Experimental and Simulation Analysis of Siloxane Mixtures Used in Organic Rankine Cycle with Thermal Stability Limits †
Abstract
:1. Introduction
2. System and Method
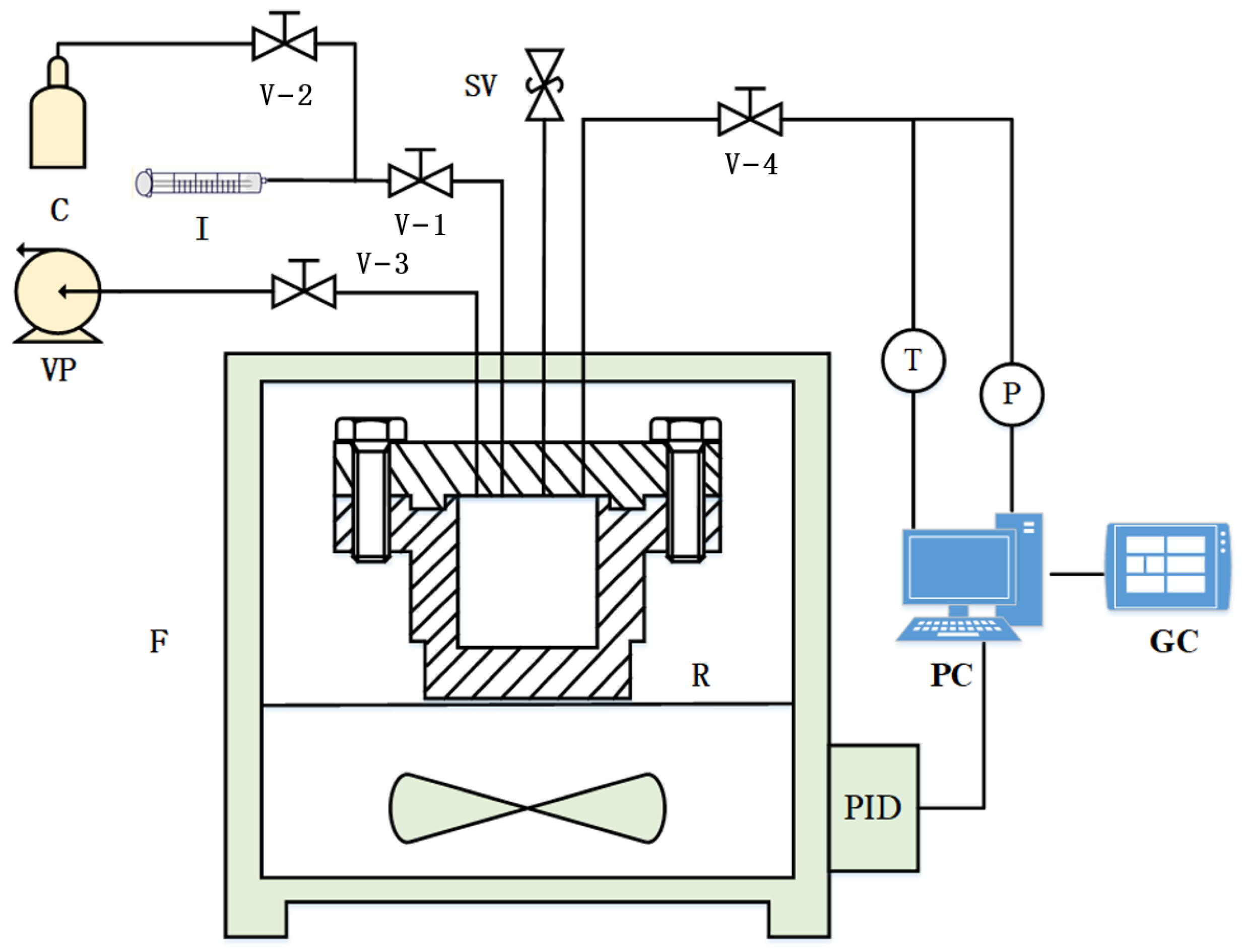
2.1. Experimental System and Method
2.2. Reaction Kinetics Model
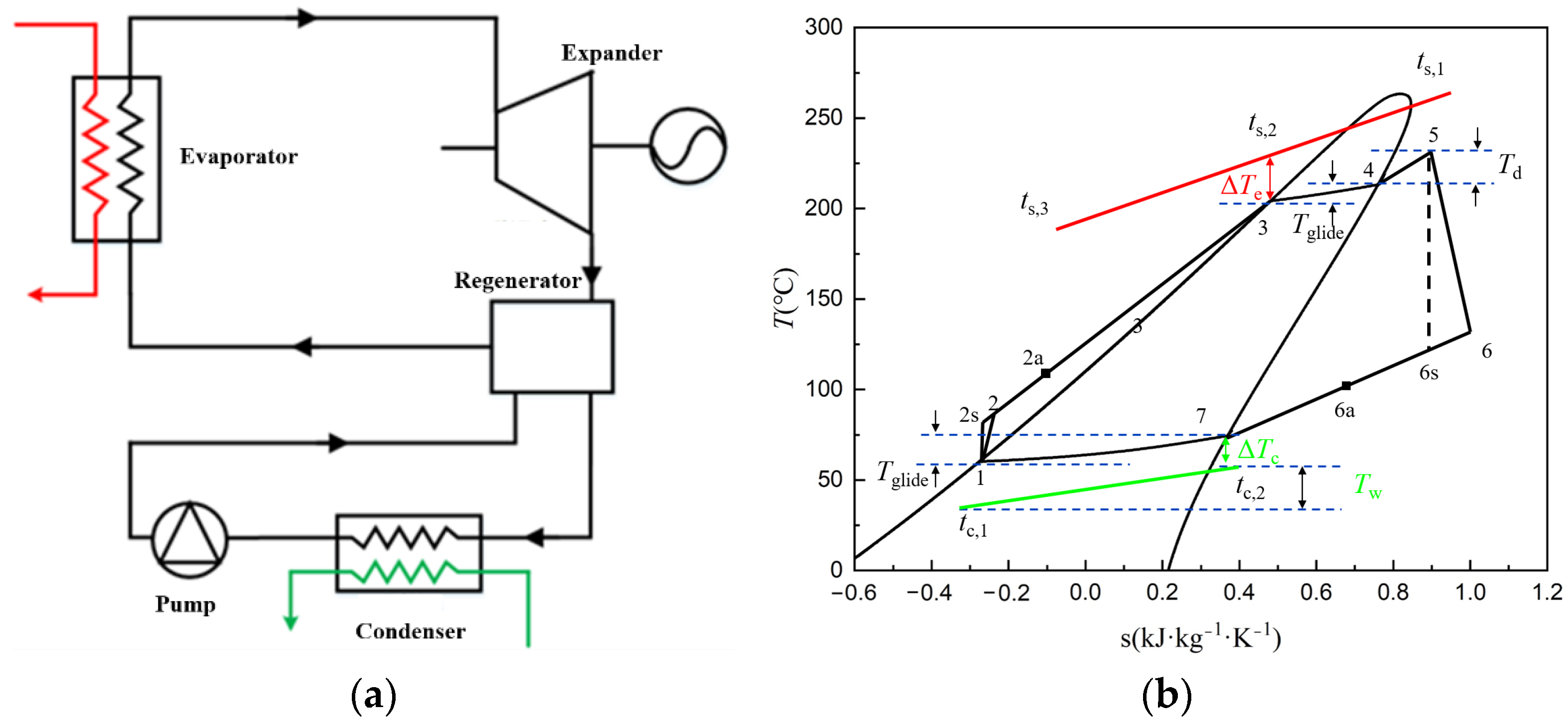
2.3. Simulation Method
- All components in the cycle operate under steady-state conditions;
- The heat exchangers incorporated into the cycle utilize a counter-flow configuration;
- Heat losses and pressure drops in the pipeline are disregarded.
3. Results and Discussion
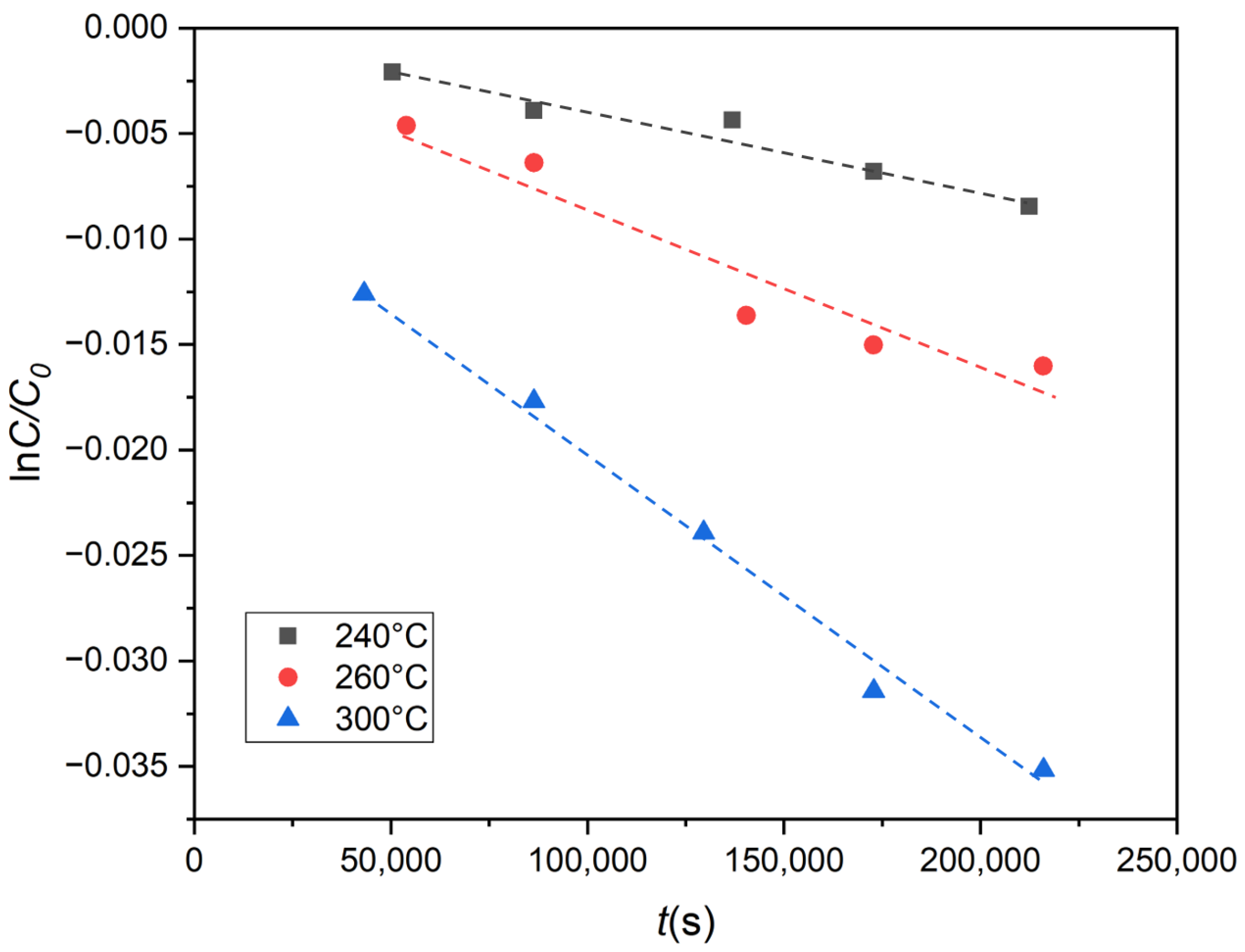
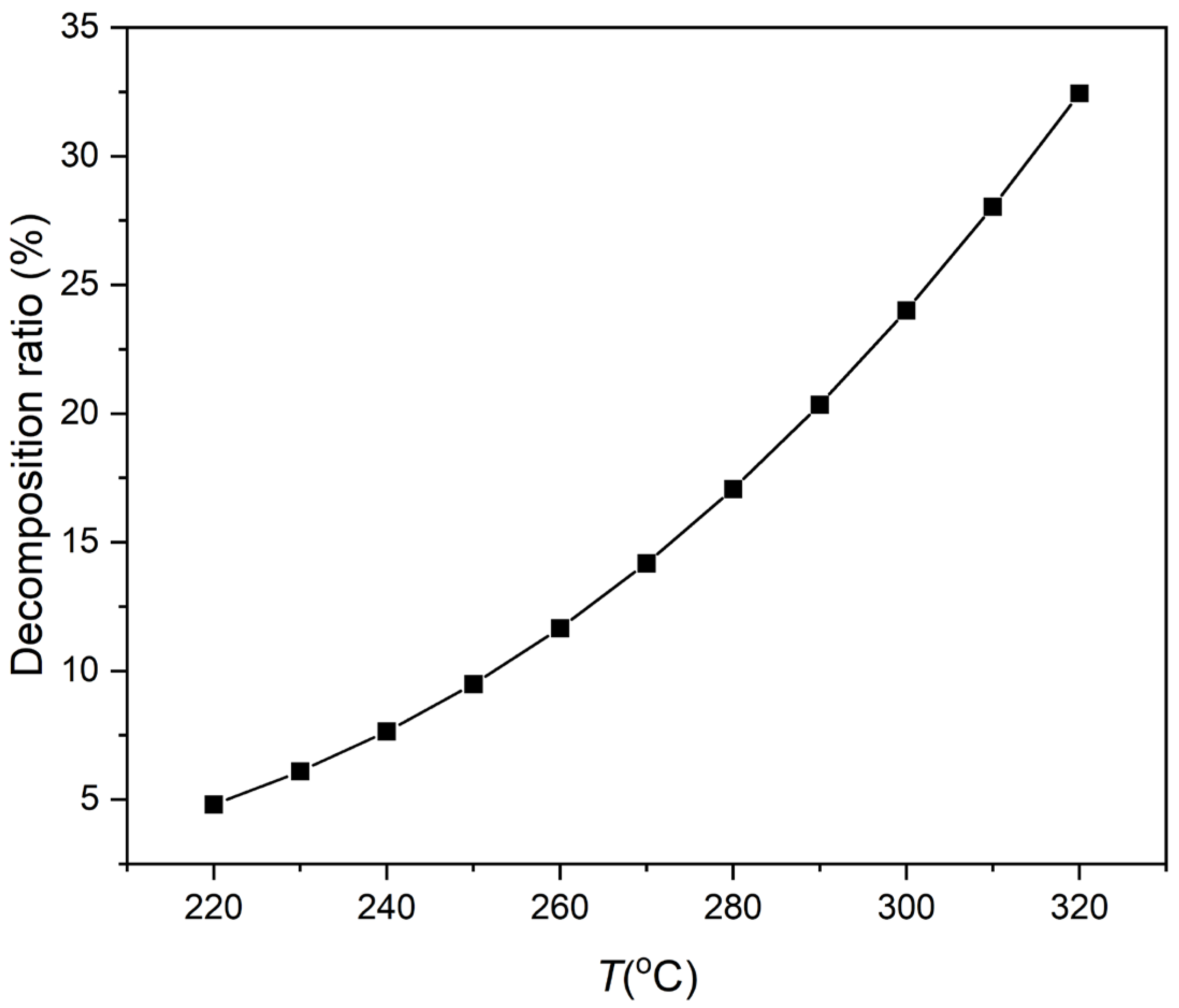
3.1. Experimental Results and Discussion
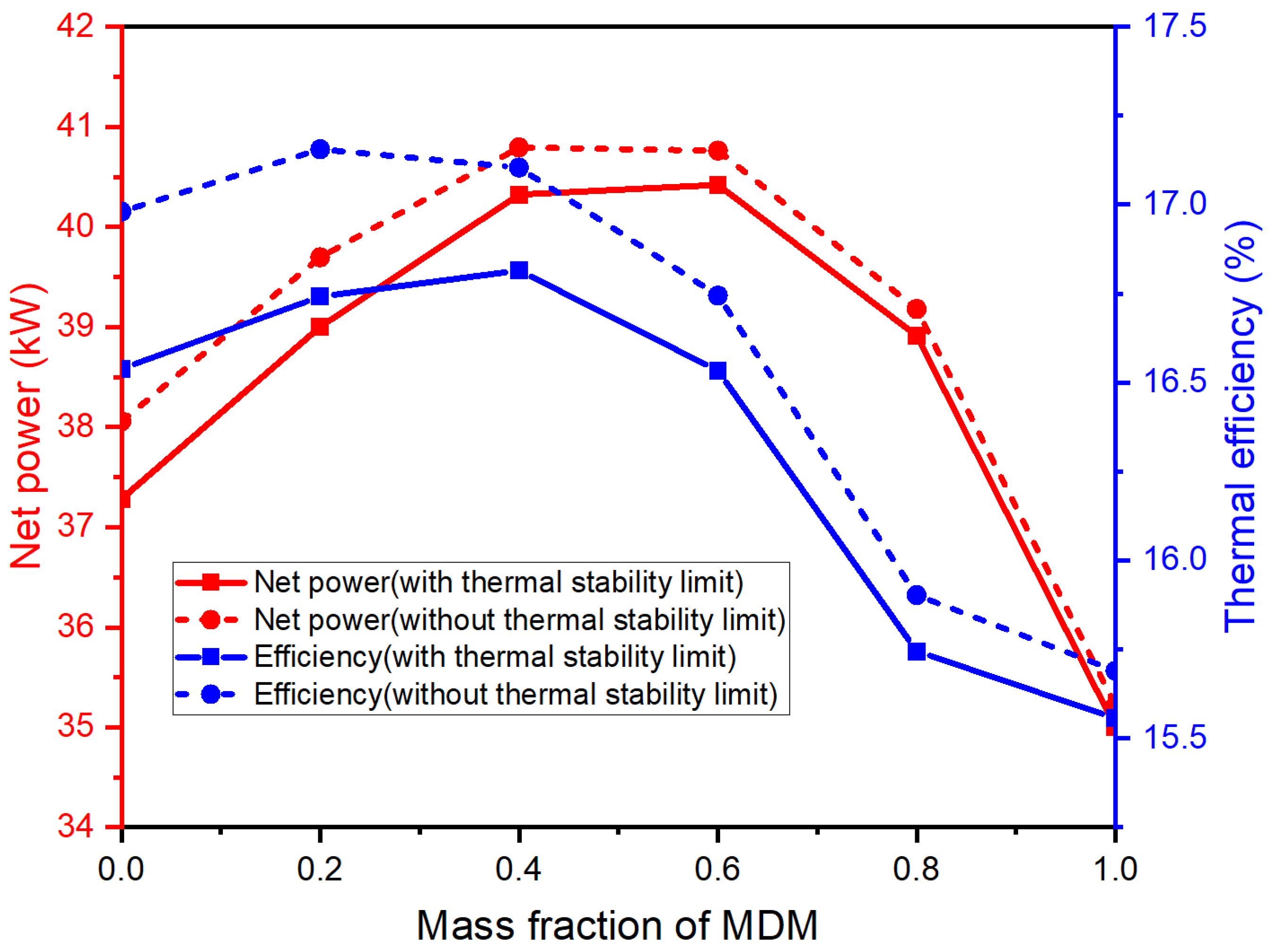
3.2. Simulation Results and Discussion
4. Conclusions
- (1)
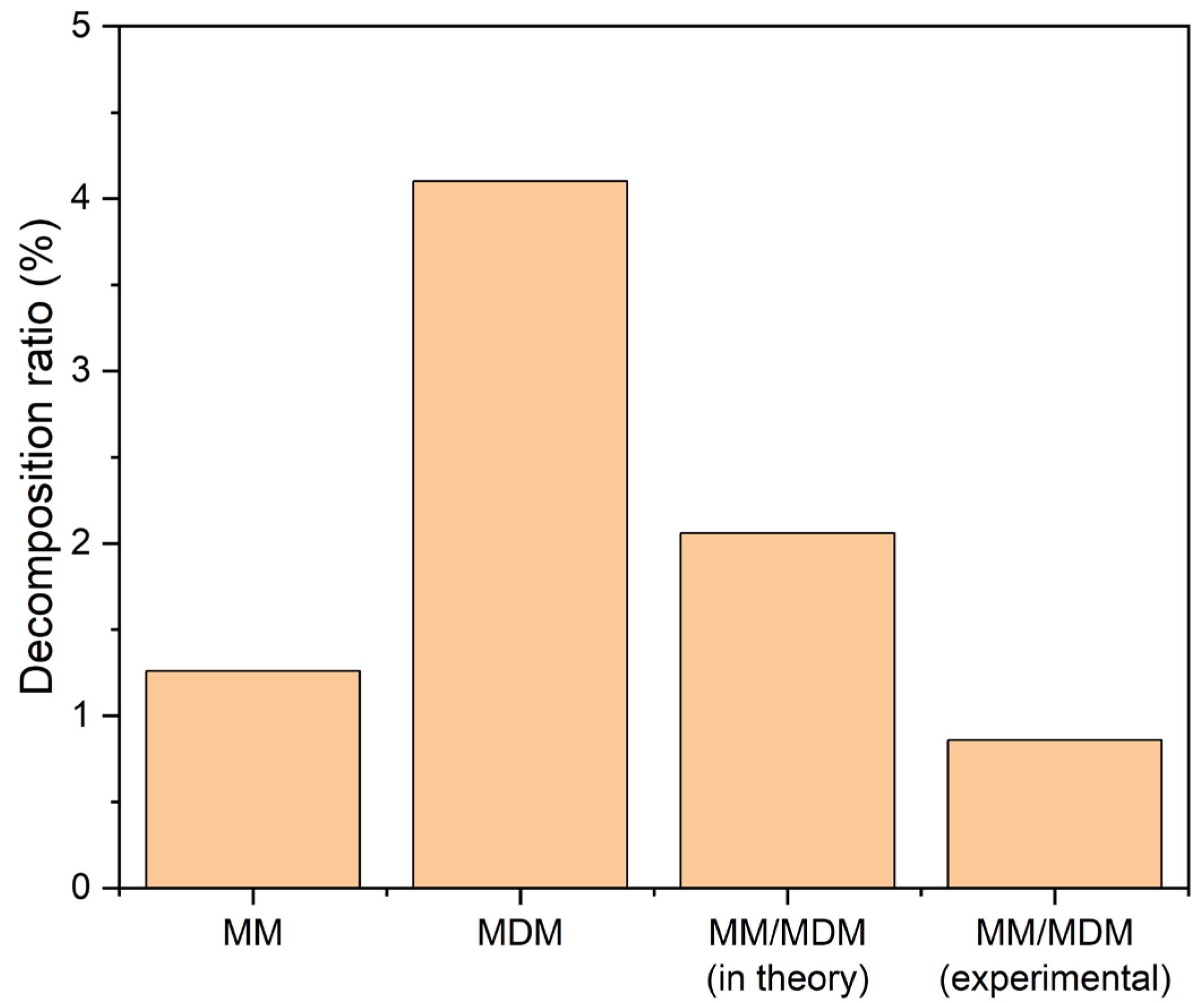
- MDM demonstrated inferior thermal stability compared to MM within the temperature range of 240~320 °C. The MM/MDM mixture had a smaller decomposition ratio than pure MM or MDM. Using MM/MDM mixture as the working fluid of high-temperature ORCs is a good choice by the thermal stability.
- (2)
- MM decomposed by just about 5% at 220 °C after a long operation period of 20 years, resulting in a limited loss of the ORC system performance. A temperature of 220 °C can be considered as the limiting highest temperature for ORCs using MM, MDM, and their mixtures as working fluids.
- (3)
- Both the net power and the thermal efficiency of the system were diminished when an evaporation temperature limit was imposed. The impact of this temperature limit on the net power was relatively minor, while the decrease in thermal efficiency was more pronounced. Different design strategies considering the thermal stability limitation should be used with the different output parameter optimization objectives.
- (4)
- Certain siloxane mixtures demonstrated superior performance compared to pure fluid, given the system model and conditions outlined in this study. The MM/MDM (0.6/0.4) emerged as an optimal working fluid selection within the scope of this work.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclatures
| C | mass fraction (–) |
| h | specific enthalpy (kJ/kg) |
| k | reaction rate constant (s−1) |
| m | mass flow (kg/s) |
| Q | heat flow rate (kW) |
| W | power output (kW) |
| x | decomposition ratio (–) |
| η | efficiency (–) |
| α | regenerative ratio (–) |
| Subscript | |
| 1–7 | state point |
| c | condenser |
| e | evaporator |
| net | net power |
| s | heat source |
| P | pump |
| T | turbine |
| th | thermal efficiency |
| wf | working fluid |
References
- Qyyum, M.A.; Khan, A.; Ali, S.; Khurram, M.S.; Mao, N.; Naquash, A.; Noon, A.A.; He, T.; Lee, M. Assessment of working fluids, thermal resources and cooling utilities for Organic Rankine Cycles: State-of-the-art comparison, challenges, commercial status, and future prospects. Energy Convers. Manag. 2022, 252, 115055. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Feng, Y.; Xi, H.; Hung, T. Assessment and working fluid comparison of steam Rankine cycle Organic Rankine cycle combined system for severe cold territories. Case Stud. Therm. Eng. 2021, 28, 101601. [Google Scholar] [CrossRef]
- Tartière, T.; Astolfi, M. A world overview of the organic Rankine cycle market. Energy Procedia 2017, 129, 2–9. [Google Scholar] [CrossRef]
- Xu, G.; Fu, J.; Quan, Y.; Wen, J.; Dong, B. Experimental investigation on heat transfer characteristics of hexamethyldisiloxane (MM) at supercritical pressures for medium/high temperature ORC applications. Int. J. Heat Mass Transf. 2020, 156, 119852. [Google Scholar] [CrossRef]
- Chitgar, N.; Hemmati, A.; Sadrzadeh, M. A comparative performance analysis, working fluid selection, and machine learning optimization of ORC systems driven by geothermal energy. Energy Convers. Manag. 2023, 286, 117072. [Google Scholar] [CrossRef]
- Javed, S.; Tiwari, A.K. Performance assessment of different Organic Rankine Cycle (ORC) configurations driven by solar energy. Process Saf. Environ. Prot. 2023, 171, 655–666. [Google Scholar] [CrossRef]
- Zhang, C.; Shu, G.; Tian, H.; Wei, H.; Liang, X. Comparative study of alternative ORC-based combined power systems to exploit high temperature waste heat. Energy Convers. Manag. 2015, 89, 541–554. [Google Scholar] [CrossRef]
- Galloni, E.; Fontana, G.; Staccone, S. Design and experimental analysis of a mini ORC (organic Rankine cycle) power plant based on R245fa working fluid. Energy 2015, 90, 768–775. [Google Scholar] [CrossRef]
- Shu, G.; Li, X.; Tian, H.; Liang, X.; Wei, H.; Wang, X. Alkanes as working fluids for high-temperature exhaust heat recovery of diesel engine using organic Rankine cycle. Appl. Energy 2014, 119, 204–217. [Google Scholar] [CrossRef]
- Eyerer, S.; Wieland, C.; Vandersickel, A.; Spliethoff, H. Experimental study of an ORC (Organic Rankine Cycle) and analysis of R1233zd-E as a drop-in replacement for R245fa for low temperature heat utilization. Energy 2016, 103, 660–671. [Google Scholar] [CrossRef]
- Dai, X.; Shi, L.; Qian, W. Review of the Working Fluid Thermal Stability for Organic Rankine Cycles. J. Therm. Sci. 2019, 28, 597–607. [Google Scholar] [CrossRef]
- Angelino, G.; Colonna di Paliano, P. Multicomponent Working Fluids for Organic Rankine Cycles (ORCs). Energy 1998, 23, 449–463. [Google Scholar] [CrossRef]
- Erhart, T.G.; Gölz, J.; Eicker, U.; Van den Broek, M. Working Fluid Stability in Large-Scale Organic Rankine Cycle-Units Using Siloxanes—Long-Term Experiences and Fluid Recycling. Energies 2016, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Shi, L.; Qian, W. Thermal stability of hexamethyldisiloxane (MM) as a working fluid for organic Rankine cycle. Int. J. Energy Res. 2018, 43, 896–904. [Google Scholar] [CrossRef]
- Preißinger, M.; Brüggemann, D. Thermal Stability of Hexamethyldisiloxane (MM) for High-Temperature Organic Rankine Cycle (ORC). Energies 2016, 9, 183. [Google Scholar] [CrossRef] [Green Version]
- Nami, H.; Ertesvåg, I.S.; Agromayor, R.; Riboldi, L.; Nord, L.O. Gas turbine exhaust gas heat recovery by organic Rankine cycles (ORC) for offshore combined heat and power applications—Energy and exergy analysis. Energy 2018, 165, 1060–1071. [Google Scholar] [CrossRef]
- Uusitalo, A.; Honkatukia, J.; Turunen-Saaresti, T. Evaluation of a small-scale waste heat recovery organic Rankine cycle. Appl. Energy 2017, 192, 146–158. [Google Scholar] [CrossRef]
- Dong, B.; Xu, G.; Cai, Y.; Li, H. Analysis of zeotropic mixtures used in high-temperature Organic Rankine cycle. Energy Convers. Manag. 2014, 84, 253–260. [Google Scholar] [CrossRef]
- Oyekale, J.; Heberle, F.; Petrollese, M.; Brüggemann, D.; Cau, G. Thermo-economic evaluation of actively selected siloxane mixtures in a hybrid solar-biomass organic Rankine cycle power plant. Appl. Therm. Eng. 2020, 165, 114607. [Google Scholar] [CrossRef]
- Keulen, L.; Gallarini, S.; Landolina, C.; Spinelli, A.; Iora, P.; Invernizzi, C.; Lietti, L.; Guardone, A. Thermal stability of hexamethyldisiloxane and octamethyltrisiloxane. Energy 2018, 165, 868–876. [Google Scholar] [CrossRef]
- Rajabloo, T.; Bonalumi, D.; Iora, P. Effect of a partial thermal decomposition of the working fluid on the performances of ORC power plants. Energy 2017, 133, 1013–1026. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Dai, X.; Shi, L.; An, Q.; Qian, W. Chemical Kinetics Method for Evaluating the Thermal Stability of Organic Rankine Cycle Working Fluids. Appl. Therm. Eng. 2016, 100, 708–713. [Google Scholar] [CrossRef]
- Lamura, M.D.P.; Ammarullah, M.I.; Hidayat, T.; Maula, M.I.; Jamari, J.; Bayuseno, A.P. Diameter ratio and friction coefficient effect on equivalent plastic strain (PEEQ) during contact between two brass solids. Cogent Eng. 2023, 10, 2218691. [Google Scholar] [CrossRef]
- Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.S.; Abdullah, A.H.; Abdul Kadir, M.R.; Ramlee, M.H. Biomechanical Effects of the Porous Structure of Gyroid and Voronoi Hip Implants: A Finite Element Analysis Using an Experimentally Validated Model. Materials 2023, 16, 3298. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Tauviqirrahman, M.; Ammarullah, M.I.; Jamari, J.; Saputra, E.; Winarni, T.I.; Kurniawan, F.D.; Shiddiq, S.A.; Heide, E. Analysis of contact pressure in a 3D model of dual-mobility hip joint prosthesis under a gait cycle. Sci. Rep. 2023, 13, 3564. [Google Scholar] [CrossRef] [PubMed]
- Lamura, M.D.P.; Hidayat, T.; Ammarullah, M.I.; Bayuseno, A.P.; Jamari, J. Study of contact mechanics between two brass solids in various diameter ratios and friction coefficient. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2023. [Google Scholar] [CrossRef]
- Ahmadi, G.; Toghraie, D.; Akbari, O. Energy, exergy and environmental (3E) analysis of the existing CHP system in a petrochemical plant. Renew. Sustain. Energy Rev. 2019, 99, 234–242. [Google Scholar] [CrossRef]
- Ahmadi, G.; Toghraie, D.; Akbari, O. Technical and environmental analysis of repowering the existing CHP system in a petrochemical plant: A case study. Energy 2018, 159, 937–949. [Google Scholar] [CrossRef]
- Irriyanto, M.Z.; Lim, H.S.; Choi, B.S.; Myint, A.A.; Kim, J. Thermal stability and decomposition behavior of HFO-1234ze(E) as a working fluid in the supercritical organic Rankine cycle. J. Supercrit. Fluids 2019, 154, 104602. [Google Scholar] [CrossRef]
- Wang, W.; Dai, X.; Shi, L. Influence of thermal stability on organic Rankine cycle systems using siloxanes as working fluids. Appl. Therm. Eng. 2022, 200, 117639. [Google Scholar] [CrossRef]



| Siloxane | Molecular Formula | Tb/°C | Tc/°C | pc/MPa |
|---|---|---|---|---|
| MM | C6H18OSi2 | 100.25 | 245.60 | 1.94 |
| MDM | C8H24O2Si3 | 152.51 | 290.94 | 1.42 |
| Parameters | Unit | Value |
|---|---|---|
| Heat source (air) temperature | ts,1/°C | 300 |
| Heat source flow | /kg·s−1 | 1 |
| Cold source (water) temperature | tc,1/°C | 25 |
| Evaporator pinch | ΔTe/°C | 10 |
| Condenser pinch | ΔTc/°C | 5 |
| Expander design isentropic efficiency | t/% | 85 |
| Pump design isentropic efficiency | /% | 90 |
| Product | Mass Fraction/% | Product | Mass Fraction/% |
|---|---|---|---|
| MDM | 90.47 | MM | 5.82 |
| MD2M | 1.85 | MD3M | 0.57 |
| MD4M | 0.28 | MD5M | 0.20 |
| D4 | 0.35 | D5 | 0.14 |
| QM4 | 0.23 | Others | 0.09 |
| k (s−1) | T (K) | Ea (kJ/mol) | A (s−1) | ||
|---|---|---|---|---|---|
| 513.15 | 533.15 | 573.15 | |||
| k | 3.76 × 10−8 | 7.76 × 10−8 | 1.36 × 10−7 | 50.50 | 5.80 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Tian, L.; Shi, L.; Dai, X. Experimental and Simulation Analysis of Siloxane Mixtures Used in Organic Rankine Cycle with Thermal Stability Limits. Processes 2023, 11, 2108. https://doi.org/10.3390/pr11072108
Wang W, Tian L, Shi L, Dai X. Experimental and Simulation Analysis of Siloxane Mixtures Used in Organic Rankine Cycle with Thermal Stability Limits. Processes. 2023; 11(7):2108. https://doi.org/10.3390/pr11072108
Chicago/Turabian StyleWang, Wenhuan, Lei Tian, Lin Shi, and Xiaoye Dai. 2023. "Experimental and Simulation Analysis of Siloxane Mixtures Used in Organic Rankine Cycle with Thermal Stability Limits" Processes 11, no. 7: 2108. https://doi.org/10.3390/pr11072108






