Abstract
Scutellaria baicalensis is the most studied species of the genus, while Scutellaria pycnoclada is a poorly studied endemic species. Ten lines of the hairy roots of S. pycnoclada were obtained using Agrobacterium rhizogenes A4. The hairy root cultures of S. pycnoclada and the previously obtained roots of S. baicalensis were cultured on liquid and agar Gamborg media. A total of 14 flavonoids were detected via HPLC MS/MS in S. pycnoclada, and 17 were detected in S. baicalensis. Among them were flavones characteristic of both the roots and the aboveground parts of the plants. S. pycnoclada had a lower diversity of methylated flavones than S. baicalensis. Moreover, tenaxin I was absent in all S. pycnoclada lines on agar medium. HPLC analysis revealed that the flavone content in the different hairy root lines was 1.4–12.7 times higher on liquid medium than on agar medium. S. baicalensis and S. pycnoclada differed significantly in the ratio of the main flavones. In S. baicalensis, baicalin (7.83 mg/g DW) and wogonoside (6.29 mg/g DW) dominated when cultured on liquid medium, and wogonin (2.08 mg/g DW) dominated when cultured on solid medium. In S. pycnoclada, baicalin predominated (52–88% of the total content). S. pycnoclada is assumed to have a different set of O-methyltransferases and less biosynthetic enzyme activity than S. baicalensis.
1. Introduction
The genus Scutellaria belongs to the family Lamiaceae, includes more than 360 plant species, and is very diverse. The genus Scutellaria is rich in flavones, which are flavonoid metabolites derived from the phenylpropanoid biosynthesis pathway. All flavonoids usually have the same basic skeleton, consisting of two 6-C rings (A and B) connected by a 3-C bridge, which usually forms a third ring (C ring), as in flavones. Scutellaria baicalensis is the best known and most studied species of the genus. It is widely used in the traditional medicine of China, Japan, and Korea. S. baicalensis has antibacterial, anticancer, antiviral, and antiallergic properties [1]. The medicinal properties of S. baicalensis have been attributed to the unique root-specific 4′-desoxyfavones, which lack the 4′-hydroxyl group on their B-rings [2]. A specialized biosynthesis pathway of these unique biologically active 4′-desoxyfavones, including the aglycones baicalein and wogonin and their glycosides baicalin and wogonoside, respectively, has been decoded [3]. Currently, the less common species of the genus Scutellaria are being actively characterized and studied. Such species include Scutellaria pycnoclada, endemic to Kyrgyzstan and Uzbekistan. It has been shown to be capable of synthesis 16 flavones, including three 4′-desoxyfavones—baicalein, baicalin, and their precursor chrysin [4]. However, not even theoretical data are available concerning what biosynthetic pathways operate in this species. Since S. baicalensis and S. pycnoclada have a number of flavones in common, S. pycnoclada may have similar medicinal properties.
S. baicalensis grows over a wide area, in contrast to S. pycnoclada, but in both cases, the range and content of flavones is influenced by external environmental conditions. Therefore, it is advisable to conduct studies under in vitro conditions. Since the synthesis of pharmacologically important flavones in skullcaps takes place in the roots, it could be especially promising to identify and investigate the cultures of the hairy roots. These are obtained via transformation with Agrobacterium rhizogenes. Hairy roots are capable of rapid, neoplastic growth on hormone-free nutrient media and have a relatively high ability of synthesis secondary metabolites. The studies on flavone composition in hairy root cultures of the genus Scutellaria are mainly focused on the determination of the content of baicalin, baicalein, wogonin, wogonoside, scutellarein, and chrysin using the HPLC method [5,6,7]. However, in addition to the secondary metabolites present in intact plants, the synthesis of a variety of compounds of different nature that are not characteristic of intact roots has been repeatedly registered in hairy roots [8,9]. Moreover, only one study, performed on several S. baicalensis hairy roots lines obtained through transformation with an A. rhizogenes strain containing the endogenous β-glucoronidase gene, showed the complex study and identification of flavones [10]. Thus, there are no data to compare the qualitative composition of the flavones of several hairy root lines to those of other species of the genus Scutellaria.
In our previous study, we obtained a hairy root culture of S. pycnoclada, in which 14 flavones were detected during its cultivation on a liquid medium [11]. It produced the highest amount of baicalin compared to all other cultivars of the genus Scutellaria, including S. baicalensis. There is evidence that the biochemical differences between hairy root lines are derived from a single strain of A. rhizogenes, which could be related to the amount of transferred genetic material, the site of the integration of T-DNA into a plant genome, or the cultivation conditions [12,13]. It should be noted that no attempt has previously been made to compare the qualitative content of flavones in hairy roots cultured on liquid and agar media. At the same time, the screening of the qualitative composition of secondary metabolites in the obtained in vitro culture lines could make it possible to identify new valuable metabolites, which could be used in biotechnology in the future for the production of functional food products.
As was shown previously, the hairy roots of S. baicalensis and S. pycnoclada cultivated on agar and in liquid nutrient media differed significantly from each other in terms of the content of the main flavones [11]. However, the qualitative composition of flavonoids in hairy roots growing on the media of different densities has not yet been compared.
The aim of the present work was to obtain several lines of the hairy roots of S. pycnoclada in order to carry out the qualitative and quantitative analysis of flavones in roots cultivated on liquid and agar nutrient media, to compare these parameters with those of the hairy roots of S. baicalensis, and to reveal the regularities of flavone biosynthesis in these cultures. Furthermore, a comparison of the range of metabolites between S. baicalensis and S. pycnoclada in the absence of data on the S. pycnoclada genome will allow a number of assumptions to be made on the biosynthesis pathways operating in the hairy roots of the latter.
2. Materials and Methods
2.1. Establishment of S. pycnoclada Transformed Root Cultures
The hairy roots of S. pycnoclada were obtained as has been described previously [11]. The seeds of Scutellaria pycnoclada Juz. were sterilized using a 0.1% diocide solution (composition: ethylmercuric chloride, cetylpyridinium chloride) for 10 min. Then, they were rinsed with sterilized water. The treated seeds were transferred onto Murashige–Skoog medium with 5 g/L sucrose and 8 g/L agar (26 ± 1 °C, 16 h of light daily). The wild strain of Agrobacterium rhizogenes A4 was used to obtain the hairy roots clones. Agrobacteria were transferred to fresh YEB medium containing 8 g/L of agar before inoculation and incubated for 48 h at 26 ± 1 °C. The bacteria were then transferred to Gamborg nutrient medium (B5) supplemented with sucrose (20 g/L) for co-cultivation with plant explants. The segments of the cotyledons and hypocotyls of 3–4 week old seedlings were used, which were injured via an insulin syringe. The explants were incubated in agrobacterial suspension for 12 h at 23 ± 1 °C on a rotary shaker (90 rpm), in the dark. Then, for the elimination of agrobacterium, the explants were transferred to B5 medium containing 500 mg/L of cefotaxime (Claforan, Wockhardt, UK), 20 g/L of sucrose, and 8 g/L of agar (26 ± 1 °C, light). Each newly emerged root was placed on a single 90 mm diameter Petri dish with hormone-free B5 medium containing 250 mg/L of cefotaxime, 20 g/L of sucrose, and 8 g/L of agar (23 ± 1 °C, dark).
The obtained root lines after two passages on agar B5 medium were placed in a liquid medium of the same composition (flask to medium ratio 100:20) with 250 mg/L of cefotaxime and 20 g/L of sucrose (23 ± 1 °C, 90 rpm, dark). The root cultures were transferred to antibiotic-free medium after four weeks of cultivation. The cultivation cycle was four weeks. In parallel, the resulting root lines were grown on B5 medium containing 20 g/L sucrose and 8 g/L agar (23 ± 1 °C, dark). The roots were also replanted every four weeks.
2.2. PCR Analysis
The total DNA from root culture lines was isolated using a modified CTAB technique in which polyvinylpyrrolidone is added to the samples to avoid polyphenols binding to the DNA strands [14]. DNA concentration in the samples was evaluated on an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
The presence of rolA, rolB, rolC, rolD, aux2, and VirD genes was assessed via PCR analysis using appropriate primers (Table 1). The rol PCR reaction was performed in a final volume of 25 µL containing 40 ng of DNA, 0.2 mM of each dNTP, 2.5 µL of 10× PCR buffer (Qiagen, Velno, The Netherlands) containing 1.5 mM MgCl2, 2.5 U Taq DNA polymerase (Qiagen), and 0.3 µL of each primer. Amplification was carried out in an MC2 programmable thermocycler (DNA Technology, Moscow, Russia). The amplification program included: initial denaturation for 2 min at 94 °C; then 5 cycles of denaturation for 20 s at 94 °C, annealing for 10 s at 54–66 °C, elongation for 10 s at 72°C; then 40 cycles of denaturation for 5 s at 94 °C, annealing for 5 s at 54–62 °C, elongation for 5 s at 72 °C; and final elongation for 2 min at 72 °C (Table 1). The amplification products were separated via electrophoresis in a 2% agarose gel in 1× TBE buffer. DNA fragment lengths were determined using a 100 bp or 1 kb DNA Ladder Mix molecular weight marker (Eurogen, Moscow, Russia). The gels were stained with ethidium bromide (5 µg/mL) for 10 min immediately after the electrophoresis had been completed. Nucleic acid fluorescence was observed in UV light at 58 wavelength 302 nm in a TM-36 transilluminator.

Table 1.
Nucleotide sequence of primers used for the detection of Agrobacterium rhizogenes genes in hairy root cultures.
2.3. HPLC-UV Analysis
The biomass of both S. pycnoclada and S. baicalensis hairy root lines taken at the end of the cultivation cycle was lyophilized. Afterwards, the samples were extracted with methanol according to the previously published procedure [11]. The extraction was performed with methanol (1:100 biomass:extractant ratio) in an FS14H ultrasonic bath (Fisher Scientific, Waltham, MA, USA) for 180 min, then 1 mL of the extract was taken and centrifuged for 10 min at 8000 rpm. The HPLC system, chromatographic column with its dimensions, injection volume, flow rate, the gradient program, and the acquisition of the UV spectra were same as described before [11]. The contents of the studied flavones were determined using the calibration curves (Figure S1).
2.4. HPLC-MS/MS Analysis
A mass spectrometric study was carried out with the obtained methanol extracts. The mass spectrometric analysis was performed on a tandem mass spectrometer by Thermo TSQ Endura (Thermo Fisher Scientific, San Jose, CA, USA). The analysis was carried out in the full-scan (for detecting peaks with maximum intensity) and MRM (multiple reaction monitoring) modes (for analyzing fragment ions, and for identifying compounds). The test substances were determined via electrospray ionization in the positive region.
2.5. Statistical Analysis
The data were statistically processed using Microsoft® Excel. The text gives the arithmetic mean values of the parameters. The bars in the chart correspond to the maximum values of the confidence intervals at the 95% confidence level according to Student’s t-test. All the experiments were repeated at least three times.
3. Results and Discussion
3.1. Establishment of Hairy Root Lines of S. pycnoclada
The roots were formed within 2–4 weeks of the co-culturing of the explants with A. rhizogenes. The resulting roots were isolated and cultured individually. During the cultivation on hormone-free media, some of the root lines became dark brown and stopped their growth. A similar result was observed, for example, when the hairy root lines of Saussurea involucrata were obtained [15]. Ten lines were used for further investigation: L0, L3, L7, L10, L12, L17, L19, L21, L22, and L23 (Figure 1). The roots of these lines were thin, with light yellow coloring on the young growing parts and a brown coloring on the older ones, as well as in S. baicalensis (Figure 1). Such root culture coloring is characteristic of members of the genus Scutellaria [11,16,17].

Figure 1.
Lines of hairy roots of S. pycnoclada (L0–L23) and hairy roots of S. baicalensis (S.b.).
3.2. The Presence of Different Genes of TL- and TR-DNA in Hairy Roots Clones of S. baicalensis and S. pycnoclada
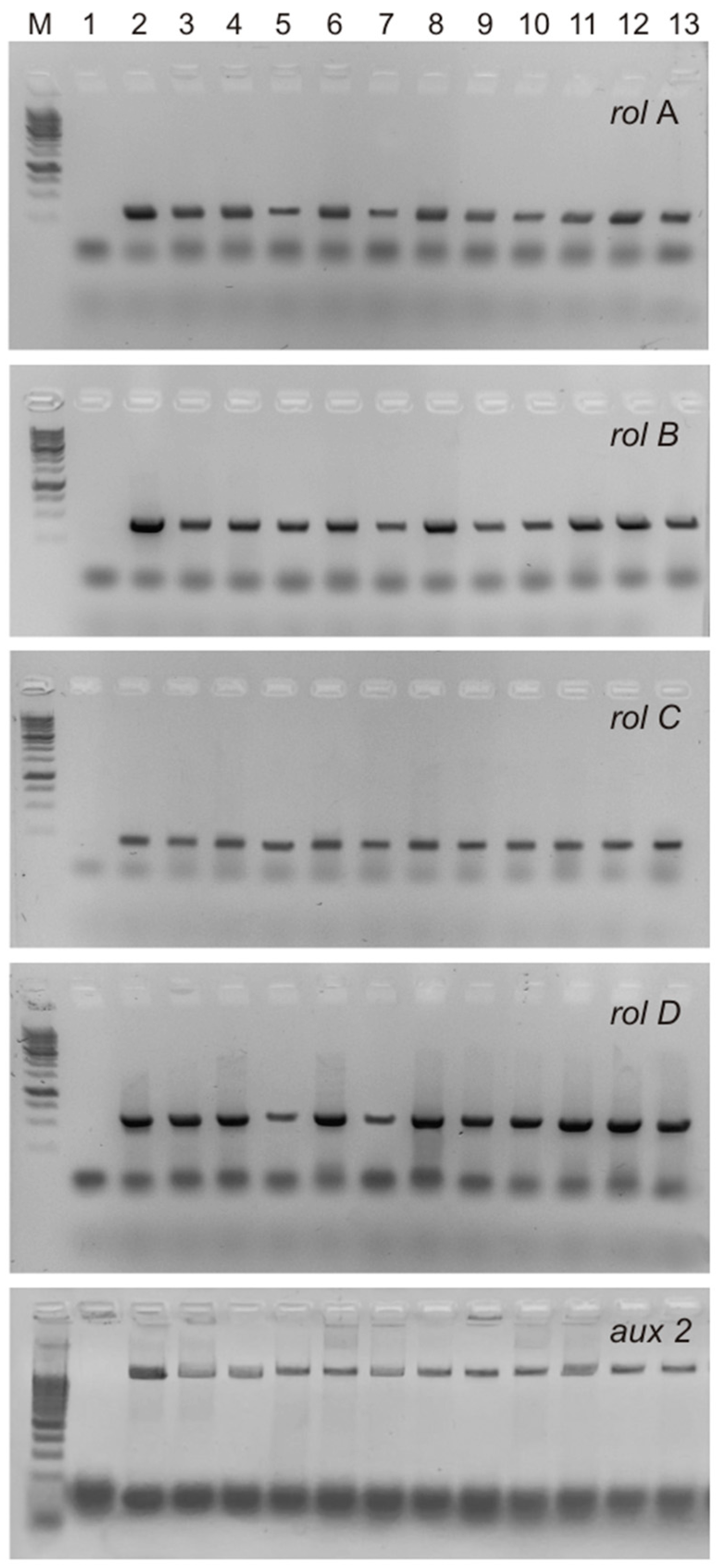
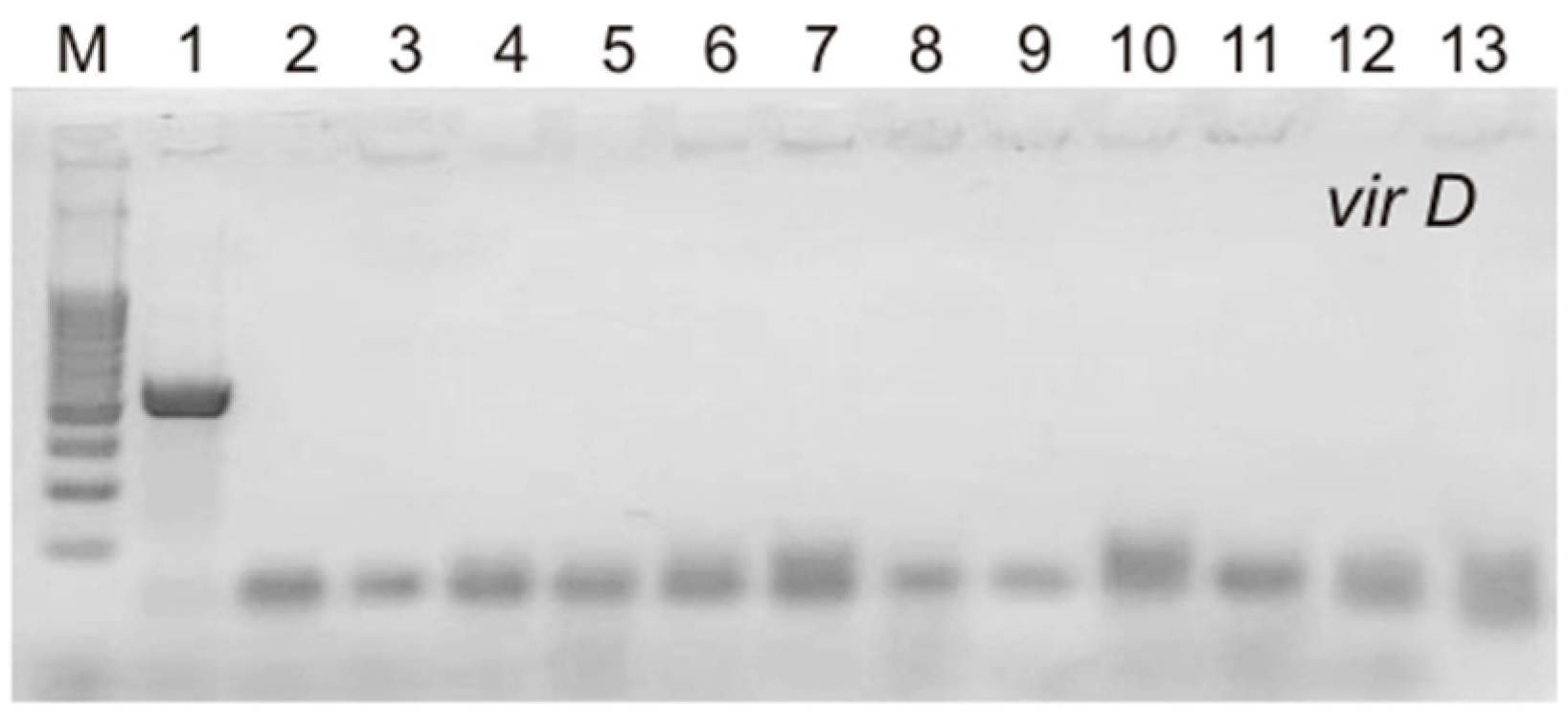
The T-DNA of the Ri plasmids of A. rhizogenes agropin strains, including the A4 strain used in this study, contains two parts, TL and TR, both of which are transferred into the plant genome independently of each other. Four rol genes (rolA, rolB, rolC, and rolD) located on the TL-DNA influence the formation of the roots, their morphology, and the level of accumulation of secondary metabolites. TR-DNA carries genes for auxin (aux1 and aux2) and agropin (ags) synthesis. It has been shown that in the genome of cultures obtained via transformation with A. rhizogenes, the TL- and TR-DNA genes could be partially or completely absent [18,19,20]. The presence of rolA, rolB, rolC, rolD, and aux2 (Figure 2) was confirmed via PCR in all hairy root lines in our study. Thus, all S. pycnoclada lines and hairy roots of S. baicalensis contained both TL-DNA and TR-DNA. None of the lines showed the presence of the virD gene, which is located on a plasmid outside T-DNA and is not transferred into the plant genome. This confirms that there was no contamination of the obtained lines with A. rhizogenes root cultures (Figure 3).
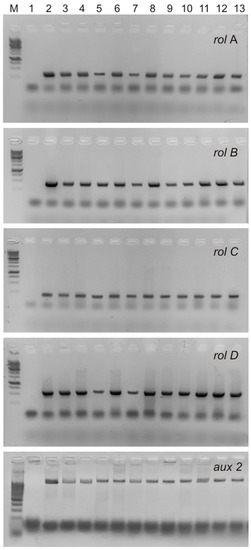
Figure 2.
PCR products of the amplification of the rol-genes and aux2 primers in hairy roots of S. pycnoclada and S. baicalensis. M—DNA marker (100 bp ladder); 1—negative control (water); 2—plasmid DNA of A. rhizogenes strain A4 (positive control); 3–12—genomic DNA of hairy roots lines of S. pycnoclada (L0, L3, L7, L10, L12, L17, L19, L21, L22, and L23, respectively); 13—genomic DNA of hairy roots of S. baicalensis.
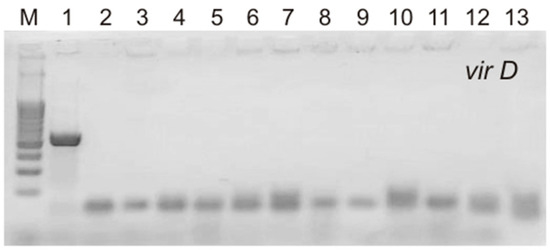
Figure 3.
PCR products of the amplification of the virD primers in hairy roots of S. pycnoclada and S. baicalensis. M—DNA marker (100 bp ladder); 1—plasmid DNA of A. rhizogenes strain A4 (positive control); 2—negative control (water); 3–12—genomic DNA of hairy roots lines of S. pycnoclada (L0, L3, L7, L10, L12, L17, L19, L21, L22, and L23, respectively); 13—genomic DNA of hairy roots of S. baicalensis.
3.3. The Qualitative Composition of Flavones
A total of 14 flavonoids were detected by HPLC MS/MS in the S. pycnoclada hairy roots and 17 in the hairy roots of S. baicalensis (Table 2). They included a number of flavones characteristic of the roots of plants of the genus Scutellaria: chrysin, baicalein, oroxylin A, wogonin, tenaxin I, scullcapflavone II, chrysin-6-C-β-d-glucoside/chrysin-8-C-β-d-glucoside, chrysin-7-O-β-d-glucuronide, oroxin A, baicalin, wogonoside, and viscidulin III 6-O-β-d-glucoside. The monomethoxyflavone oroxylin A and polymethoxyflavone scullcapflavone II were missing in the hairy roots of S. pycnoclada. The flavonoids synthesized in the above-ground part of the plants were also recorded in the hairy roots of both S. pycnoclada and S. baicalensis: apigenin, apigetrin, scutellarin, and isocarthamidin-7-O-β-d-glucuronide/carthamidin-7-O-β-d-glucuronide. Naringenin was only detected in S. baicalensis roots. S. baicalensis is known to have two flavone biosynthesis pathways: the above-ground pathway involving FNSII-1 (flavones-synthase) enzyme and the root-specific pathway involving FNSII-2. FNSII-1 converts naringenin to apigenin in the aerial parts of the plant. FNSII-2 is evolutionarily non-functional FNSII and can only convert pinocembrin to chrysin and is highly expressed in roots [3]. The presence of both FNSII isoforms was later shown in Scutellaria barbata [21]. Thus, the two flavone biosynthesis pathways are active in both S. baicalensis and S. pycnoclada hairy roots. No data are currently available on the range of flavone biosynthesis enzymes in S. pycnoclada. However, a comparison of the flavone composition of S. baicalensis and S. pycnoclada leads to a number of assumptions. Firstly, enzymes of both flavone biosynthesis pathways are present and expressed in the hairy roots of S. pycnoclada, as in S. baicalensis. Secondly, as S. pycnoclada has a narrow range of methylated flavones, namely the monomethoxyflavone wogonin, its glucoronide wogonoside, and the trioxymethylated flavone tenaxin I, it probably has a different set of O-methyltransferases (OMT) than S. baicalensis. Two types of OMTs have been shown to be involved in flavone biosynthesis in S. baicalensis. The second type of OMTs are Mg2+-dependent and are also known as phenylpropanoid and flavonoid OMTs (PFOMTs). In the roots of S. baicalensis, SbPFOMT2 and SbPFOMT5 can effectively O-methylate the C6, C8, and C3 positions of flavones to form monomethoxyflavones, including oroxylin A and wogonin [22,23]. Since these flavones have been recorded in the hairy roots of S. pycnoclada, the analogues of SbPFOMT2 and SbPFOMT5 are probably also present in them. Three type I OMTs, called flavonoid OMTs (SbFOMTs), were found in S. baicalensis. SbFOMT3 is a 7-OMT that converts baicalein to 7-methoxybaicalein [24]. SbFOMT6 is a 7-OMT that can use both baicalein and norwogonin as its substrates. SbFOMT5 can methylate the hydroxyl groups in baicalein at the C5, C6, and C7 positions. The combination of SbPFOMT5 and SbFOMT6 or SbPFOMT5 plus SbFOMT5 can result in the formation of scullcapflavone I and tenaxin I, respectively. Only tenaxin I was found among the polymethoxylated flavones in the hairy roots of S. pycnoclada; therefore, only one type I OMT, the SbPFOMT5 analogue, is probably present or expressed in them. Thirdly, the presence of oroxin A—baicalein 7-O-glucoside in S. pycnoclada indicates the presence of glucosyltrasferases (UGATs), responsible of transferring the sugar residue at position 7 to baicalein [24]. It should be noted that in an earlier study by Malikov and Yuldashev [4] on the analysis of flavones in intact S. pycnoclada plants, metoxylated wogonin, tenaxin I, and wogonoside were not reported along with oroxin A and viscidulin III 6-O-β-d-glucoside. This could be due to the different activity of the corresponding enzymes of their biosynthesis in the plant under natural conditions and in the obtained hairy roots.

Table 2.
Identification of flavones in the hairy roots of S. pycnoclada and S. baicalensis via HPLC-MS/MS.
The qualitative composition of flavones in the hairy roots of S. baicalensis when cultured on both liquid and agar media was the same. S. pycnoclada lines growing on liquid medium also did not differ in their flavone composition, but when cultured on agar medium they lacked tenaxin I (Table 3). This could be due to the absence or low expression of the SbFOMT5 analogue in S. pycnoclada roots when cultured on agar medium. Moreover, in the L10 line wogonoside was present only in trace amounts. This could be a consequence of the low biosynthetic activity of this line as well as abnormalities caused by the effect of the T-DNA insertion position. Finding out the cause requires further investigation.

Table 3.
Flavones in S. pycnoclada and S. baicalensis hairy roots cultured on liquid and agar media.
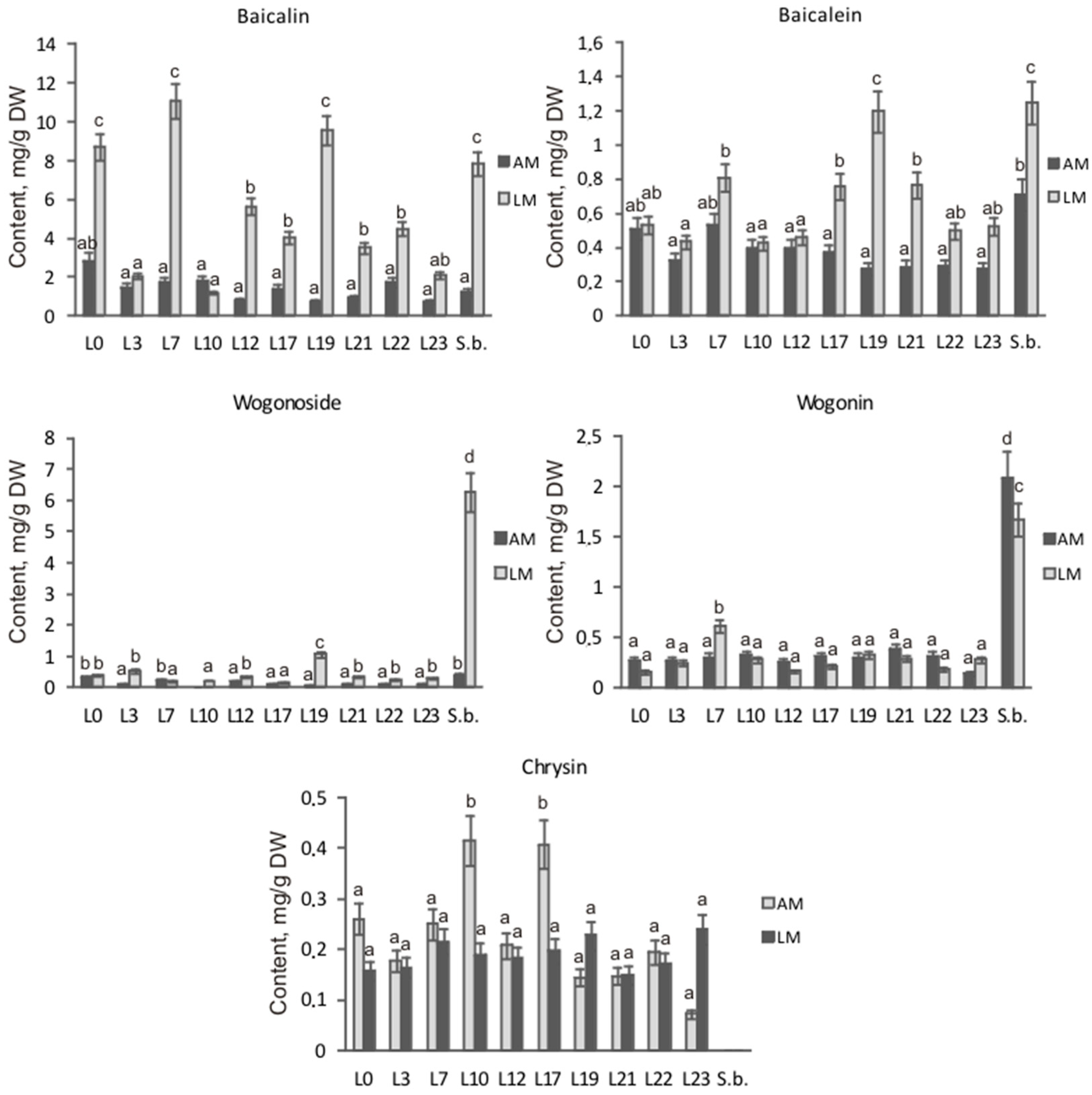
3.4. Content of the Main Flavones
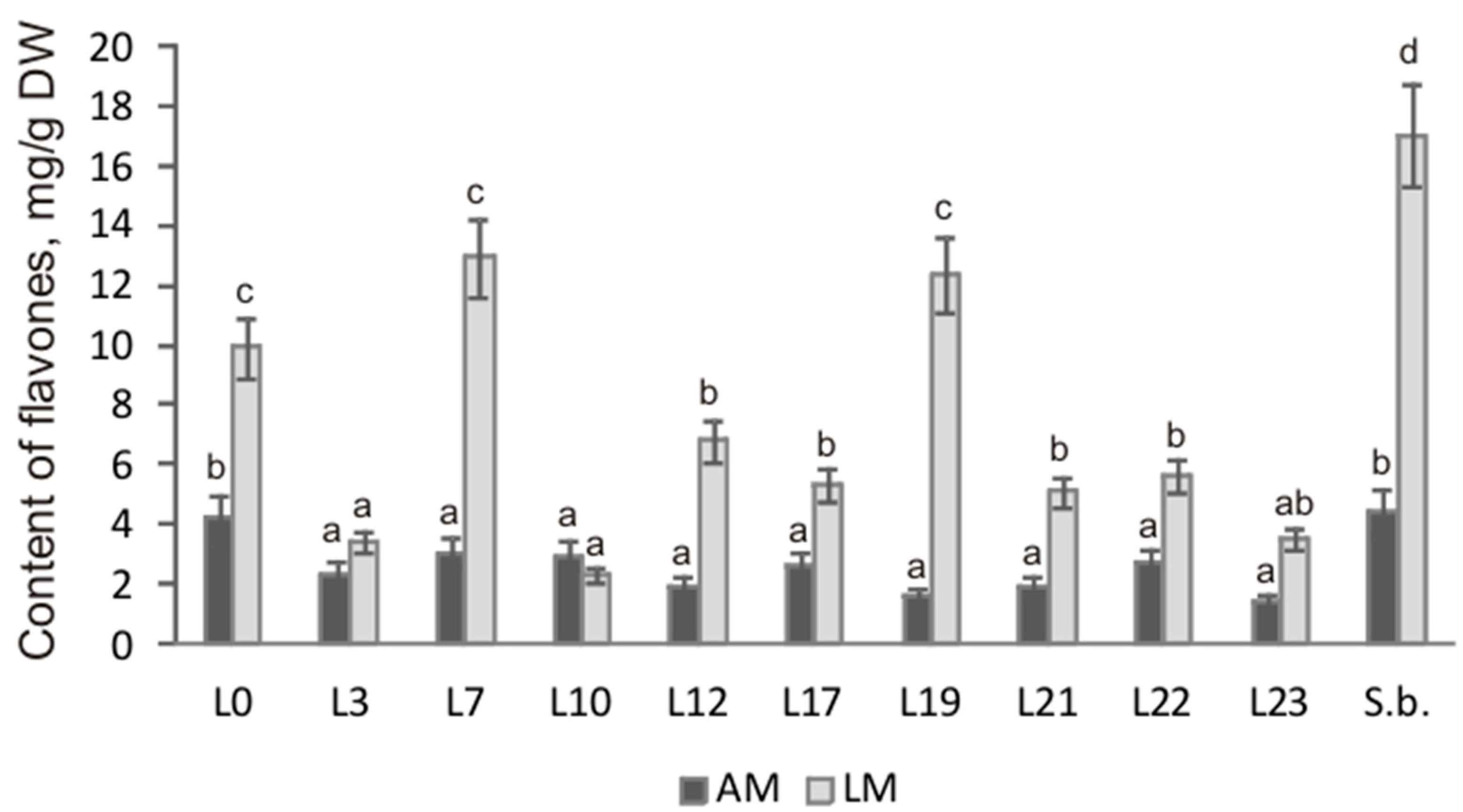
By means of HPLC analysis, it was shown that cultures of hairy roots of S. pycnoclada and S. baicalensis differed significantly in the content of main root-specific flavones both among themselves and depending on the culture medium (Figure 4). The highest total content of the main flavones, 17.04 mg/g DW, was observed in S. baicalensis hairy roots culturing on the liquid medium. The maximum flavone content was slightly lower in S. pycnoclada (lines L7 and L19 were 12.93 and 12.37 mg/g DW, respectively). Generally, the flavone content in the different lines of hairy roots was 1.4–12.7 times higher on liquid medium than on agar medium. The exception was line L10, in which a change in the cultivation conditions had no significant effect on the total flavone content. Remarkably, a similar result was obtained in our previous work for the hairy roots of S. przewalskii (Stepanova et al., 2021). Normally, using a liquid nutrient medium provides a higher level of secondary metabolite synthesis than culturing on an agar medium. This has been shown repeatedly, e.g., for plants of the genus Scutellaria: S. alpina, S. baicalensis, and S. lateriflora [11,25,26].
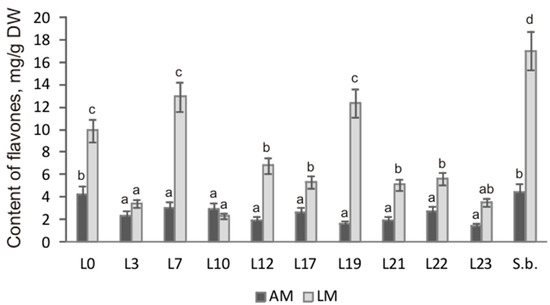
Figure 4.
Total content of the main flavones in the hairy roots of S. pycnoclada (L0–L23) and S. baicalensis (S.b.). AM—agar medium; LM—liquid medium. Different letters indicate a significant difference between the means (one-way ANOVA, p < 0.05).
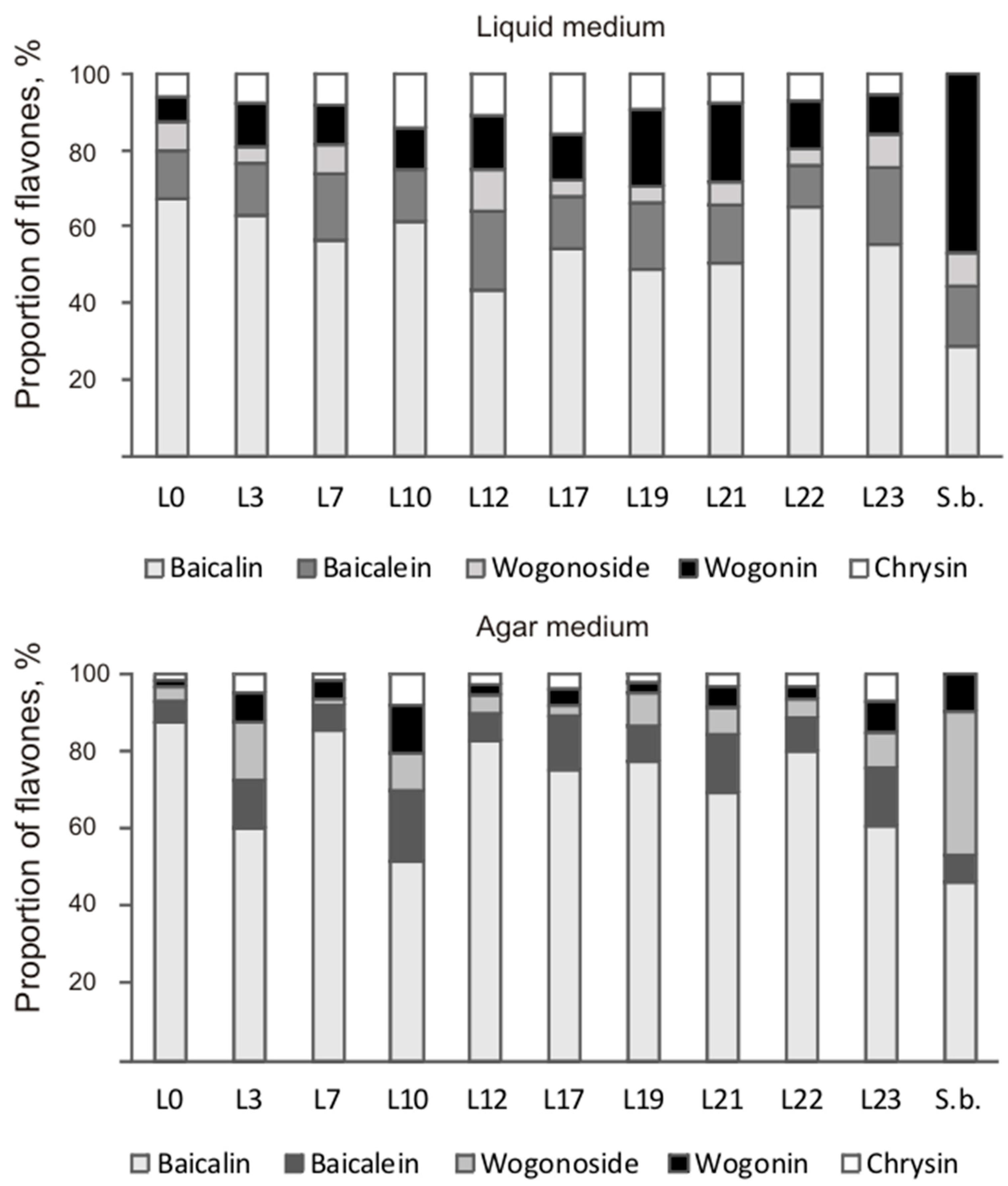
Despite similar total flavones content in the hairy roots of both species, they differed dramatically in the ratio of the main root-specific flavones (Figure 5). The content of wogonoside—6.29 mg/g DW—in hairy roots of S. baicalensis was similar to that of baicalin—7.83 mg/g DW—on liquid medium, while on solid medium, the predominant flavone was wogonin (2.08 mg/g DW). The main contribution in total content of flavones of the obtained S. pycnoclada hairy root lines on both liquid and solid media was made by baicalin (52–88% of the total content). In lines L0, L7, and L19, the baicalin content was significantly higher on liquid medium than in S. baicalensis culture (Figure 6). The content of methoxylated wogonoside and wogonin was low in all resulting S. pycnoclada hairy root lines regardless of the cultivation medium (0–0.38 mg/g DW). The exception was line L19, which contained 1.06 mg/g DW wogonoside when cultured on liquid medium. It should be mentioned that the predominance of the synthesis of baicalin and baicalein over that of wogonoside and wogonin in the roots is characteristic of most of the studied plant species of the genus Scutellaria. However, in hairy roots obtained by transformation with A. rhizogenes strain A4, an equivalent or greater wogonoside content is usually observed than that of baicalin [5,7,11,27]. Baicalein is known to be formed in S. baicalensis through the 6-C hydroxylation of chrysin via SbF6H. The formation of wogonin occurs in two stages. Firstly, chrysin is converted to norwogonin by SbF8H through 8-C hydroxylation. Then, 8-O-methyltransferase (8-OMT) transfers the methyl group to norwogonin [3,22]. Thus, an increase in wogonoside and wogonin content in the hairy roots of S. baicalensis is probably associated with a change in the regulation of root-specific flavone biosynthesis and its shift towards this branch. Although baicalin is the predominant flavone in the obtained S. pycnoclada hairy root lines, the fact that wogonoside and wogonin were not previously recorded in the intact plants could indirectly indicate an increased expression of SbF8H and 8-OMT analogues in them. The presence of only trace amounts of chrysin in the hairy roots of S. baicalensis combined with the high content of root-specific flavones derived from it suggests a higher biosynthetic enzyme activity than in the obtained S. pycnoclada lines.
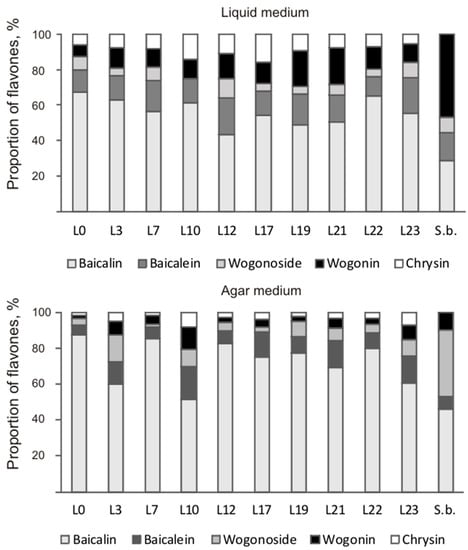
Figure 5.
The ratio of the main flavones in S. pycnoclada (L0–L23) and S. baicalensis (S.b.) hairy roots cultured on liquid and agar media.
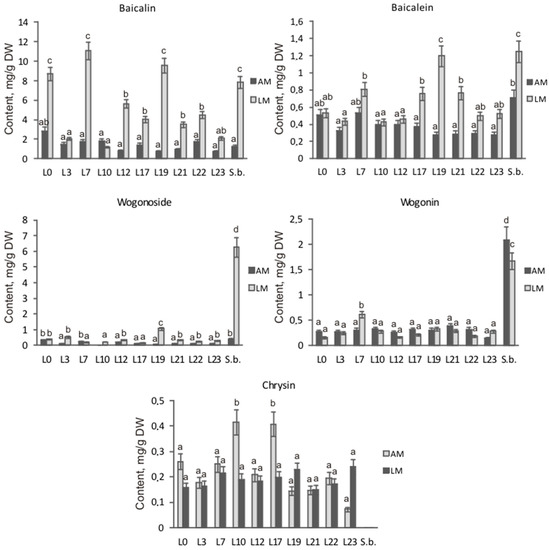
Figure 6.
Content of the main flavones in the hairy roots of S. pycnoclada (L0–L23) and S. baicalensis (S.b.) cultured on liquid and agar media. AM—agar medium; LM—liquid medium. Different letters indicate a significant difference between the means (one-way ANOVA, p < 0.05).
Aglycon flavones are known to be more hydrophilic and to have greater physiological activity than glucuronides. We have previously shown that the ratio of aglycones in the total flavones content was significantly higher when growing S. baicalensis hairy roots and calli on solid media than on liquid media. A similar result was obtained by other researchers in an in vitro culture of Scutellaria alpine shoots cultivated on liquid and agar nutrient media [25]. In the present study, all hairy root lines of S. pycnoclada, except L3, L10, and L23, had 1.9 to 3.8 times higher percentage of aglycones on an agar medium than on a liquid medium, and S. baicalensis had a 3.7 times higher percentage on agar medium. The conversion of baicalein/wogonin to baicalin/wogonoside in S. baicalensis is performed via baicalein 7-O-glucuronosyltransferase (UBGAT) and baicalinase (endogenous β-glucuronidase or sGUS) [28,29,30]. UBGAT catalyzes the transfer reaction of a glucuronic acid residue to baicalein/wogonin, resulting in the formation of baicalin/wogonoside. The reverse transition is carried out by detaching the glycosidic part of baicalin and wogonoside via sGUS. We have identified no correlation between total aglycone content and sGUS activity in the in vitro cultures of S. baicalensis when cultivated on solid media [26]. Therefore, the higher proportion of aglycones in hairy roots grown on agar media could be related to a lower substrate availability rather than to their cultivation on liquid media and, as a consequence, a lower UBGAT activity. It should be noted that the lack of a significant effect of cultivation conditions on the flavones content, as in L3, L10, and L23 lines, had already been shown in previous works for S. przewalskii hairy roots [11]. Further research should be undertaken to uncover the cause of this phenomenon.
4. Conclusions
A qualitative and quantitative analysis of flavones in the hairy roots of both S. baicalensis and S. pycnoclada during the cultivation on liquid and agar media showed that above-ground as well as the root-specific flavone biosynthesis pathways are operating in these plants. While the total flavone content in the hairy roots of both species was similar, S. pycnoclada lines showed a narrow range of methylated flavones, probably due to a lower diversity of O-methyltransferases in them than in S. baicalensis. On liquid medium, baicalin and wogonoside dominated in the hairy roots of S. baicalensis, while wogonin was dominant on solid medium. Unmethylated baicalin dominated in the S. pycnoclada hairy root lines on both liquid and solid media. Thus, in the hairy roots of S. baicalensis, the wogonin and wogonoside biosynthesis branch was more active than in S. pycnoclada. The presence of only trace amounts of chrysin in the hairy roots of S. baicalensis with a high content of root-specific flavones produced from it suggests a higher biosynthetic enzyme activity than in the obtained S. pycnoclada lines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11072102/s1, Figure S1: Calibration curve for HPLC-UV.
Author Contributions
A.I.S. and A.Y.S. developed the concept, designed the experiments, and wrote the article; E.A.G. analyzed the data; A.Y.S. and Y.M.P. carried out the experimental work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Science of the Russian Federation (Topic No. 121050500047-5).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are grateful to: Malunova, M.V. (K.A. Timiryazev Institute of Plant Physiology, Russian Academy of Sciences); Lelishentsev A.A. (Bioanalytical Laboratory, Institute of Clinical Research and Pharmaceutical Expertise).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shah, M.; Mubin, S.; Hassan, S.S.U.; Tagde, P.; Ullah, O.; Rahman, M.H.; Al-Harrasi, A.; Rehman, N.U.; Murad, W. Phytochemical profiling and bio-potentiality of genus Scutellaria: Biomedical approach. Biomolecules 2022, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, Y.; Wang, G.; Hill, L.; Weng, J.K.; Chen, X.Y.; Xue, H.; Martin, C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016, 2, e1501780. [Google Scholar] [CrossRef]
- Malikov, V.M.; Yuldashev, M.P. Phenolic compounds of plants of the Scutellaria genus. distribution, structure, and properties. Chem. Nat. Compd. 2002, 38, 473–519. [Google Scholar] [CrossRef]
- Kovács, G.; Kuzovkina, I.; Szoke, É.; Kursinszki, L. HPLC determination of flavonoids in hairy-root cultures of Scutellaria baicalensis Georgi. Chromatographia 2004, 60, 81–85. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Trivedi, M.; Guang, Z.C.; Guo, G.Q.; Zheng, G.C. Agrobacterium rhizogenes mediated transformation of Scutellaria baicalensis and production of flavonoids in hairy roots. Biol. Plant. 2008, 52, 26–35. [Google Scholar] [CrossRef]
- Wilczańska-Barska, A.; Królicka, A.; Głód, D.; Majdan, M.; Kawiak, A.; Krauze-Baranowska, M. Enhanced accumulation of secondary metabolites in hairy root cultures of Scutellaria lateriflora following elicitation. Biotechnol. Lett. 2012, 34, 1757–1763. [Google Scholar] [CrossRef]
- Wysokinska, H.; Chmiel, A. Transformed root cultures for biotechnology. Acta Biotechnol. 1997, 17, 131–159. [Google Scholar] [CrossRef]
- Abhyankar, G.; Rao, K.V.; Reddy, V.D. Genomic and metabolomic fingerprinting of Phyllanthus amarus (Schumm & Thonn) hairy root clones. Ann. Phytomed. 2013, 2, 74–88. [Google Scholar]
- Nishikawa, K.; Ishimaru, K. Flavonoids in root cultures of Scutellaria baicalensis. J. Plant Physiol. 1997, 151, 633–636. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Solov’eva, A.I.; Malunova, M.V.; Salamaikina, S.A.; Panov, Y.M.; Lelishentsev, A.A. Hairy roots of Scutellaria spp. (Lamiaceae) as promising producers of antiviral flavones. Molecules 2021, 26, 3927. [Google Scholar] [CrossRef]
- Amselem, J.; Tepfer, M. Molecular basis for novel root phenotypes induced by Agrobacterium rhizogenes A4 on cucumber. Plant Mol. Biol. 1992, 19, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Endogenous production of specific flavonoids and verbascoside in agar and agitated microshoot cultures of Scutellaria lateriflora L. and biotransformation potential. Plant Cell Tissue Organ Cult. 2020, 142, 471–482. [Google Scholar] [CrossRef]
- Nunes, C.F.; Ferreira, J.L.; Nunes-Fernandes, M.C.; de Souza Breves, S.; Generoso, A.L.; Fontes-Soares, B.D.; Carvalho-Dias, M.S.; Pasqual, M.; Borem, A.; de Almeida Cancado, G.M. An improved method for genomic DNA extraction from strawberry leaves. Ciênc. Rural 2011, 41, 1383–1389. [Google Scholar] [CrossRef]
- Li, F.X.; Jin, Z.P.; Zhao, D.X.; Cheng, L.Q.; Fu, C.X.; Ma, F. Overexpression of the Saussurea medusa chalcone isomerase gene in S. involucrata hairy root cultures enhances their biosynthesis of apigenin. Phytochemistry 2006, 67, 553–560. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, Y.S.; Kim, Y.; Uddin, M.R.; Kim, Y.B.; Kim, H.H.; Park, S.Y.; Lee, M.Y.; Chung, S.O.; Park, S.U. Comparative analysis of flavonoids and polar metabolites from hairy roots of Scutellaria baicalensis and Scutellaria lateriflora. World J. Microbiol. Biotechnol. 2014, 30, 887–892. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Enhanced flavonoid production in hairy root cultures of Scutellaria bornmuelleri by elicitor induced over-expression of MYB7 and FNS∏2 genes. Plant Physiol. Biochem. 2020, 148, 35–44. [Google Scholar] [CrossRef]
- Jouanin, L.; Guerche, P.; Pamboukdjian, N.; Tourneur, C.; Casse Delbart, F.; Tourneur, J. Structure of T-DNA in plants regenerated from roots transformed by Agrobacterium rhizogenes strain A4. Mol. Gen. Genet. 1987, 206, 387–392. [Google Scholar] [CrossRef]
- Alpizar, E.; Dechamp, E.; Lapeyre-Montes, F.; Guilhaumon, C.; Bertrand, B.; Jourdan, C.; Lashermes, P.; Etienne, H. Agrobacterium rhizogenes-transformed roots of coffee (Coffea arabica): Conditions for long-term proliferation, and morphological and molecular characterization. Ann. Bot. 2008, 101, 929–940. [Google Scholar] [CrossRef]
- Taneja, J.; Jaggi, M.; Wankhede, D.P.; Sinha, A.K. Effect of loss of T-DNA genes on MIA biosynthetic pathway gene regulation and alkaloid accumulation in Catharanthus roseus hairy roots. Plant Cell Rep. 2010, 29, 1119–1129. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, R.; Pu, X.; Xu, R.; Wang, J.; Zheng, S.; Zeng, Y.; Chen, J.; He, C.; Song, J. Comparative genome analysis of Scutellaria baicalensis and Scutellaria barbata reveals the evolution of active flavonoid biosynthesis. Genom. Proteom. Bioinform. 2020, 18, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, J.; Cui, M.Y.; Liu, J.; Fang, Y.; Yan, M.; Qiu, W.; Shang, H.; Xu, Z.; Yidiresi, R.; et al. The reference genome sequence of Scutellaria baicalensis provides insights into the evolution of wogonin biosynthesis. Mol Plant. 2019, 12, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.Y.; Lu, A.R.; Li, J.X.; Liu, J.; Fang, Y.M.; Pei, T.L.; Zhong, X.; Wi, Y.K.; Kong, Y.; Qiu, W.Q.; et al. Two types of O-methyltransferase are involved in biosynthesis of anticancer methoxylated 4’-deoxyflavones in Scutellaria baicalensis Georgi. Plant Biotechnol. J. 2022, 20, 129–142. [Google Scholar] [CrossRef]
- Pei, T.; Yan, M.; Li, T.; Li, X.; Yin, Y.; Cui, M.; Fang, Y.; Liu, J.; Kong, Y.; Xu, P.; et al. Characterization of UDP-glycosyltransferase family members reveals how major flavonoid glycoside accumulates in the roots of Scutellaria baicalensis. BMC Genom. 2022, 23, 169. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. In vitro cultures of Scutellaria alpine as a source of pharmacologically active metabolites. Acta Physiol. Plant. 2016, 38, 7. [Google Scholar] [CrossRef]
- Solov’eva, A.I.; Evsyukov, S.V.; Sidorov, R.A.; Stepanova, A.Y. Correlation of endogenous β-glucuronidase activity with differentiation of in vitro cultures of Scutellaria baicalensis. Acta Physiol. Plant. 2020, 42, 169. [Google Scholar] [CrossRef]
- Kuzovkina, I.N.; Prokof’eva, M.Y.; Umralina, A.R.; Chernysheva, T.P. Morphological and biochemical characteristics of genetically transformed roots of Scutellaria andrachnoides. Russ. J. Plant Physiol. 2014, 61, 697–706. [Google Scholar] [CrossRef]
- Ikegami, F.; Matsunae, K.; Hisamitsu, M.; Kurihara, T.; Yamamoto, T.; Murakoshi, I. Purifcation and properties of a plant b-D-glucuronidase form Scutellaria root. Biol. Pharm. Bull. 1995, 18, 1531–1534. [Google Scholar] [CrossRef]
- Morimoto, S.; Tateishi, N.; Matsuda, T.; Tanaka, H.; Taura, F.; Furuya, N.; Matsuyama, N.; Shoyama, Y. Novel hydrogen peroxide metabolism in suspension cells of Scutellaria baicalensis Georgi. J. Biol. Chem. 1998, 273, 12606–12611. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Chen, J.; Liang, X. Purifcation and characterization of baicalin-b-D-glucuronidase hydrolyzing baicalin to baicalein from fresh roots of Scutellaria viscidula Bge. Process. Biochem. 2005, 40, 1911–1915. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).