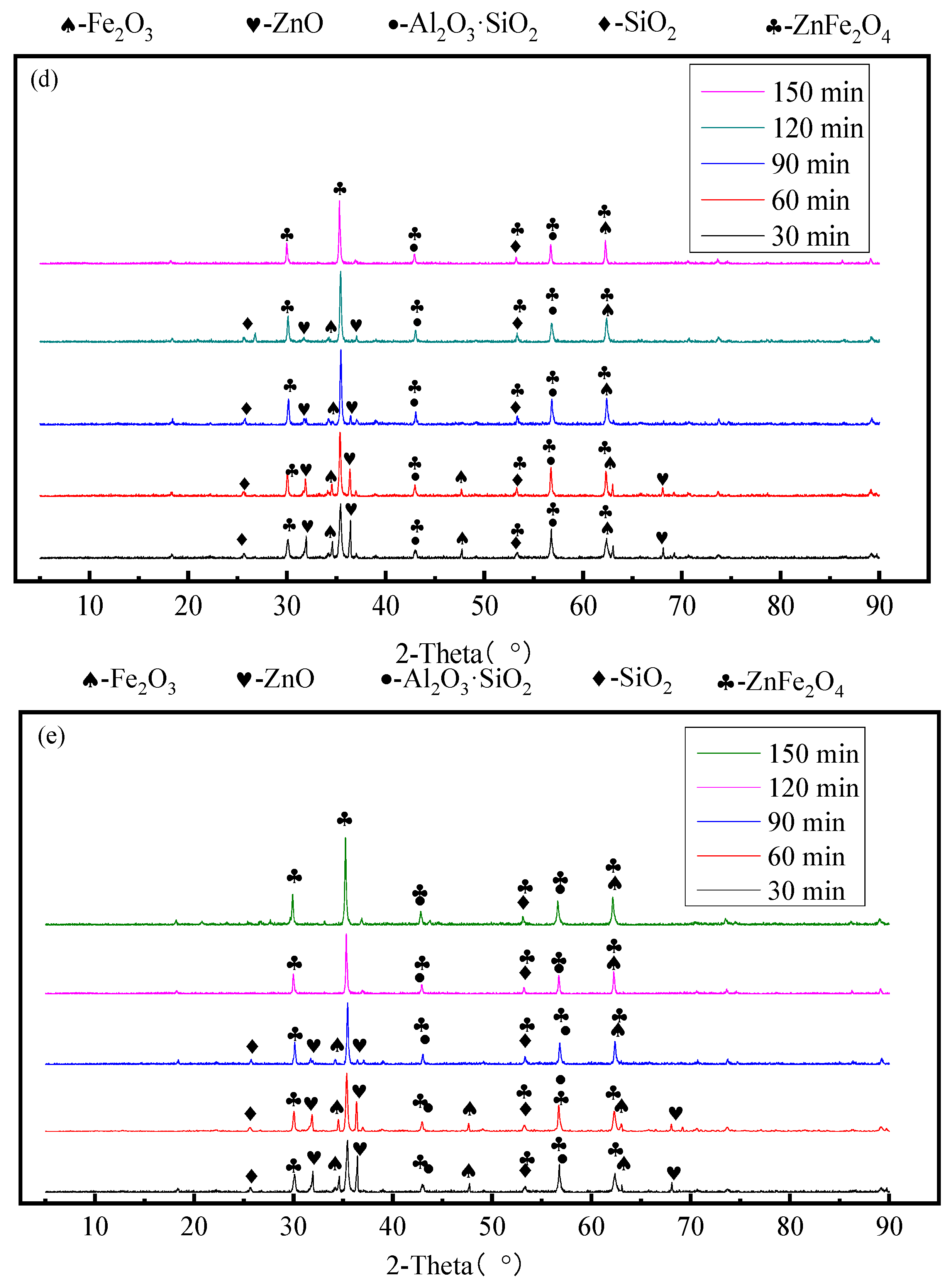
3.3. Effect of Mechanical Activation Time
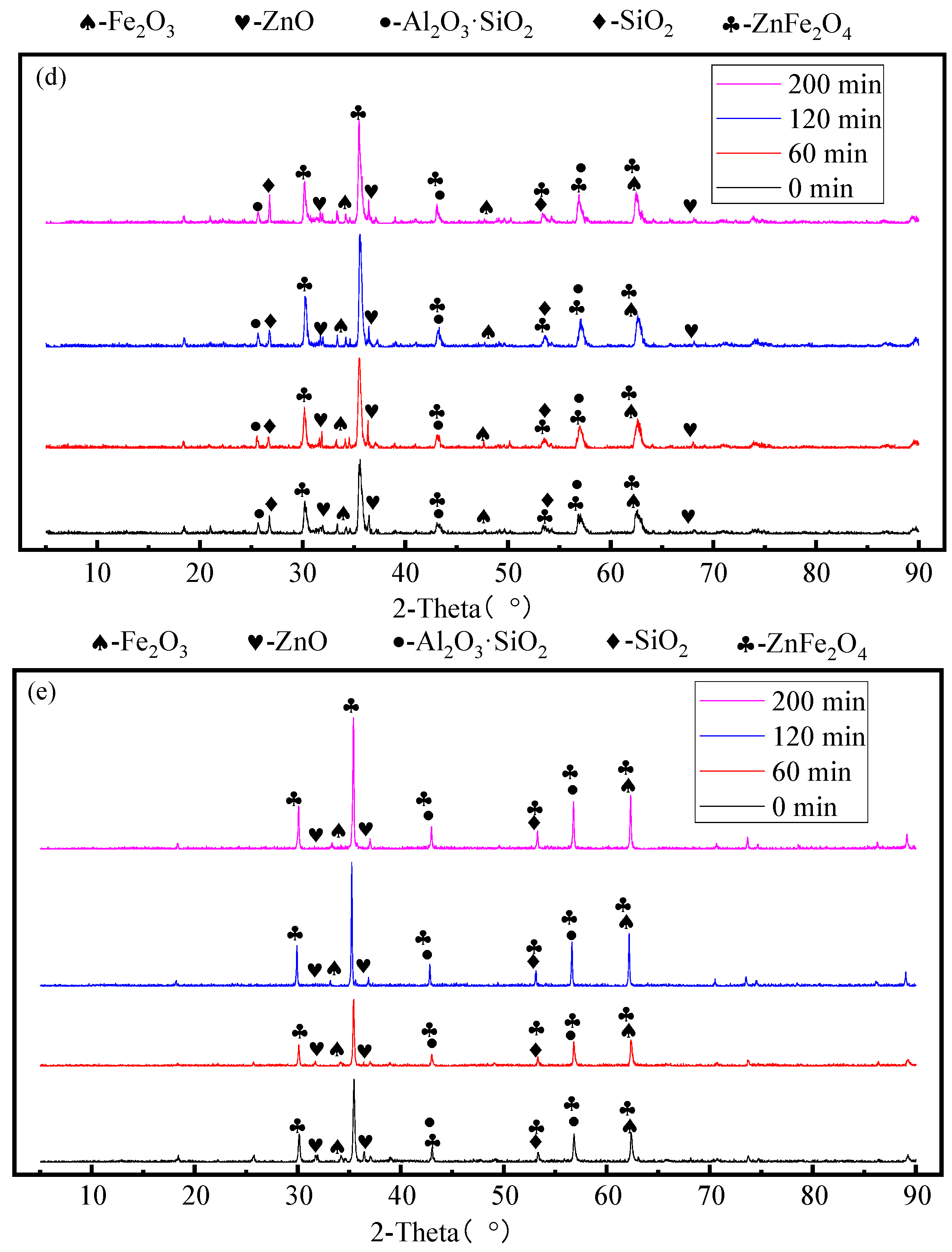
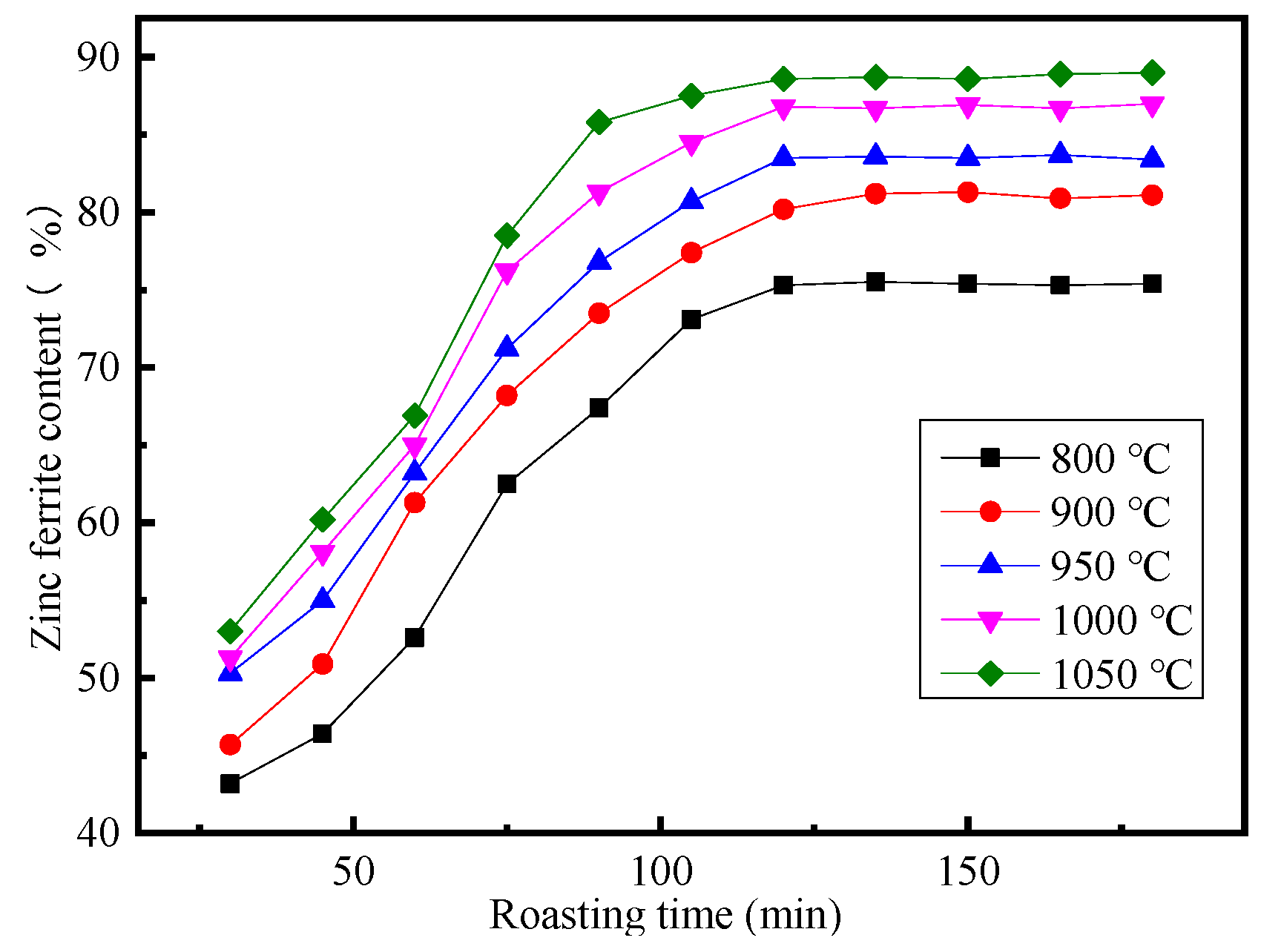
3.4. Effect of Roasting Time
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pabllo, H.C.S.; Marcondes, L.C. Mineralogical and textural evolution of the Alvo 118 copper-bearing gossan: Implications for supergene metallogenesis in Carajás Mineral Province. Brazil. J. S. Am. Earth Sci. 2023, 121, 104108. [Google Scholar]
- Andreu, E.; Torró, L.; Proenza, J.A.; Domenech, C.; García-Casco, A.; Villanova de Benavent, C.; Chavez, C.; Espaillat, J.; Lewis, J.F. Weathering profile of the Cerro de Maimón VMS deposit (Dominican Republic): Textures, mineralogy, gossan evolution and mobility of gold and silver. Ore Geol. Rev. 2015, 65, 165–179. [Google Scholar] [CrossRef]
- Pires, G.L.C.; Renac, C.; Bongiolo, E.M.; Neumann, R. Gossan mineralogy, textures, and gold enrichment over the Au (As, Bi, Ag) deposit in the Buracão Area (Brasília Fold Belt, Brazil): Implications for gold prospecting in weathering profiles. J. Geochem. Explor. 2020, 218, 106615. [Google Scholar] [CrossRef]
- Kříbek, B.; Zachariáš, J.; Knésl, I.; Míková, J.; Mihaljevič, M.; Veselovský, F.; Bamba, O. Geochemistry, mineralogy, and isotope composition of Pb, Zn, and Cu in primary ores, gossan and barren ferruginous crust from the Perkoa base metal deposit, Burkina Faso. J. Geochem. Explor. 2016, 168, 49–64. [Google Scholar] [CrossRef]
- Baggio, S.B.; Hartmann, L.A.; Massonne, H.J.; Theye, T.; Antunes, L.M. Silica gossan as a prospective guide for amethyst geode deposits in the Ametista do Sul mining district, Paraná volcanic province, southern Brazil. J. Geochem. Explor. 2015, 159, 213–226. [Google Scholar] [CrossRef]
- Yesares, L.; Sáez, R.; Ruiz De Almodóvar, G.R.; Nieto, J.M.; Gómez, C.; Ovejero, G. Mineralogical evolution of the Las Cruces gossan cap (Iberian Pyrite Belt): From subaerial to underground conditions. Ore Geol. Rev. 2017, 80, 377–405. [Google Scholar] [CrossRef]
- Laakso, K.; Rivard, B.; Rogge, D. Enhanced detection of gossans using hyperspectral data: Example from the Cape Smith Belt of northern Quebec, Canada. ISPRS J. Photogramm. 2016, 114, 137–150. [Google Scholar] [CrossRef]
- Velasco, F.; Herrero, J.M.; Suárez, S.; Yusta, I.; Alvaro, A.; Tornos, F. Supergene features and evolution of gossans capping massive sulphide deposits in the Iberian pyrite Belt. Ore Geol. Rev. 2013, 53, 181–203. [Google Scholar] [CrossRef]
- Costa, M.L.; Angélica, R.S.; Costa, N.C. The geochemical association Au–As–B-(Cu)−Sn−W in latosol, colluvium, lateritic iron crust, and gossan in Carajás, Brazil: Importance for primary ore identification. J. Geochem. Explor. 1999, 67, 33–49. [Google Scholar] [CrossRef]
- Assawincharoenkij, T.; Hauzenberger, C.; Ettinger, K.; Sutthirat, C. Mineralogical and geochemical characterization of waste rocks from a gold mine in northeastern Thailand: Application for environmental impact protection. Environ. Sci. Pollut. Res. Int. 2018, 25, 3488–3500. [Google Scholar] [CrossRef]
- Li, J.M. Experimental study on cyanide leaching of a gossan type gold and silver ore in Qinghai. Gold Sci. Technol. 2015, 23, 88–93. (In Chinese) [Google Scholar]
- Celep, O.; Serbest, V. Characterization of an iron oxy/hydroxide (gossan type) bearing refractory gold and silver ore by diagnostic leaching. Trans. Nonferrous Met. Soc. China 2015, 25, 1286–1297. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, X.C.; Liu, H. Mineral phase transition and heavy metal release during the reduction of iron caps by Shewanella oneidensis MR-1. Bull. Mineral. Rock Geochem. 2015, 34, 316–322. (In Chinese) [Google Scholar]
- Hankare, P.P.; Patil, R.P.; Jadhav, A.V.; Garadkar, K.M.; Sasikala, R. Enhanced photocatalytic degradation of methyl red and thymol blue using titania-alumina-zinc ferrite nanocomposite. Appl. Catal. B 2011, 107, 333–339. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Jinka, K.M.; Bajaj, H.C. Nanosized sulfated zinc ferrite as catalyst for the synthesis of nopol and other fine chemicals. Catal. Today 2012, 198, 98–105. [Google Scholar] [CrossRef]
- Matinise, N.; Kaviyarasu, K.; Mongwaketsi, N.; Khamlich, S.; Kotsedi, L.; Mayedwa, N.; Maaza, M. Green synthesis of novel zinc iron oxide(ZnFe2O4) nanocomposite via Moringa oleifera natural extract for electrochemical applications. Appl. Surf. Sci. 2018, 446, 66–73. [Google Scholar] [CrossRef]
- Raut, S.D.; Awasarmol, V.V.; Ghule, B.G.; Shaikh, S.F.; Gore, S.K.; Sharma, R.P.; Pawar, P.P.; Mane, R.S. γ-irradiation induced zinc ferrites and their enhanced room-temperature ammonia gas sensing properties. Mater. Res. Express 2018, 5, 035702. [Google Scholar] [CrossRef]
- Sai, R.; Kulkarni, S.D.; Yamaguchi, M.; Bhat, N.; Shivashankar, S.A. Integrated X-band inductor with a nanoferrite film core. IEEE Magn. Lett. 2017, 8, 3703904. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Raeisi Shahraki, R.; Seyyed Ebrahimi, S.A.; Masoudpanah, S.M. Magnetic properties of zinc ferrite nanoparticles synthesized by coprecipitation method. J. Supercond. Nov. Magn. 2014, 27, 1587–1592. [Google Scholar] [CrossRef]
- Sangita, N.P.; Pratik, A.N.; Arvind, V.N.; Shankar, R.T.; Arun, V.B. Preparation techniques for zinc ferrites and their applications: A review. Mater. Today Proc. 2022, 60, 2194–2208. [Google Scholar]
- Sun, X.M.; Li, Z.W.; Li, X.H.; Zhang, Z.J. Preparation of mesoporous zinc ferrite flame retardant with different scales and its performance in epoxy resin. Polym. Test. 2022, 110, 107549. [Google Scholar] [CrossRef]
- Rachna, S.; Singh, N.B.; Agarwal, A. Preparation, characterization, properties, and applications of nano zinc ferrite. Mater. Today Proc. 2018, 5, 9148–9155. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, P.; Ma, S. Study on properties of gossan ore from zinc sulfide deposit. J. Phys. Conf. Ser. 2021, 2044, 012081. [Google Scholar] [CrossRef]


| Component | Zn | Fe2O3 | SiO2 | Al2O3 | MgO | CaO |
|---|---|---|---|---|---|---|
| Content (%) | 8.99 | 68.32 | 10.32 | 5.6 | 1.35 | 0.56 |
| Component | Na2O | K2O | SO3 | TiO2 | Mn | Pb |
| Content (%) | 0.20 | 0.12 | 0.90 | 0.25 | 1.46 | 1.38 |
| Particle Size (mm) | −1 | −0.1 | −0.088 | −0.074 | −0.065 |
|---|---|---|---|---|---|
| Content of ZnFeO4 (%) | 75.3 | 80.1 | 85.3 | 88.6 | 88.4 |
| Component | Zn | Fe2O3 | SiO2 | Al2O3 | MgO | CaO |
|---|---|---|---|---|---|---|
| Content (%) | 12.32 | 73.56 | 5.81 | 2.6 | 0.78 | 0.68 |
| Component | Na2O | K2O | SO3 | TiO2 | Mn | Pb |
| Content (%) | 0.15 | 0.10 | 0.86 | 0.35 | 0.96 | 1.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, Z.; Huo, X.; Li, H.; Liao, S.; Ma, S.; Li, H. Study on the Preparation of ZnFeO4 by Roasting Zinc-Containing Gossan Ore. Processes 2023, 11, 1991. https://doi.org/10.3390/pr11071991
Yang J, Li Z, Huo X, Li H, Liao S, Ma S, Li H. Study on the Preparation of ZnFeO4 by Roasting Zinc-Containing Gossan Ore. Processes. 2023; 11(7):1991. https://doi.org/10.3390/pr11071991
Chicago/Turabian StyleYang, Jinlin, Zongyu Li, Xingnan Huo, Hangyu Li, Shizhen Liao, Shaojian Ma, and Hengjun Li. 2023. "Study on the Preparation of ZnFeO4 by Roasting Zinc-Containing Gossan Ore" Processes 11, no. 7: 1991. https://doi.org/10.3390/pr11071991
APA StyleYang, J., Li, Z., Huo, X., Li, H., Liao, S., Ma, S., & Li, H. (2023). Study on the Preparation of ZnFeO4 by Roasting Zinc-Containing Gossan Ore. Processes, 11(7), 1991. https://doi.org/10.3390/pr11071991








