Impact of Pulsed Electric Field Treatment on the Process Kinetics and Selected Properties of Air and Dehumidified Air-Dried Mushrooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Pulsed Electric Field (PEF) Pre-Treatment
2.3. Convective Drying
2.4. Dry Matter Content
2.5. Hygroscopic Properties
2.6. Rehydration Properties
2.7. Color
2.8. Total Phenolic Content (TPC)
2.9. Anti-Oxidant Activity (DPPH and ABTS Assays)
2.10. Statistical Analysis
3. Results and Discussion
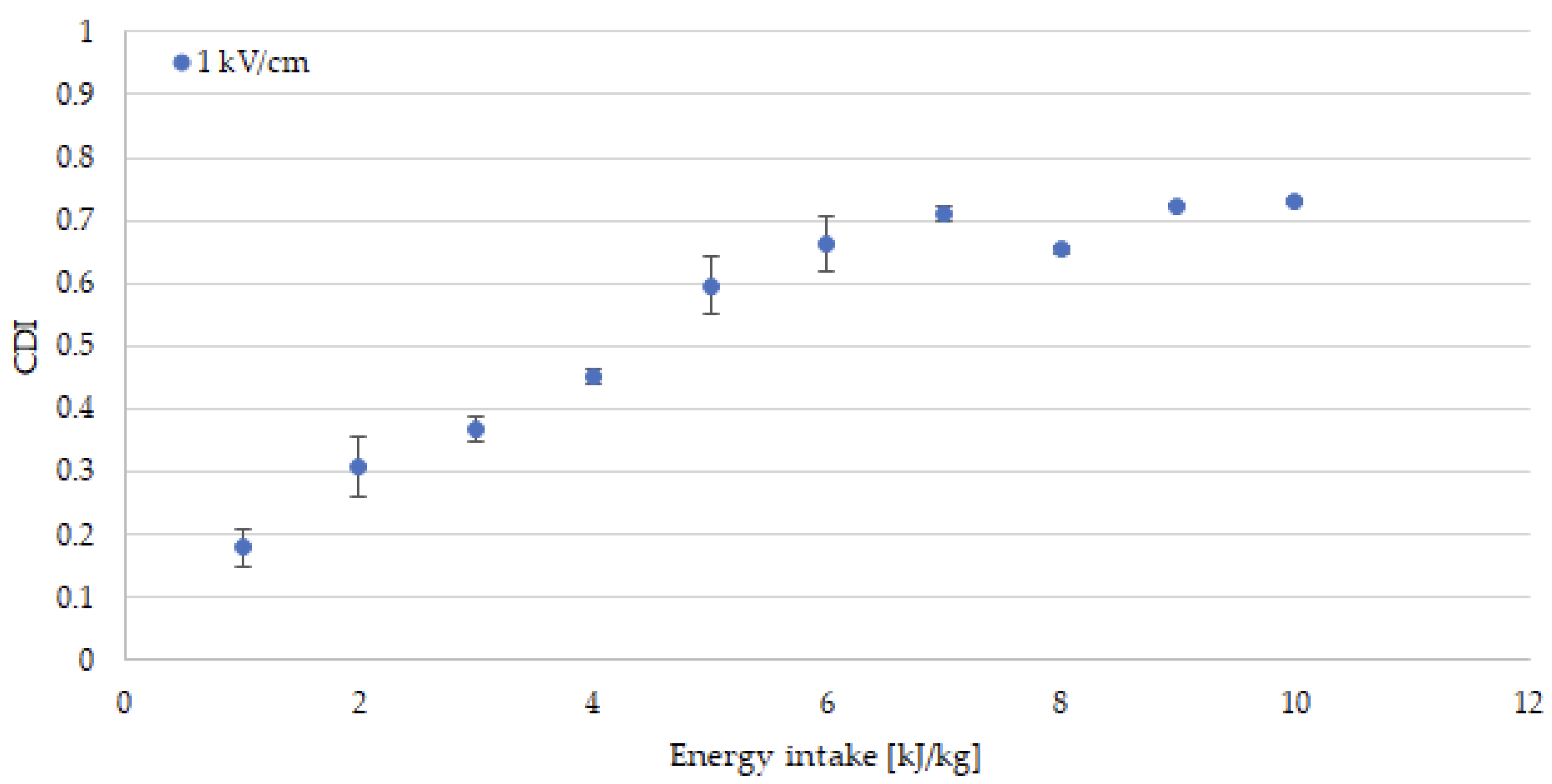
3.1. Drying Kinetics
3.2. Dry Matter Content
3.3. Hygroscopic Properties
3.4. Rehydration Properties
3.5. Color
3.6. Total Phenolic Content and Anti-Oxidant Activity
4. Cluster Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; Bekhit, A.E.-D.; Liu, Z.W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Löffler, M.J. Generation and Application of High Intensity Pulsed Electric Fields. In Pulsed Electric Fields Technology for the Food Industry; Raso, J., Heinz, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 55–106. [Google Scholar]
- Mahnič-Kalamiza, S.; Miklavčič, D. The Phenomenon of Electroporation. In Pulsed Electric Fields Technology for the Food Industry; Raso, J., Heinz, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 107–141. [Google Scholar]
- Dellarosa, N.; Tappi, S.; Ragni, L.; Laghi, L.; Rocculi, P.; Rosa, M.D. Metabolic response of fresh-cut apples induced by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2016, 38, 356–364. [Google Scholar] [CrossRef]
- Nowacka, M.; Dadan, M.; Janowicz, M.; Wiktor, A.; Witrowa-Rajchert, D.; Mandal, R.; Pratap-Singh, A.; Janiszewska-Turak, E. Effect of nonthermal treatments on selected natural food pigments and color changes in plant material. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5097–5144. [Google Scholar] [CrossRef] [PubMed]
- Mahnič-Kalamiza, S.; Vorobiev, E.; Miklavčič, D. Electroporation in food processing and biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Dellarosa, N.; Frontuto, D.; Laghi, L.; Rosa, M.D.; Lyng, J.G. The impact of pulsed electric fields and ultrasound on water distribution and loss in mushrooms stalks. Food Chem. 2017, 236, 94–100. [Google Scholar] [CrossRef]
- Wiktor, A.; Parniakov, O.; Witrowa-Rajchert, D. Drying Improving by Pulsed Electric Fields. In Pulsed Electric Fields Technology for the Food Industry; Raso, J., Heinz, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 385–397. [Google Scholar]
- Mirzaei-Baktash, H.; Hamdami, N.; Torabi, P.; Fallah-Joshaqani, S.; Dalvi-Isfahan, M. Impact of different pretreatments on drying kinetics and quality of button mushroom slices dried by hot-air or electrohydrodynamic drying. LWT 2022, 155, 112894. [Google Scholar] [CrossRef]
- Chen, X.D.; Mujumdar, A.S. Food drying fundamentals. In Drying Technologies in Food Processing; Chen, X.D., Mujumdar, A.S., Eds.; Blackwell Publishing Ltd.: Singapore, 2008; pp. 1–7. [Google Scholar]
- Matys, A.; Wiktor, A.; Dadan, M.; Witrowa-Rajchert, D. Influence of Ultrasound and the Conditions of Convective Drying with Dehumidified Air on the Course of the Process and Selected Properties of Apple Tissue. Foods 2021, 10, 1840. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M.; Wiktor, A.; Sobczynska, A.; Witrowa-Rajchert, D. Ultrasound to improve drying processes and prevent thermolabile nutrients degradation. In Design and Optimization of Innovative Food Processing Techniques Assisted by Ultrasound; Barba, F.J., Cravotto, G., Chemat, F., Lorenzo Rodriguez, J.M., Munekata, P.E.S., Eds.; Academic Press: London, UK, 2021; pp. 55–110. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M. The assessment of the possibility of using ethanol and ultrasound to design the properties of dried carrot tissue. Appl. Sci. 2021, 11, 689. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Sledz, M.; Wiktor, A.; Rybak, K.; Nowacka, M.; Witrowa-Rajchert, D. The impact of ultrasound and steam blanching pre-treatments on the drying kinetics, energy consumption and selected properties of parsley leaves. Appl. Acoust. 2016, 103, 148–156. [Google Scholar] [CrossRef]
- Association of Official Analytical Collaboration International. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Rockville, MD, USA, 2002. [Google Scholar]
- Śledź, M.; Nowacka, M.; Wiktor, A.; Witrowa-Rajchert, D. Selected chemical and physico-chemical properties of microwave-convective dried herbs. Food Bioprod. Process. 2013, 91, 421–428. [Google Scholar] [CrossRef]
- Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Quality of Red Bell Pepper Subjected to Freeze-Drying Preceded by Traditional and Novel Pretreatment. Foods 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.M.P.Q.; da Silva, M.V. Food Dehydration: Fundamentals, Modelling and Applications. In Transport Phenomena and Drying of Solids and Particulate Materials; Delgado, J.M.P.Q., Barbosa de Lima, A.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 69–94. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Shynkaryk, N.V.; Vorobiev, E. Pulsed electric field enhanced drying of potato tissue. J. Food Eng. 2007, 78, 606–613. [Google Scholar] [CrossRef]
- Liu, C.; Pirozzi, A.; Ferrari, G.; Vorobiev, E.; Grimi, N. Effects of Pulsed Electric Fields on Vacuum Drying and Quality Characteristics of Dried Carrot. Food Bioprocess Technol. 2020, 13, 45–52. [Google Scholar] [CrossRef]
- Kaya, A.; Aydın, O.; Demirtaş, C. Drying Kinetics of Red Delicious Apple. Biosyst. Eng. 2007, 96, 517–524. [Google Scholar] [CrossRef]
- Kaya, A.; Aydın, O.; Kolaylı, S. Effect of different drying conditions on the vitamin C (ascorbic acid) content of Hayward kiwifruits (Actinidia deliciosa Planch). Food Bioprod. Process. 2010, 88, 165–173. [Google Scholar] [CrossRef]
- Kaya, A.; Aydin, O.; Demirtas, C.; Akgün, M. An experimental study on the drying kinetics of quince. Desalination 2007, 212, 328–343. [Google Scholar] [CrossRef]
- Alam, M.R.; Lyng, J.G.; Frontuto, D.; Marra, F.; Cinquanta, L. Effect of Pulsed Electric Field Pretreatment on Drying Kinetics, Color, and Texture of Parsnip and Carrot. J. Food Sci. 2018, 83, 2159–2166. [Google Scholar] [CrossRef]
- Liu, C.; Grimi, N.; Lebovka, N.; Vorobiev, E. Convective air, microwave, and combined drying of potato pre-treated by pulsed electric fields. Dry. Technol. 2019, 37, 1704–1713. [Google Scholar] [CrossRef]
- Ostermeier, R.; Giersemehl, P.; Siemer, C.; Töpfl, S.; Jäger, H. Influence of pulsed electric field (PEF) pre-treatment on the convective drying kinetics of onions. J. Food Eng. 2018, 237, 110–117. [Google Scholar] [CrossRef]
- Ostermeier, R.; Parniakov, O.; Töpfl, S.; Jäger, H. Applicability of Pulsed Electric Field (PEF) Pre-Treatment for a Convective Two-Step Drying Process. Foods 2020, 9, 512. [Google Scholar] [CrossRef]
- Won, Y.-C.; Min, S.C.; Lee, D.-U. Accelerated Drying and Improved Color Properties of Red Pepper by Pretreatment of Pulsed Electric Fields. Dry. Technol. 2015, 33, 926–932. [Google Scholar] [CrossRef]
- Yamada, T.; Yamakage, K.; Takahashi, K.; Takaki, K.; Orikasa, T.; Kamagata, J.; Aoki, H. Influence of Drying Rate on Hot Air Drying Processing of Fresh Foods Using Pulsed Electric Field. IEEJ Trans. Electr. Electron. Eng. 2020, 15, 1123–1125. [Google Scholar] [CrossRef]
- Sharma, B.H.P.D. Drying Characteristics of Button Mushroom. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 503–512. [Google Scholar] [CrossRef]
- Wiktor, A.; Landfeld, A.; Matys, A.; Novotná, P.; Dadan, M.; Kováříková, E.; Nowacka, M.; Mulenko, M.; Witrowa-Rajchert, D.; Strohalm, J.; et al. Selected Quality Parameters of Air-Dried Apples Pretreated by High Pressure, Ultrasounds and Pulsed Electric Field—A Comparison Study. Foods 2021, 10, 1943. [Google Scholar] [CrossRef] [PubMed]
- Rybak, K.; Parniakov, O.; Samborska, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Energy and Quality Aspects of Freeze-Drying Preceded by Traditional and Novel Pre-Treatment Methods as Exemplified by Red Bell Pepper. Sustainability 2021, 13, 2035. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Wiktor, A.; Mykhailyk, V.; Samborska, K.; Gondek, E.; Witrowa-Rajchert, D.; Toepfl, S.; Parniakov, O. Pulsed electric field pre-treatment improves microstructure and crunchiness of freeze-dried plant materials: Case of strawberry. LWT 2020, 134, 110266. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Lewicki, P.P. Rehydration properties of dried plant tissues. Int. J. Food Sci. Technol. 2006, 41, 1040–1046. [Google Scholar] [CrossRef]
- Sacilik, K.; Elicin, A.K. The thin layer drying characteristics of organic apple slices. J. Food Eng. 2006, 73, 281–289. [Google Scholar] [CrossRef]
- Khraisheh, M.A.M.; McMinn, W.A.M.; Magee, T.R.A. Quality and structural changes in starchy foods during microwave and convective drying. Food Res. Int. 2004, 37, 497–503. [Google Scholar] [CrossRef]
- Ashtiani, S.-H.M.; Sturm, B.; Nasirahmadi, A. Effects of hot-air and hybrid hot air-microwave drying on drying kinetics and textural quality of nectarine slices. Heat Mass Transf. 2018, 54, 915–927. [Google Scholar] [CrossRef]
- Dehghannya, J.; Farshad, P.; Heshmati, M.K. Three-stage hybrid osmotic–intermittent microwave–convective drying of apple at low temperature and short time. Dry. Technol. 2018, 36, 1982–2005. [Google Scholar] [CrossRef]
- Wiktor, A.; Nowacka, M.; Dadan, M.; Rybak, K.; Lojkowski, W.; Chudoba, T.; Witrowa-Rajchert, D. The effect of pulsed electric field on drying kinetics, color, and microstructure of carrot. Dry. Technol. 2016, 34, 1286–1296. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The impact of pulsed electric field treatment on selected bioactive compound content and color of plant tissue. Innov. Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Swaston, UK, 2018; pp. 295–323. [Google Scholar] [CrossRef]
- Wiktor, A.; Pratap-Singh, A.; Parniakov, O.; Mykhailyk, V.; Mandal, R.; Witrowa-Rajchert, D. PEF as an alternative tool to prevent thermolabile compound degradation during dehydration processes. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Barba, F.J., Parniakov, O., Wiktor, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 155–202. [Google Scholar] [CrossRef]
- Matys, A.; Witrowa-Rajchert, D.; Parniakov, O.; Wiktor, A. Application of pulsed electric field prior to vacuum drying: Effect on drying time and quality of apple tissue. Res. Agric. Eng. 2022, 68, 93–101. [Google Scholar] [CrossRef]











| Temperature [°C] | PEF Input [kJ/kg] | Drying Time [min] | Drying Time Change [%] | |
|---|---|---|---|---|
| CD | DA | |||
| 55 | 0 | 336 | 209 | 38 |
| 1 | 351 | 227 | 35 | |
| 3 | 288 | 287 | 3 | |
| 5 | 301 | 190 | 37 | |
| 70 | 0 | 191 | 142 | 26 |
| 1 | 194 | 152 | 22 | |
| 3 | 207 | 152 | 27 | |
| 5 | 212 | 173 | 18 | |
| 85 | 0 | 140 | 126 | 10 |
| 1 | 126 | 112 | 11 | |
| 3 | 139 | 115 | 17 | |
| 5 | 123 | 108 | 12 | |
| Material | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|
| 55CD | 79.9 ± 3.2 d | −0.6 ± 0.5 a | 17.1 ± 0.9 a | 17.1 ± 0.9 a | 10.3 ± 2.8 a |
| PEF1_55CD | 77.8 ± 2.4 d | +2.0 ± 0.6 b | 24.9 ± 1.4 c | 25.0 ± 1.4 bc | 16.4 ± 2.4 a |
| PEF3_55CD | 50.5 ± 3.5 a | +6.4 ± 1.5 de | 21.3 ± 1.6 bc | 22.3 ± 1.8 bc | 40.5 ± 3.5 d |
| PEF5_55CD | 44.9 ± 8.1 a | +7.0 ± 0.6 de | 21.6 ± 2.2 bc | 22.7 ± 2.2 bc | 46.2 ± 7.4 d |
| 70CD | 75.1 ± 8.2 cd | −0.8 ± 0.5 a | 12.3 ± 1.5 a | 12.3 ± 1.5 a | 14.5 ± 8.3 ab |
| PEF1_70CD | 78.7 ± 6.5 d | +0.1 ± 0.4 ab | 15.5 ± 0.7 a | 15.5 ± 0.7 a | 11.1 ± 6.4 a |
| PEF3_70CD | 61.9 ± 6.5 bc | +4.2 ± 1.8 b | 21.0 ± 2.9 b | 21.5 ± 3.0 b | 29.1 ± 6.9 c |
| PEF5_70CD | 46.2 ± 3.1 a | +8.0 ± 1.0 f | 22.1 ± 2.2 bc | 23.6 ± 2.2 bc | 45.1 ± 3.0 d |
| 85CD | 77.6 ± 7.1 d | −0.7 ± 0.3 a | 13.9 ± 0.7 a | 13.9 ± 0.7 a | 11.9 ± 7.1 a |
| PEF1_85CD | 70.8 ± 6.6 cd | +1.3 ± 0.5 ab | 16.8 ± 1.9 a | 16.9 ± 2.0 a | 19.2 ± 6.7 ab |
| PEF3_85CD | 64.3 ± 6.1 c | +4.9 ± 1.7 cd | 23.0 ± 2.5 bc | 23.5 ± 2.5 bc | 27.6 ± 5.9 bc |
| PEF5_85CD | 52.8 ± 3.0 ab | +7.7 ± 1.0 f | 24.1 ± 2.9 bc | 25.3 ± 3.0 c | 39.2 ± 3.1 d |
| 55DA | 87.1 ± 1.5 B | +0.2 ± 0.4 A | 14.3 ± 0.5 A | 14.3 ± 0.5 A | 2.9 ± 1.4 A |
| PEF1_55DA | 81.9 ± 3.4 B | +0.6 ± 0.4 A | 17.0 ± 1.1 AB | 17.0 ± 1.1 ABC | 8.5 ± 3.5 AB |
| PEF3_55DA | 61.6 ± 4.5 A | +4.5 ± 1.0 BC | 21.3 ± 3.2 B | 22.2 ± 3.4 C | 29.1 ± 4.8 CD |
| PEF5_55DA | 60.6 ± 6.9 A | +3.7 ± 1.4 BC | 18.4 ± 1.6 AB | 18.6 ± 1.9 ABC | 29.7 ± 7.9 CD |
| 70DA | 87.6 ± 3.2 B | 0.0 ± 0.6 A | 14.3 ± 2.8 A | 14.3 ± 2.8 A | 3.9 ± 2.4 A |
| PEF1_70DA | 68.8 ± 7.4 A | +3.4 ± 1.3 B | 18.0 ± 1.5 AB | 18.3 ± 1.6 ABC | 21.7 ± 7.4 C |
| PEF3_70DA | 59.4 ± 9.8 A | +5.4 ± 1.6 BC | 20.9 ± 6.0 B | 20.7 ± 5.5 BC | 32.8 ± 9.5 D |
| PEF5_70DA | 68.5 ± 7.2 A | +3.0 ± 1.7 B | 16.4 ± 2.6 AB | 15.8 ± 1.9 AB | 18.8 ± 3.1 BC |
| 85DA | 84.3 ± 1.9 B | +0.4 ± 0.3 A | 15.9 ± 0.8 AB | 15.9 ± 0.8 AB | 5.9 ± 1.8 A |
| PEF1_85DA | 58.7 ± 8.3 A | +4.3 ± 1.5 BC | 16.3 ± 1.6 AB | 16.9 ± 1.8 ABC | 31.4 ± 8.5 CD |
| PEF3_85DA | 64.5 ± 4.2 A | +3.2 ± 0.4 B | 16.2 ± 1.6 AB | 16.5 ± 1.5 ABC | 25.5 ± 4.1 CD |
| PEF5_85DA | 67.1 ± 3.1 A | +3.1 ± 0.6 B | 18.5 ± 0.9 AB | 18.8 ± 0.8 ABC | 23.3 ± 2.8 CD |
| Material | TPC [mg GAE/g d.m.] | EC50 DPPH [mg d.m./mL] | EC50 ABTS [mg d.m./mL] |
|---|---|---|---|
| 55CD | 11.5 ± 0.5 abc | 0.84 ± 0.02 bcd | 0.19 ± 0.00 ab |
| PEF1_55CD | 12.4 ± 0.6 abcd | 0.71 ± 0.03 ab | 0.26 ± 0.00 cd |
| PEF3_55CD | 12.0 ± 0.2 abc | 0.76 ± 0.02 ab | 0.27 ± 0.01 d |
| PEF5_55CD | 12.7 ± 0.1 bcd | 0.71 ± 0.01 a | 0.25 ± 0.00 cd |
| 70CD | 10.9 ± 0.7 ab | 0.76 ± 0.02 ab | 0.21 ± 0.01 abc |
| PEF1_70CD | 12.7 ± 0.5 bcd | 0.78 ± 0.01 ab | 0.24 ± 0.01 bcd |
| PEF3_70CD | 17.6 ± 0.7 e | 0.70 ± 0.01 a | 0.18 ± 0.02 a |
| PEF5_70CD | 13.9 ± 0.7 d | 0.79 ± 0.01 abc | 0.23 ± 0.00 bcd |
| 85CD | 10.6 ± 0.2 a | 0.74 ± 0.03 ab | 0.21 ± 0.02 abc |
| PEF1_85CD | 13.1 ± 0.3 cd | 0.75 ± 0.00 ab | 0.23 ± 0.00 bcd |
| PEF3_85CD | 13.2 ± 0.1 cd | 0.97 ± 0.03 d | 0.24 ± 0.01 cd |
| PEF5_85CD | 13.2 ± 0.2 cd | 0.91 ± 0.05 cd | 0.26 ± 0.01 cd |
| 55DA | 12.1 ± 0.1 C | 0.60 ± 0.02 A | 0.26 ± 0.05 A |
| PEF1_55DA | 9.0 ± 0.1 ABC | 0.98 ± 0.03 CDE | 0.73 ± 0.04 BCD |
| PEF3_55DA | 5.6 ± 0.1 A | 0.97 ± 0.01 CDE | 1.11 ± 0.00 D |
| PEF5_55DA | 8.8 ± 0.0 ABC | 0.98 ± 0.00 CDE | 0.76 ± 0.04 BCD |
| 70DA | 12.3 ± 0.0 C | 0.60 ± 0.02 AB | 0.61 ± 0.04 ABC |
| PEF1_70DA | 5.9 ± 0.1 AB | 1.26 ± 0.05 E | 0.81 ± 0.07 BCD |
| PEF3_70DA | 10.1 ± 1.9 C | 0.65 ± 0.17 AB | 0.52 ± 0.21 AB |
| PEF5_70DA | 6.0 ± 0.3 AB | 1.18 ± 0.05 DE | 0.96 ± 0.07 CD |
| 85DA | 11.1 ± 0.2 C | 0.69 ± 0.01 ABC | 0.69 ± 0.06 ABCD |
| PEF1_85DA | 9.7 ± 0.2 BC | 0.90 ± 0.00 ABCD | 0.71 ± 0.02 ABCD |
| PEF3_85DA | 10.1 ± 0.2 C | 0.96 ± 0.01 BCDE | 0.76 ± 0.02 BCD |
| PEF5_85DA | 5.7 ± 0.2 AB | 1.13 ± 0.01 DE | 0.96 ± 0.03 CD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadan, M.; Barańska, A.; Matys, A.; Rybak, K.; Witrowa-Rajchert, D.; Wiktor, A.; Nowacka, M. Impact of Pulsed Electric Field Treatment on the Process Kinetics and Selected Properties of Air and Dehumidified Air-Dried Mushrooms. Processes 2023, 11, 2101. https://doi.org/10.3390/pr11072101
Dadan M, Barańska A, Matys A, Rybak K, Witrowa-Rajchert D, Wiktor A, Nowacka M. Impact of Pulsed Electric Field Treatment on the Process Kinetics and Selected Properties of Air and Dehumidified Air-Dried Mushrooms. Processes. 2023; 11(7):2101. https://doi.org/10.3390/pr11072101
Chicago/Turabian StyleDadan, Magdalena, Alicja Barańska, Aleksandra Matys, Katarzyna Rybak, Dorota Witrowa-Rajchert, Artur Wiktor, and Małgorzata Nowacka. 2023. "Impact of Pulsed Electric Field Treatment on the Process Kinetics and Selected Properties of Air and Dehumidified Air-Dried Mushrooms" Processes 11, no. 7: 2101. https://doi.org/10.3390/pr11072101
APA StyleDadan, M., Barańska, A., Matys, A., Rybak, K., Witrowa-Rajchert, D., Wiktor, A., & Nowacka, M. (2023). Impact of Pulsed Electric Field Treatment on the Process Kinetics and Selected Properties of Air and Dehumidified Air-Dried Mushrooms. Processes, 11(7), 2101. https://doi.org/10.3390/pr11072101










