Membrane-Based Micro-Volume Dialysis Method for Rapid and High-Throughput Protein Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
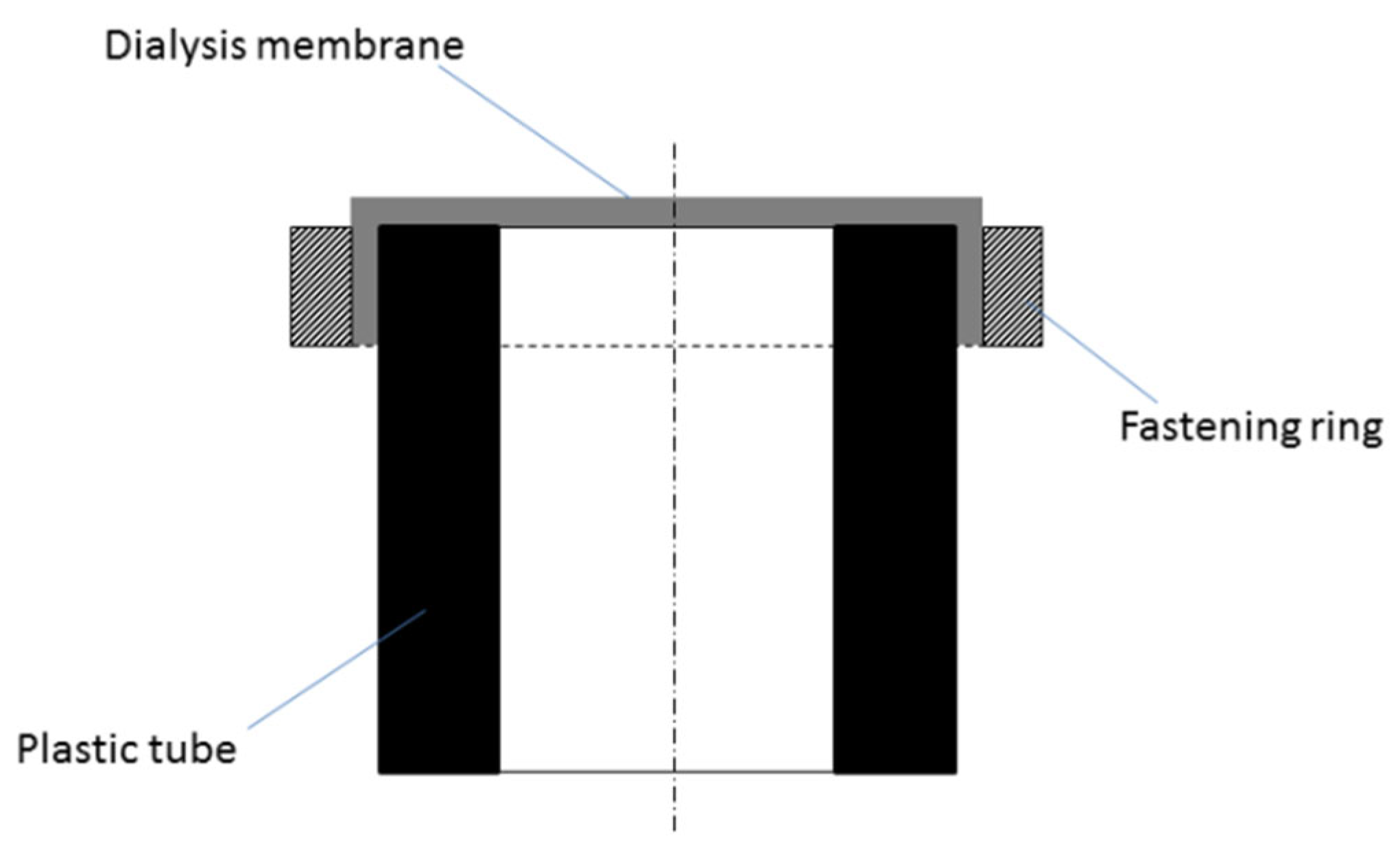
2.2. Membrane Device
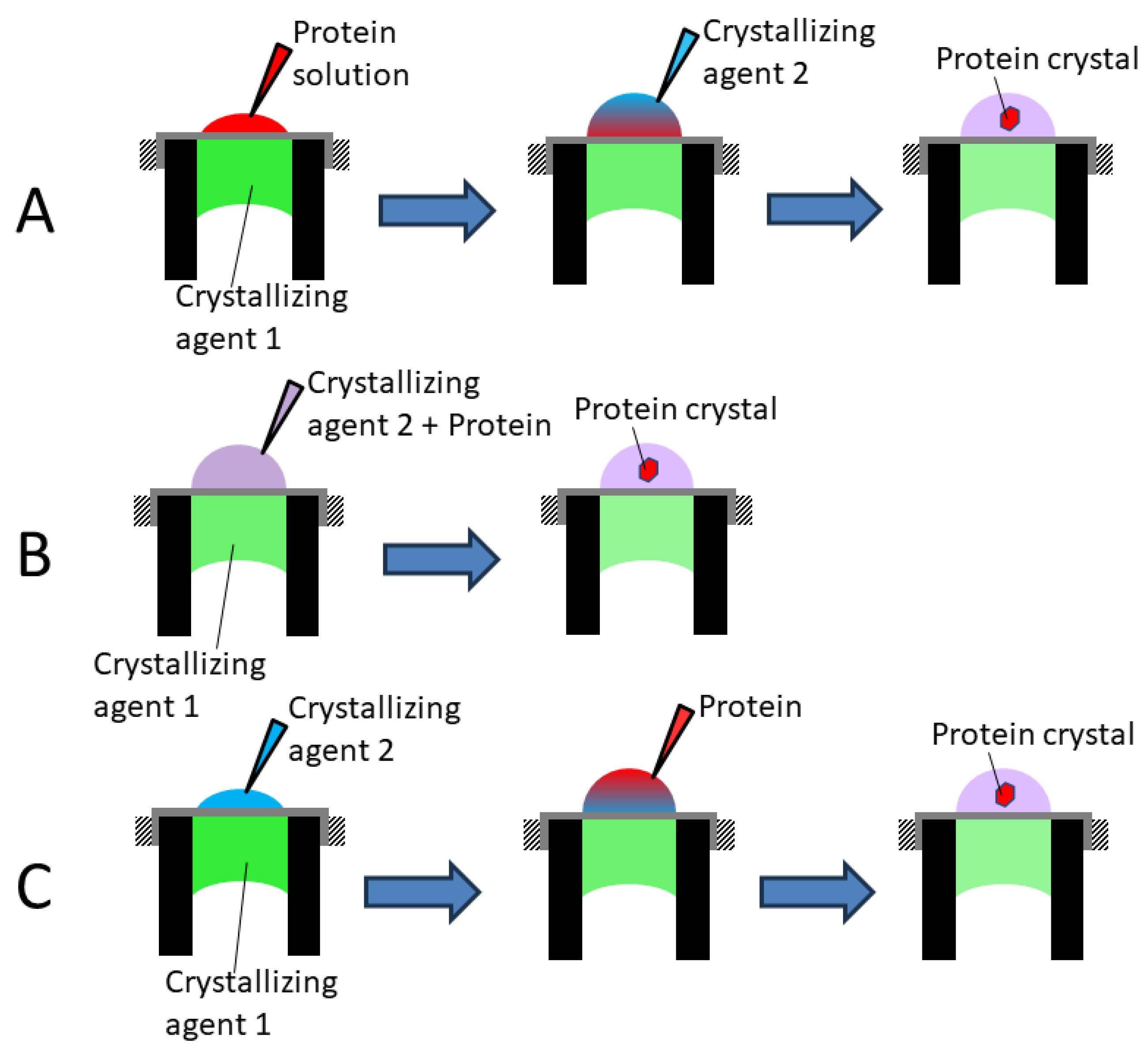
2.3. Lysozyme Crystallization
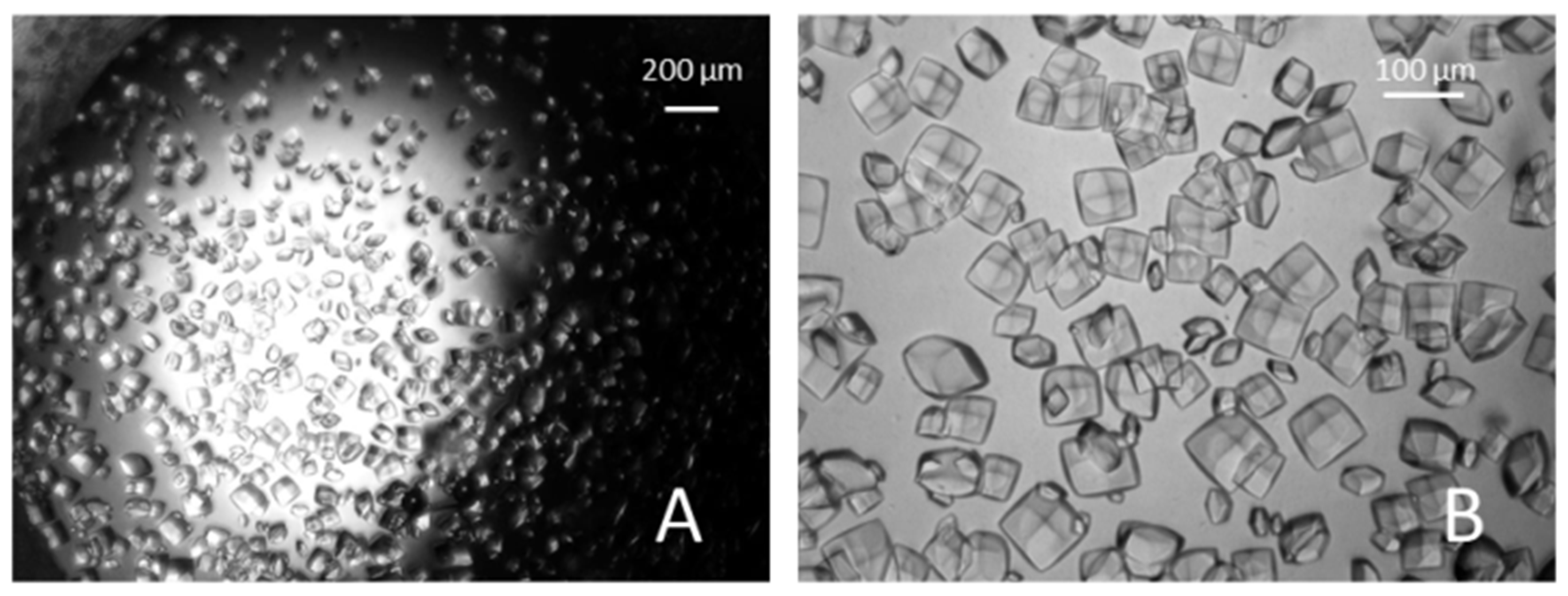
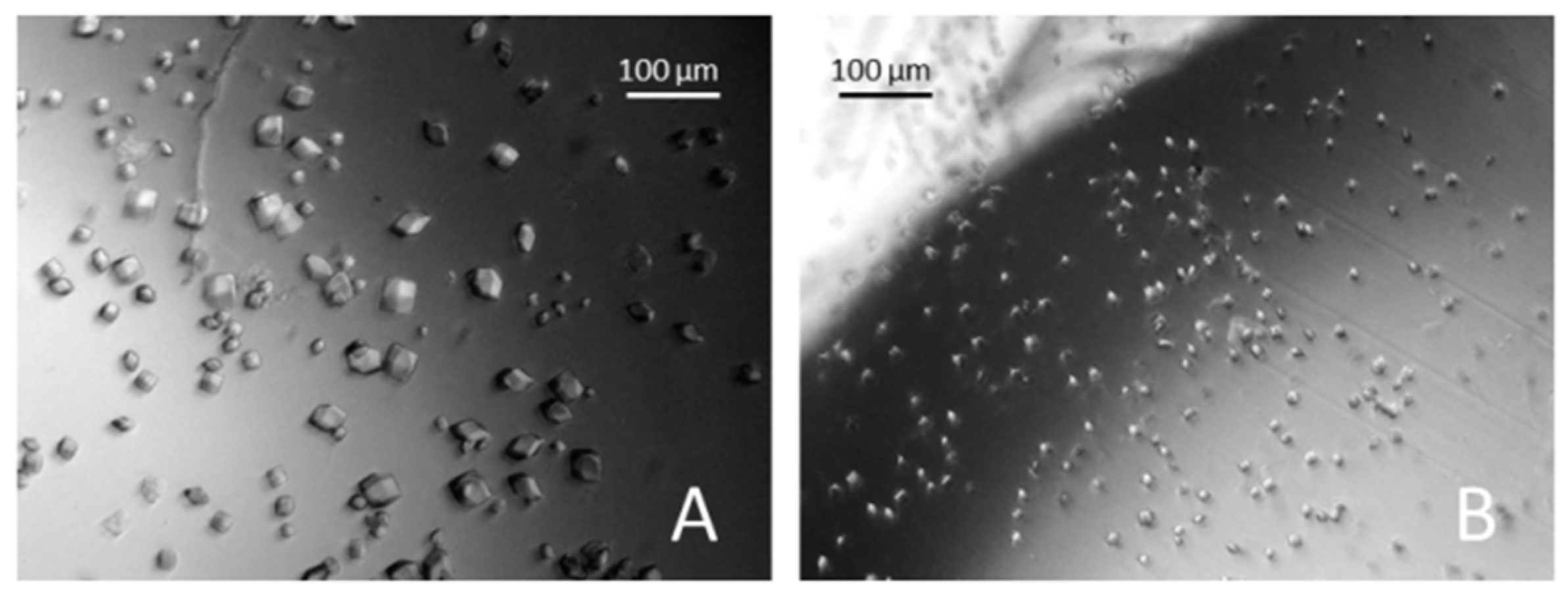
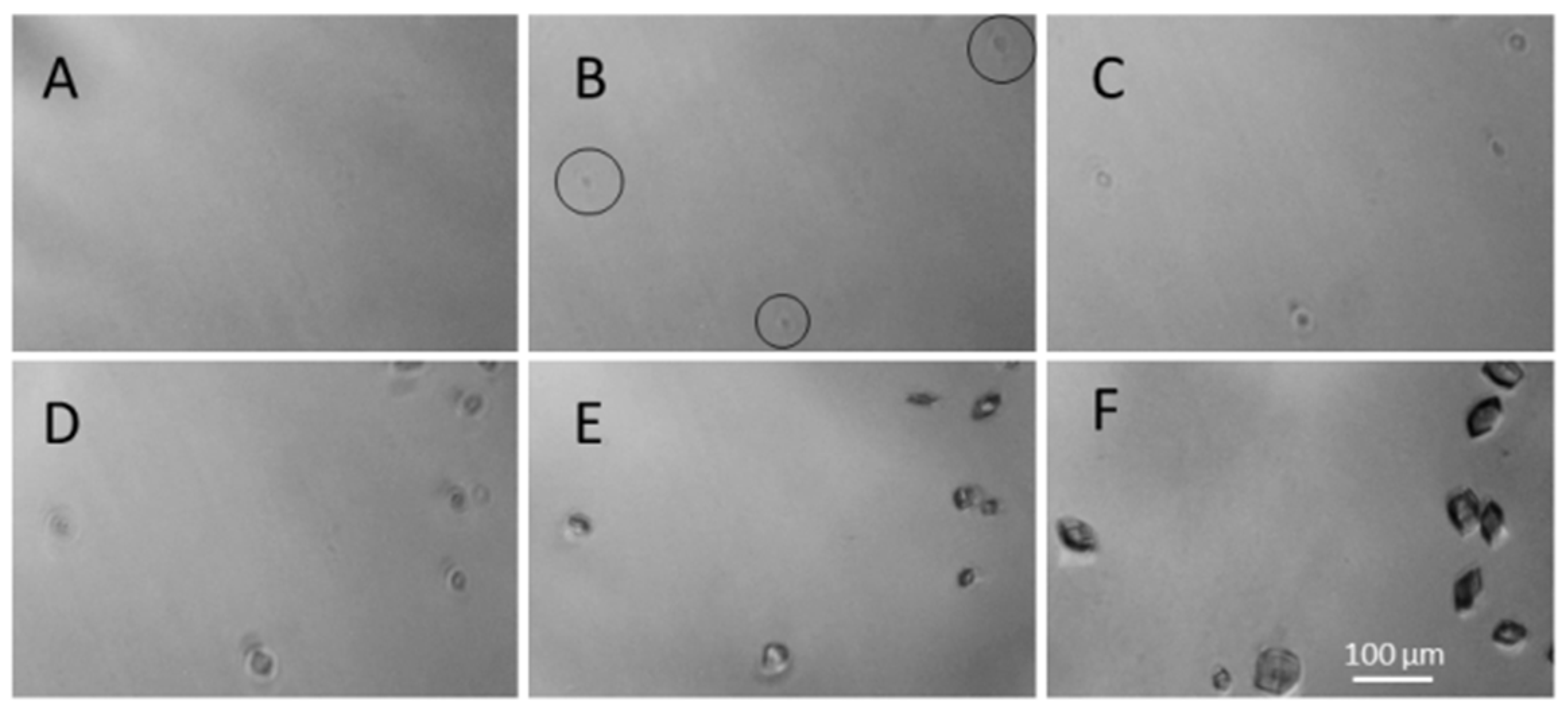
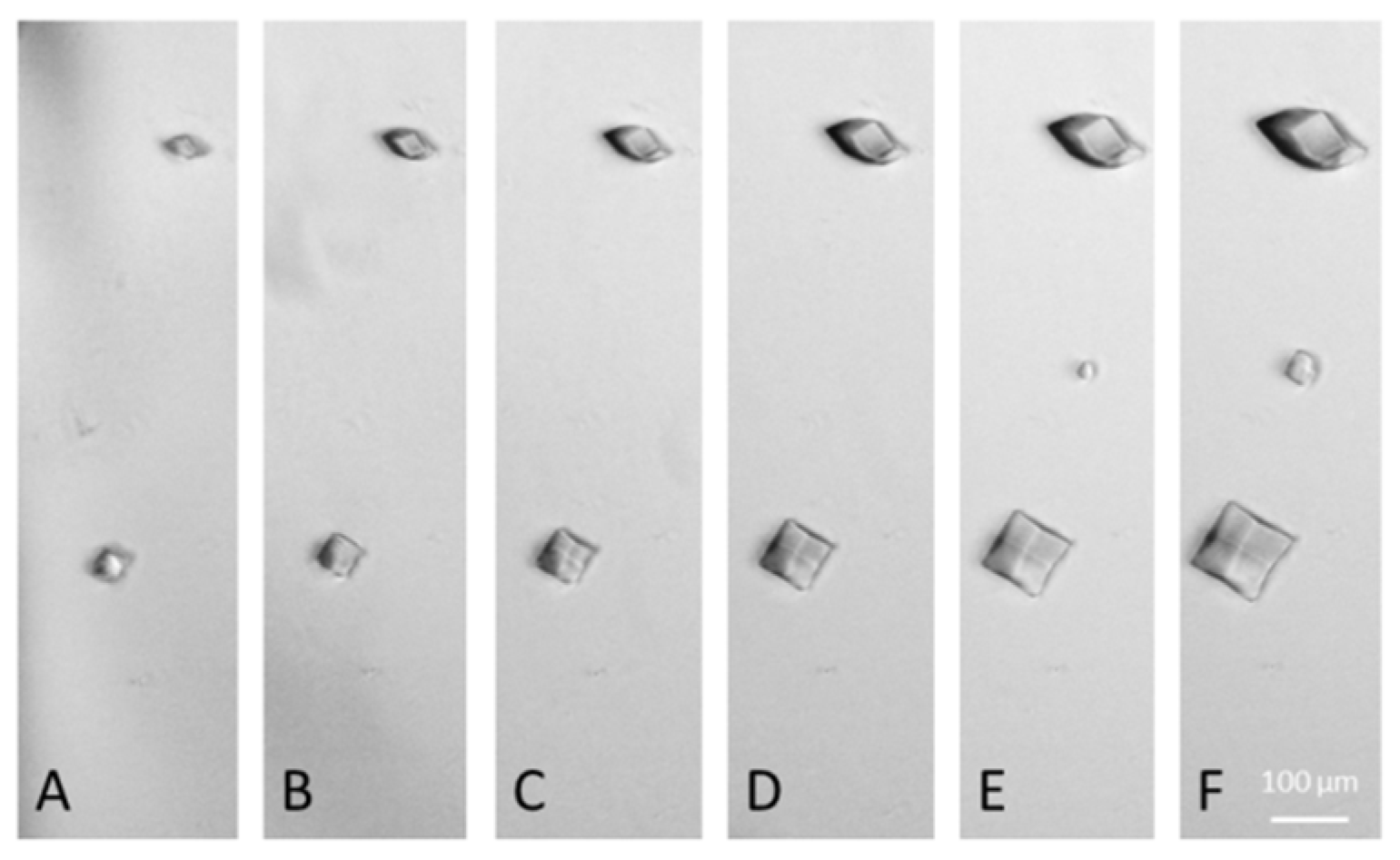
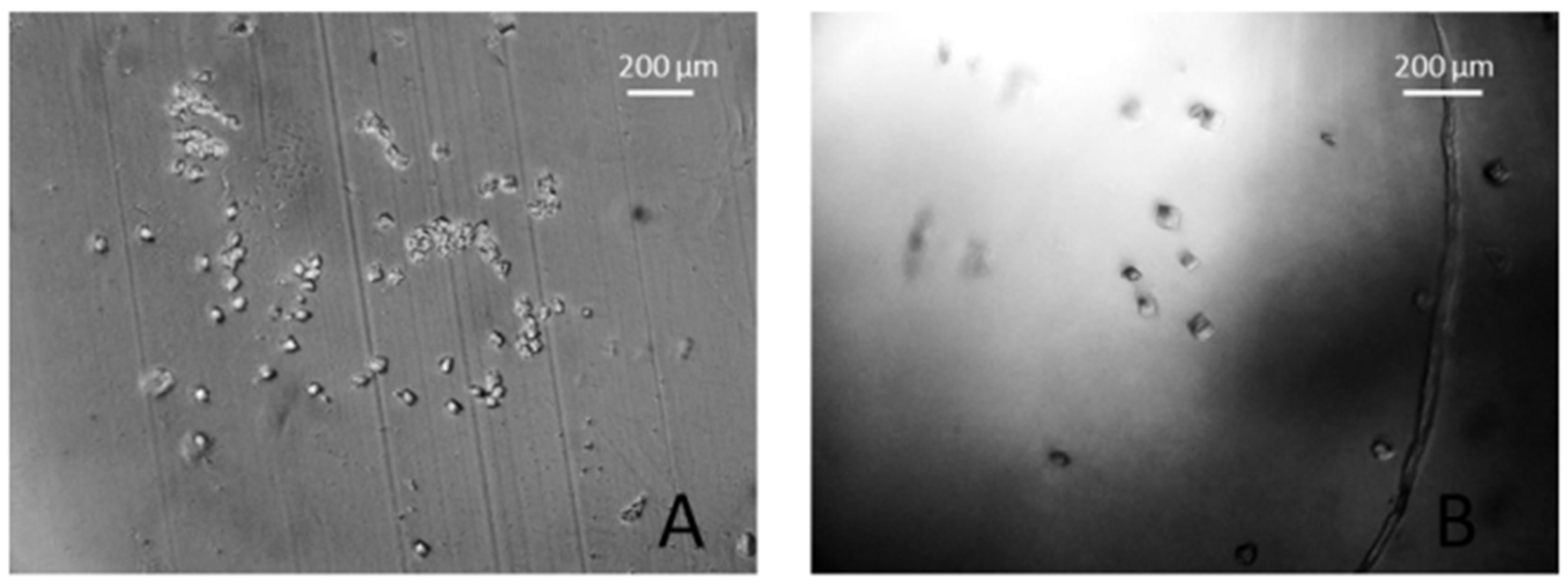
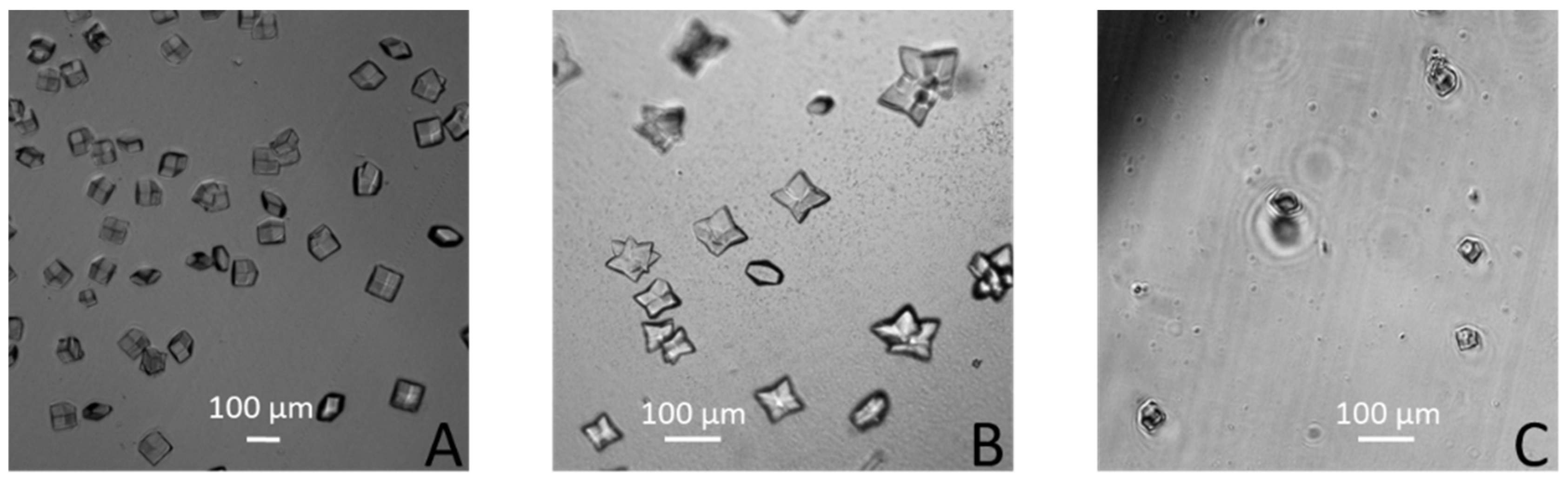
3. Results and Discussions
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, I.D. Protein crystallization for structure-based drug design. In Structure-Based Drug Discovery; Humana Press: Totowa, NJ, USA, 2012; pp. 67–91. [Google Scholar]
- Fraser, J.S.; van den Bedem, B.H.; Samelson, A.J.; Lang, P.T.; Holton, J.M.; Echols, N.; Alber, T. Accessing protein conformational ensembles using room-temperature X-ray crystallography. Proc. Natl. Acad. Sci. USA 2011, 108, 16247–16252. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Kenneth, A.; Costanzi, S. New insights for drug design from the X-ray crystallographic structures of G-protein-coupled receptors. Mol. Pharmacol. 2012, 82, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPherson, A. Introduction to protein crystallization. Methods 2004, 34, 254–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPherson, A.; Gavira, J.A. Introduction to protein crystallization. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, S.; Saridakis, E.; Govada, L.; Chayen, N.E. Porous nucleating agents for protein crystallization. Nat. Protoc. 2014, 9, 1621–1633. [Google Scholar] [CrossRef]
- Garman, E.F. Developments in X-ray crystallographic structure determination of biological macromolecules. Science 2014, 343, 1102–1108. [Google Scholar] [CrossRef]
- McPherson, A. Crystallization of Biological Macromolecules; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1999. [Google Scholar]
- Santarsiero, B.D.; Yegian, D.T.; Lee, C.C.; Spraggon, G.; Gu, J.; Scheibe, D.; Uber, D.C.; Cornell, E.W.; Nordmeyer, R.A.; Kolbe, W.F.; et al. An approach to rapid protein crystallization using nanodroplets. J. Appl. Crystallogr. 2002, 35, 278–281. [Google Scholar] [CrossRef]
- Stevens, R.C. High-throughput protein crystallization. Curr. Opin. Struct. Biol. 2000, 10, 558–563. [Google Scholar] [CrossRef]
- Chayen, N.E. High-throughput protein crystallization. Adv. Protein Chem. Struct. Biol. 2009, 77, 1–22. [Google Scholar]
- Zheng, B.; Roach, L.S.; Ismagilov, R.F. Screening of protein crystallization conditions on a microfluidic chip using nanoliter-size droplets. J. Am. Chem. Soc. 2003, 125, 11170–11171. [Google Scholar] [CrossRef] [Green Version]
- Leng, J.; Salmon, J.B. Microfluidic crystallization. Lab Chip 2009, 9, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Dhouib, K.; Malek, C.K.; Pfleging, W.; Gauthier-Manuel, B.; Duffait, R.; Thuillier, G.; Ferrigno, R.; Jacquamet, L.; Ohana, J.; Ferrer, J.L.; et al. Microfluidic chips for the crystallization of biomacromolecules by counter-diffusion and on-chip crystal X-ray analysis. Lab Chip 2009, 9, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, K.; Hu, X.; Li, G.; Jin, Q.; Zhao, J. A Centrifugal Microfluidic Device for Screening Protein Crystallization Conditions by Vapor Diffusion. Sens. Actuators B Chem. 2015, 219, 105–111. [Google Scholar] [CrossRef]
- Weber, P.; Pissis, C.; Navaza, R.; Mechaly, A.E.; Saul, F.; Alzari, P.M.; Haouz, A. High-throughput crystallization pipeline at the crystallography core facility of the institut Pasteur. Molecules 2019, 24, 4451. [Google Scholar] [CrossRef] [Green Version]
- Sutanto, F.; Shaabani, S.; Oerlemans, R.; Eris, D.; Patil, P.; Hadian, M.; Wang, M.; Sharpe, M.E.; Groves, M.R.; Dömling, A. Combining High-Throughput Synthesis and High-Throughput Protein Crystallography for Accelerated Hit Identification. Angew. Chem. 2021, 133, 18379–18387. [Google Scholar] [CrossRef]
- Blay, V.; Tolani, B.; Ho, S.P.; Arkin, M.R. High-throughput screening: Today’s biochemical and cell-based approaches. Drug Discov. Today 2020, 25, 1807–1821. [Google Scholar] [CrossRef]
- Hirata, K.; Yamashita, K.; Ueno, G.; Kawano, Y.; Hasegawa, K.; Kumasaka, T.; Yamamoto, M. ZOO: An automatic data-collection system for high-throughput structure analysis in protein microcrystallography. Acta Crystallogr. Sect. D Struct. Biol. 2019, 75, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Jedrzejczak, R.; Maltseva, N.I.; Wilamowski, M.; Endres, M.; Godzik, A.; Michalska, M.; Joachimiak, A. Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2. Protein Sci. 2020, 29, 1596–1605. [Google Scholar] [CrossRef]
- Tyler, A.R.; Ragbirsingh, R.; McMonagle, C.J.; Waddell, P.G.; Heaps, S.E.; Steed, J.W.; Thaw, P.; Hall, M.J.; Probert, M.R. Encapsulated nanodroplet crystallization of organic-soluble small molecules. Chem 2020, 6, 1755–1765. [Google Scholar] [CrossRef]
- Junius, N.; Jaho, S.; Sallaz-Damaz, Y.; Borel, F.; Salmon, J.B.; Budayova-Spano, M. A microfluidic device for both on-chip dialysis protein crystallization and in situ X-ray diffraction. Lab. Chip 2020, 20, 296–310. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zhou, J.; Dong, J.; Su, Z.; Huang, L. Revealing the effects of microwell sizes on the crystal growth kinetics of active pharmaceutical ingredients by deep learning. Chem. Eng. J. 2022, 428, 131986. [Google Scholar] [CrossRef]
- Yang, H.; Chen, W.; Peczulis, P.; Heng, J.Y.Y. Development and workflow of a continuous protein crystallization process: A case of lysozyme. Cryst. Growth Des. 2019, 19, 983–991. [Google Scholar] [CrossRef]
- Lieske, J.; Cerv, M.; Kreida, S.; Komadina, D.; Fischer, J.; Barthelmess, M.; Fischer, P. On-chip crystallization for serial crystallography experiments and on-chip ligand-binding studies. IUCrJ 2019, 6, 714–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aherne, M.; Lyons, J.A.; Caffrey, M. A fast, simple and robust protocol for growing crystals in the lipidic cubic phase. J. Appl. Crystallogr. 2012, 45, 1330–1333. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Ito, K.; Hayakawa, R.; Ataka, M. Size and number density of precrystalline aggregates in lysozyme crystallization process. J. Chem. Phys. 1999, 111, 10330–10337. [Google Scholar] [CrossRef]
- Yang, C.G.; Liu, Y.H.; Di, Y.D.; Xu, Z.R. Generation of two-dimensional concentration-gradient droplet arrays on a two-layer chip for screening of protein crystallization conditions. Microfluid. Nanofluidics 2015, 18, 493–501. [Google Scholar] [CrossRef]
- Hayashi, Y.; Maruyama, M.; Yoshimura, M.; Okada, S.; Yoshikawa, H.Y.; Sugiyama, S.; Adachi, H.; Matsumura, H.; Inoue, T.; Takano, K.; et al. Spiral growth can enhance both the normal growth rate and quality of tetragonal lysozyme crystals grown under a forced solution flow. Cryst. Growth Des. 2015, 15, 2137–2143. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Ching, C.B. Toward further understanding of lysozyme crystallization: Phase diagram, protein–protein interaction, nucleation kinetics, and growth kinetics. Cryst. Growth Des. 2010, 10, 548–558. [Google Scholar] [CrossRef]
- Saikumar, M.V.; Glatz, C.E.; Larson, M.A. Lysozyme crystal growth and nucleation kinetics. J. Cryst. Growth 1998, 187, 277–288. [Google Scholar] [CrossRef]
- Ielasi, F.S.; Hirtz, M.; Sekula-Neuner, S.; Laue, T.; Fuchs, H.; Willaert, R.G. Dip-Pen Nanolithography-Assisted Protein Crystallization. J. Am. Chem. Soc. 2014, 137, 154–157. [Google Scholar] [CrossRef]
- Akella, S.V.; Mowitz, A.; Heymann, M.; Fraden, S. Emulsion-Based Technique To Measure Protein Crystal Nucleation Rates of Lysozyme. Cryst. Growth Des. 2014, 14, 4487–4509. [Google Scholar] [CrossRef]
- Neugebauer, P.; Khinast, J.G. Continuous Crystallization of Proteins in a Tubular Plug-Flow Crystallizer. Cryst. Growth Des. 2015, 15, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R. An osmolyte-based micro-volume ultrafiltration technique. Lab. Chip 2014, 14, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Görisch, H. Drop dialysis: Time course of salt and protein exchange. Anal. Biochem. 1988, 173, 393–398. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, R. Membrane-Based Micro-Volume Dialysis Method for Rapid and High-Throughput Protein Crystallization. Processes 2023, 11, 2148. https://doi.org/10.3390/pr11072148
Ghosh R. Membrane-Based Micro-Volume Dialysis Method for Rapid and High-Throughput Protein Crystallization. Processes. 2023; 11(7):2148. https://doi.org/10.3390/pr11072148
Chicago/Turabian StyleGhosh, Raja. 2023. "Membrane-Based Micro-Volume Dialysis Method for Rapid and High-Throughput Protein Crystallization" Processes 11, no. 7: 2148. https://doi.org/10.3390/pr11072148
APA StyleGhosh, R. (2023). Membrane-Based Micro-Volume Dialysis Method for Rapid and High-Throughput Protein Crystallization. Processes, 11(7), 2148. https://doi.org/10.3390/pr11072148





