Study on Sulfuric Acid Leaching and Purification of Zinc Ferrite by Roasting Zinc-Containing Gossan Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Principles and Characterization Methods
2.2.1. Test Methods and Principles
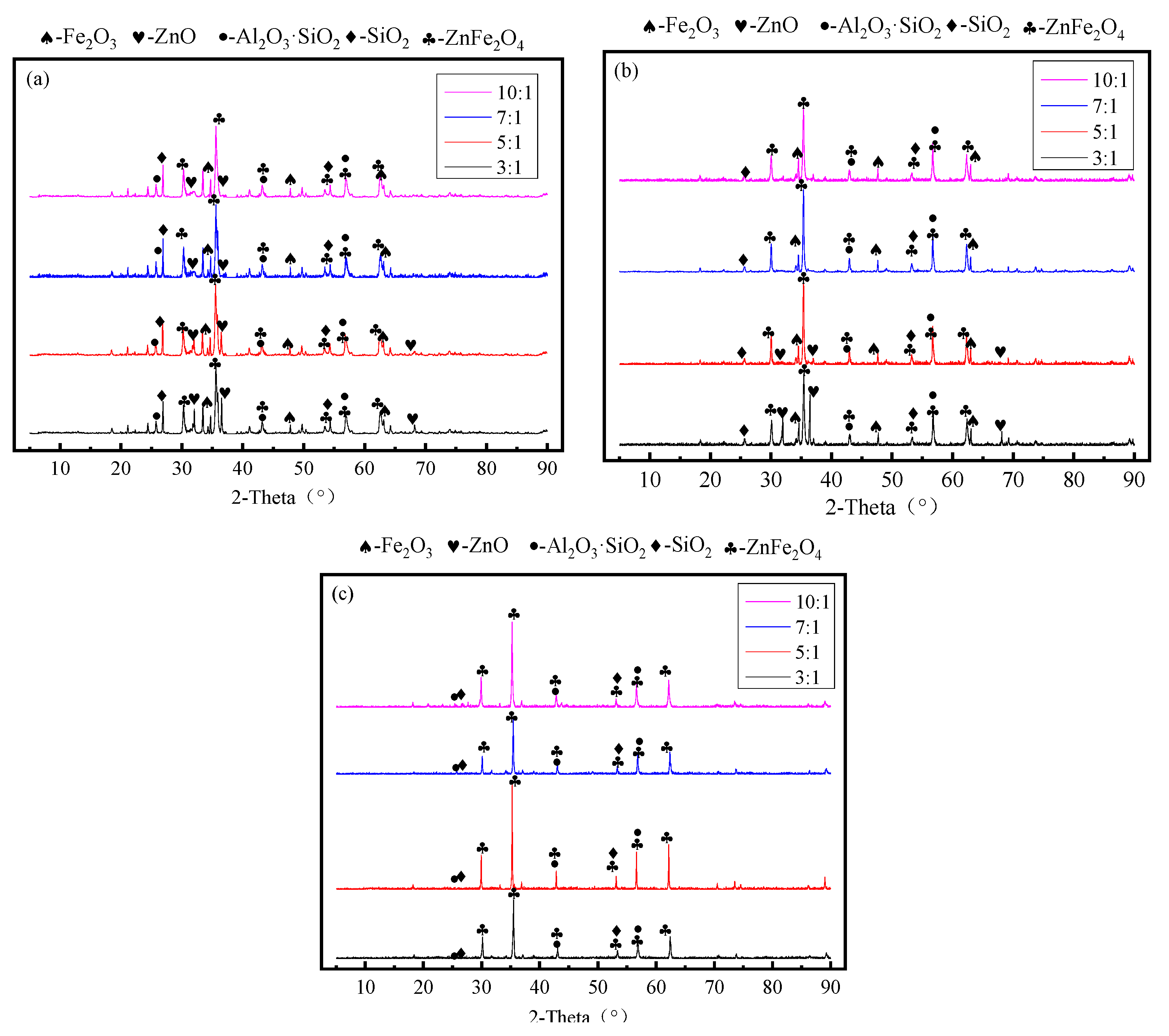
2.2.2. Characterization Methods
3. Results
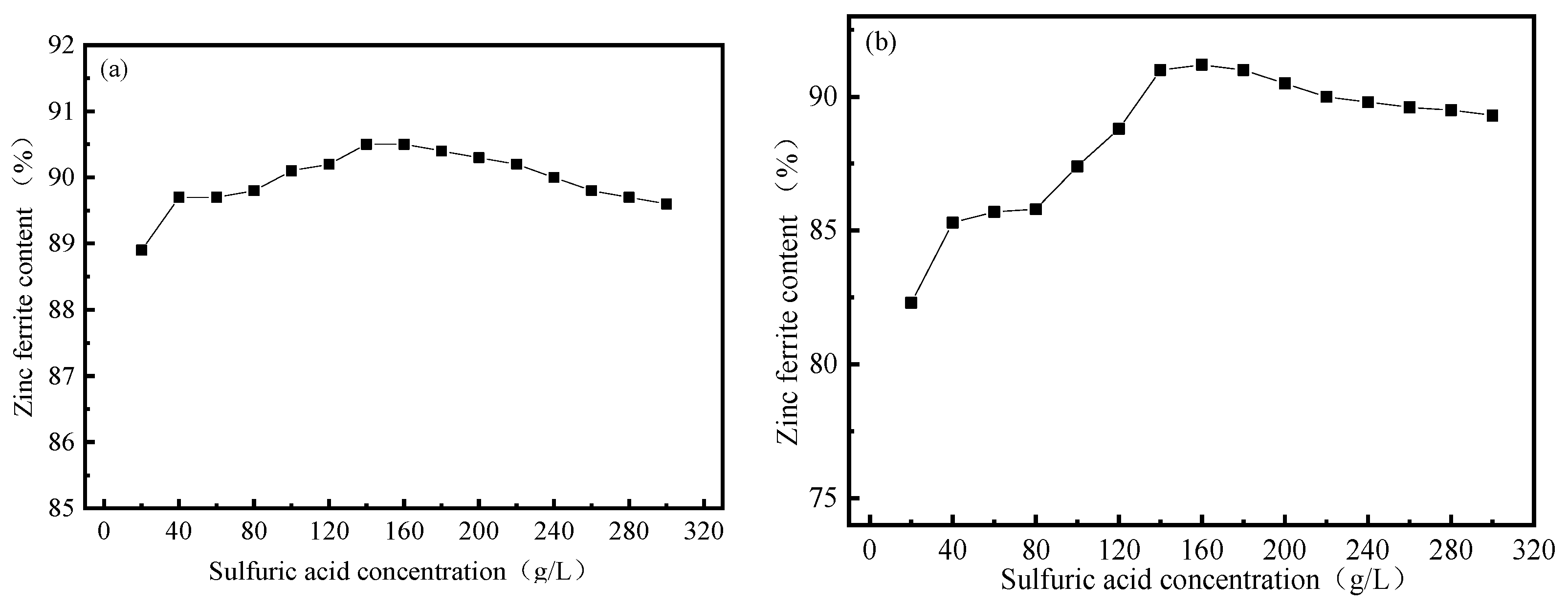
3.1. Effect of Sulfuric Acid Concentration
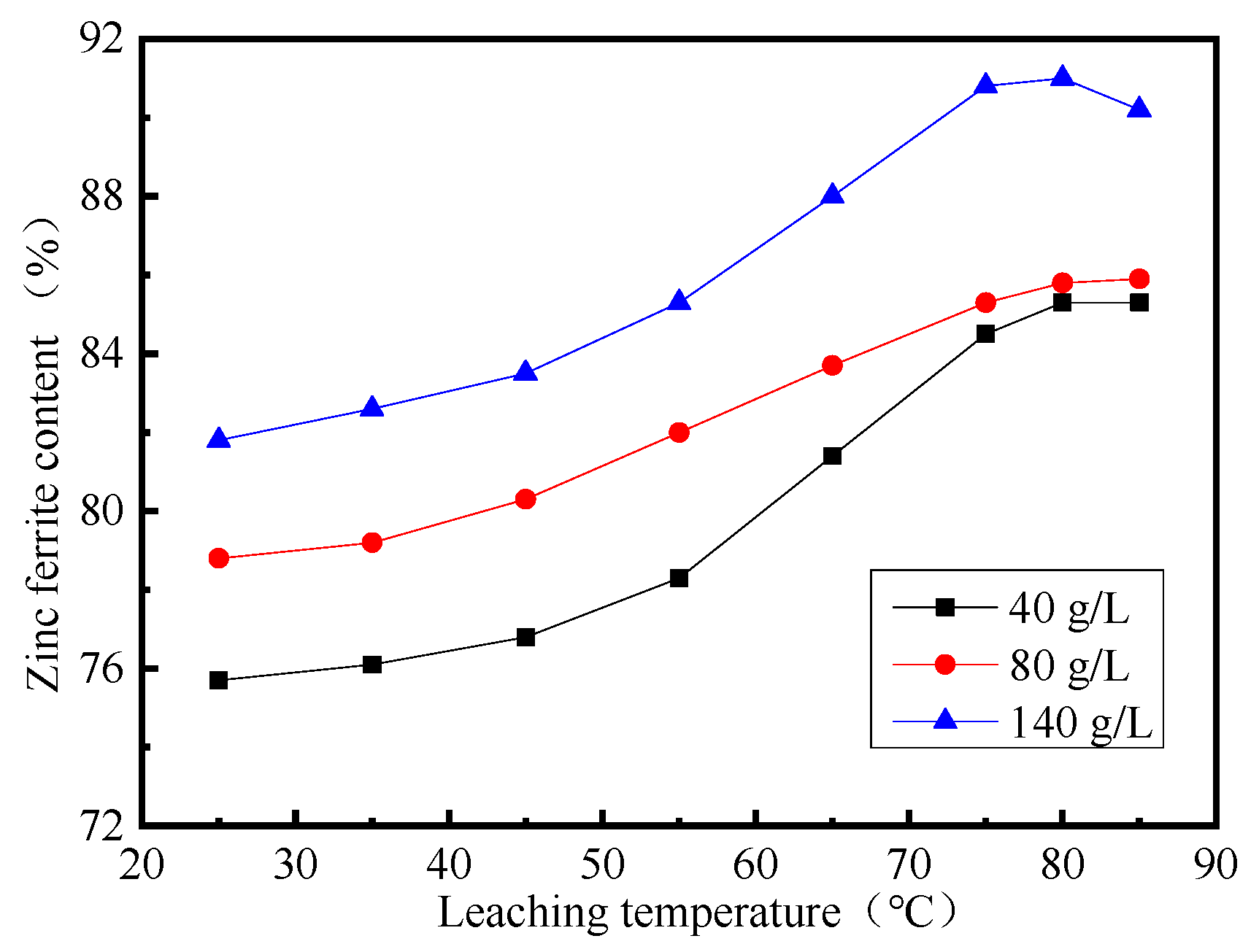
3.2. Effect of Leaching Temperature
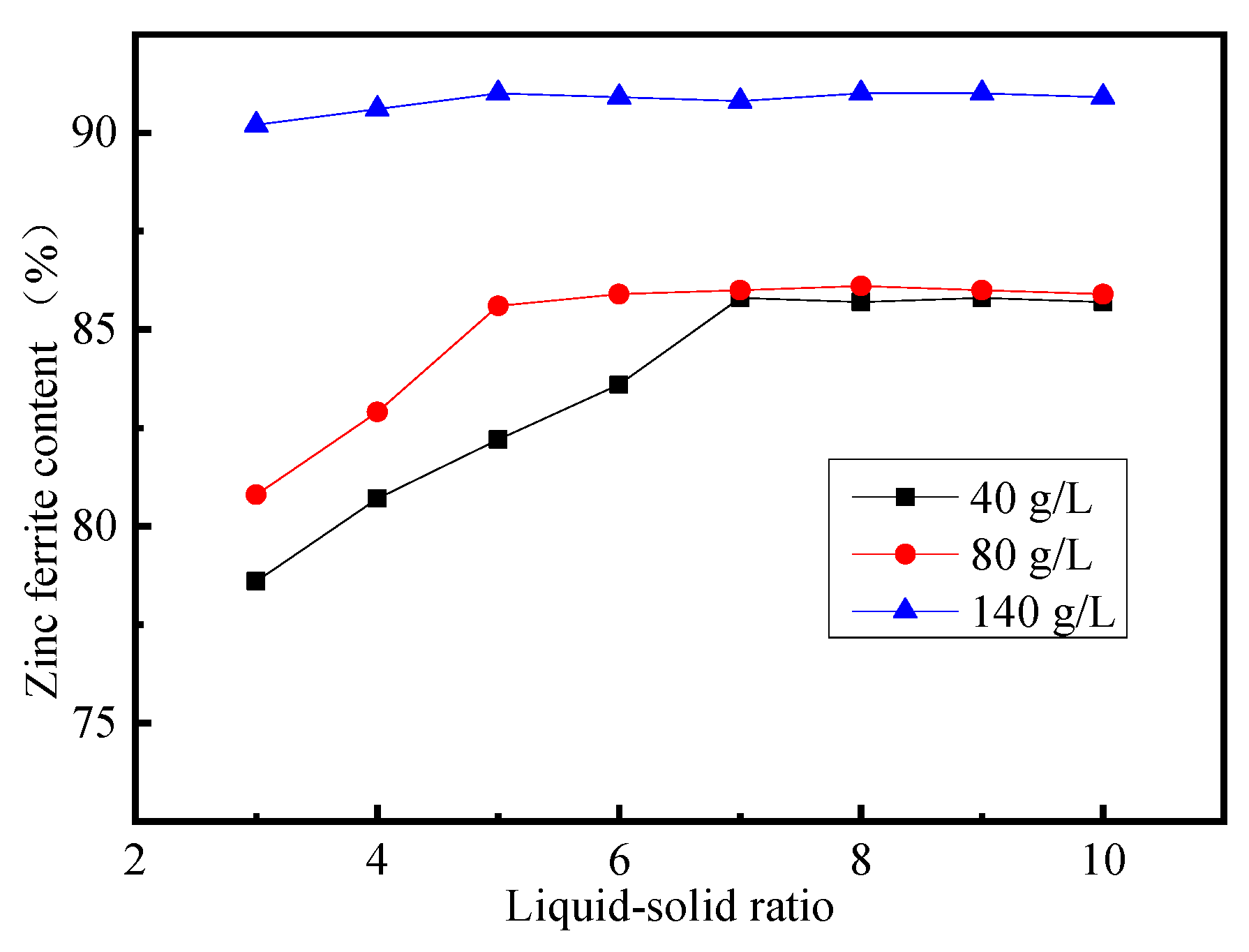
3.3. Effect of Liquid–Solid Ratio
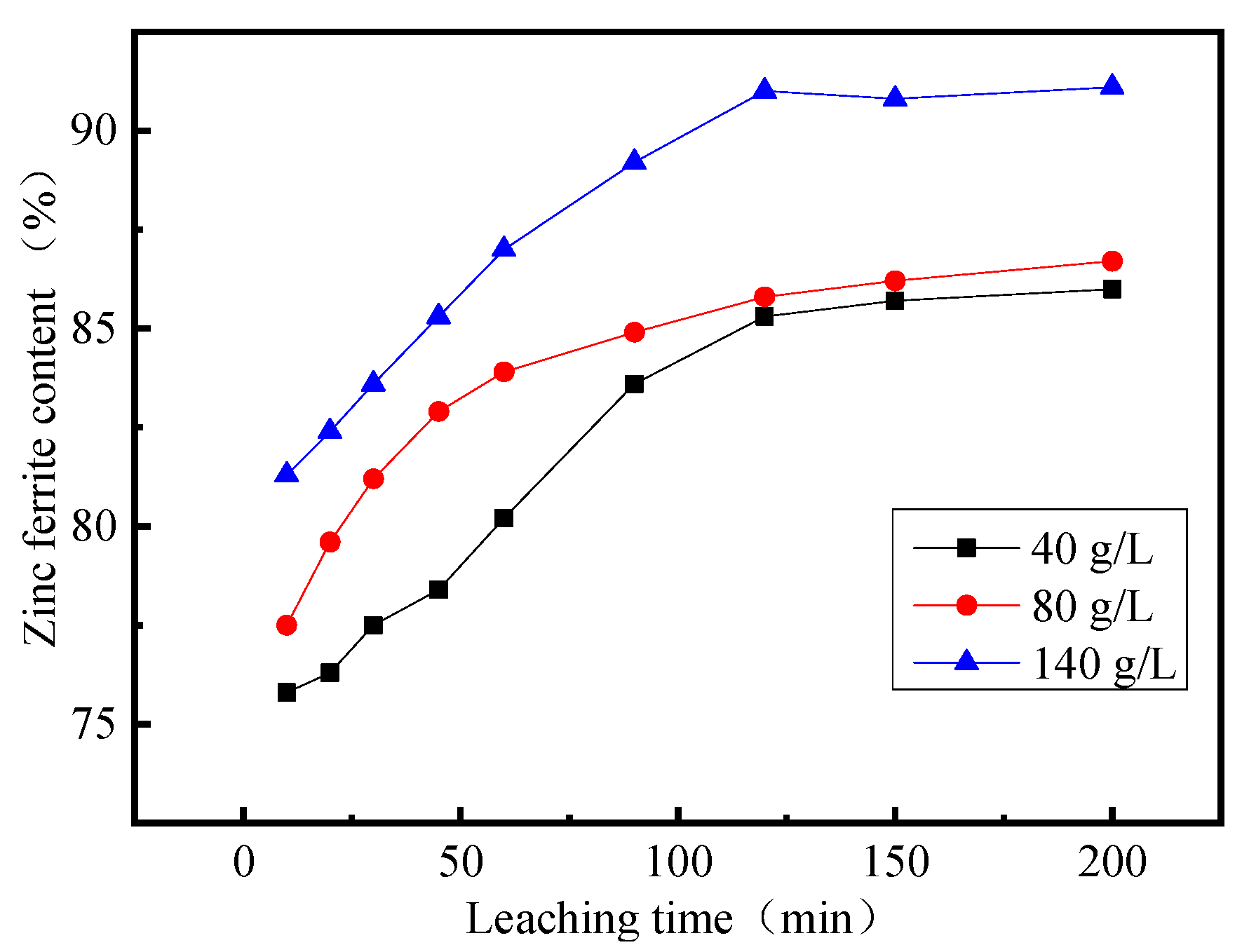
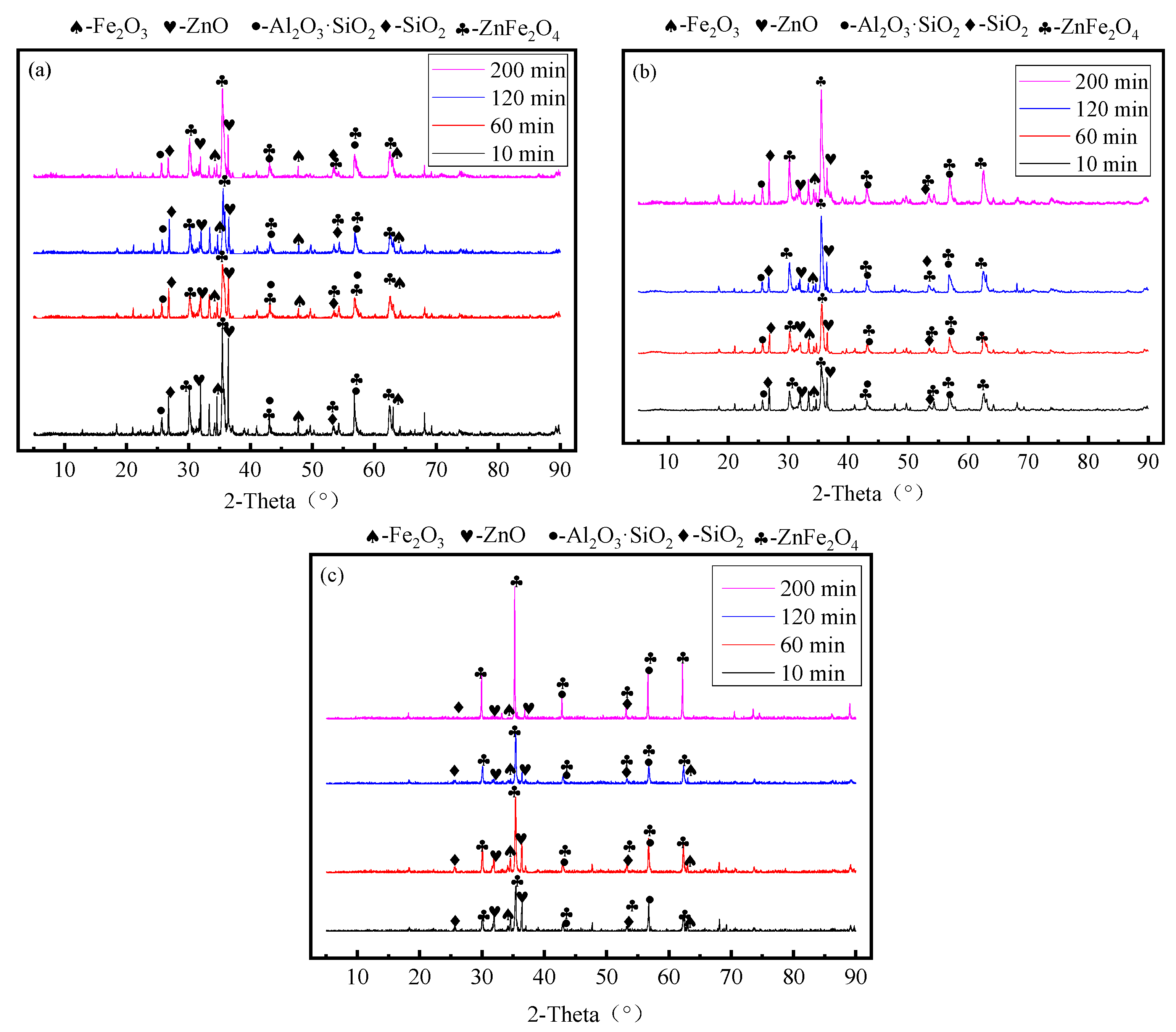
3.4. Effect of Leaching Time
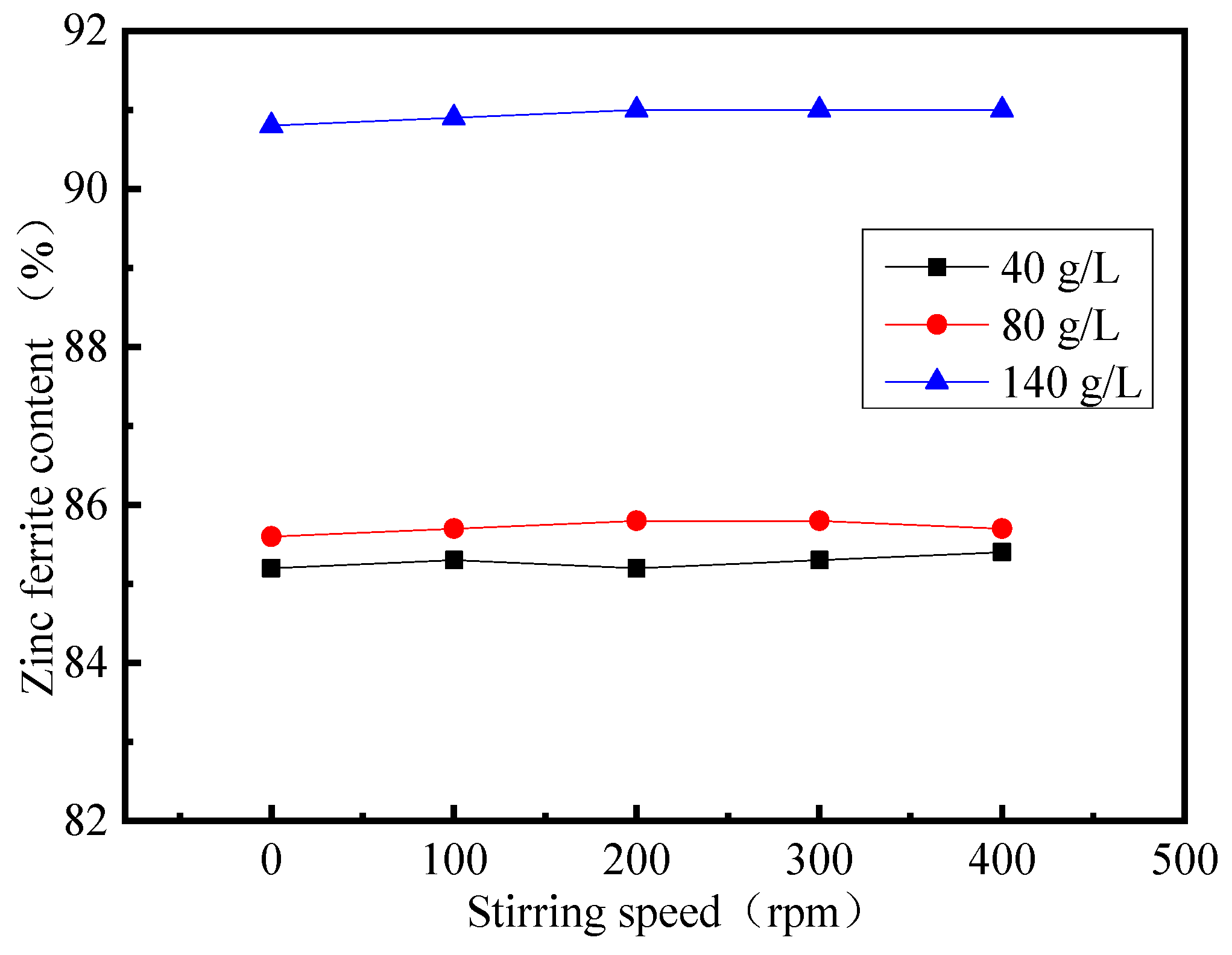
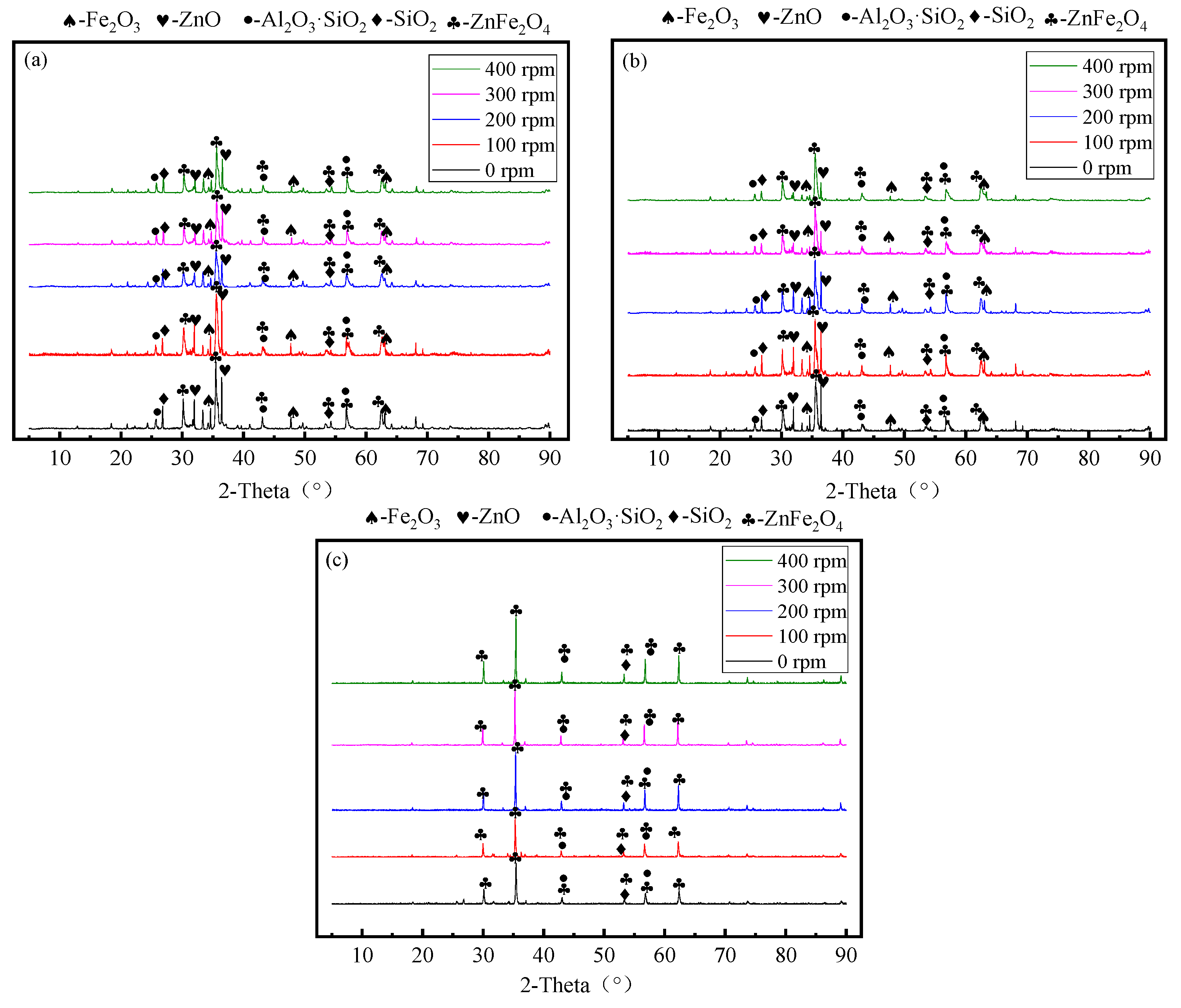
3.5. Effect of Stirring Speed
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, S.; Li, H.; Yang, X.; Xu, W.; Deng, X.; Yang, J. Study on Impact Crushing Characteristics of Minerals Based on Drop Weight Tests. Minerals 2023, 13, 632. [Google Scholar] [CrossRef]
- Hayes, S.M.; Root, R.A.; Perdrial, N.; Maier, R.M.; Chorover, J. Surficial weathering of iron sulfide mine tailings under semi-arid climate. Geochim. Cosmochim. Acta 2014, 141, 240–257. [Google Scholar] [CrossRef] [Green Version]
- Kříbek, B.; Zachariáš, J.; Knésl, I.; Míková, J.; Mihaljevič, M.; Veselovský, F.; Bamba, O. Geochemistry, mineralogy, and isotope composition of Pb, Zn, and Cu in primary ores, gossan and barren ferruginous crust from the Perkoa base metal deposit, Burkina Faso. J. Geochem. Explor. 2016, 168, 49–64. [Google Scholar] [CrossRef]
- Baggio, S.B.; Hartmann, L.A.; Massonne, H.J.; Theye, T.; Antunes, L.M. Silica gossan as a prospective guide for amethyst geode deposits in the Ametista do Sul mining district, Paraná volcanic province, southern Brazil. J. Geochem. Explor. 2015, 159, 213–226. [Google Scholar] [CrossRef]
- Yesares, L.; Sáez, R.; Ruiz De Almodóvar, G.; Nieto, J.M.; Gómez, C.; Ovejero, G. Mineralogical evolution of the Las Cruces gossan cap (Iberian Pyrite Belt): From subaerial to underground conditions. Ore Geol. Rev. 2017, 80, 377–405. [Google Scholar] [CrossRef]
- Laakso, K.; Rivard, B.; Rogge, D. Enhanced detection of gossans using hyperspectral data: Example from the Cape Smith Belt of northern Quebec, Canada. ISPRS J. Photogramm. Remote Sens. 2016, 114, 137–150. [Google Scholar] [CrossRef]
- Velasco, F.; Herrero, J.M.; Suárez, S.; Yusta, I.; Alvaro, A.; Tornos, F. Supergene features and evolution of gossans capping massive sulphide deposits in the Iberian Pyrite Belt. Ore Geol. Rev. 2013, 53, 181–203. [Google Scholar] [CrossRef]
- Costa, M.L.; Angélica, R.S.; Costa, N.C. The geochemical association Au-As-B-(Cu)-Sn-W in latosol, colluvium, lateritic iron crust and gossan in Carajás, Brazil: Importance for primary ore identification. J. Geochem. Explor. 1999, 67, 33–49. [Google Scholar] [CrossRef]
- Assawincharoenkij, T.; Hauzenberger, C.; Ettinger, K.; Sutthirat, C. Mineralogical and geochemical characterization of waste rocks from a gold mine in northeastern Thailand: Application for environmental impact protection. Environ. Sci. Pollut. Res. Int. 2018, 25, 3488–3500. [Google Scholar] [CrossRef]
- Lorenzo-Tallafigo, J.; Iglesias-González, N.; Mazuelos, A.; Romero, R.; Carranza, F. An alternative approach to recover lead, silver and gold from black gossan (polymetallic ore). Study of biological oxidation and lead recovery stages. J. Clean. Prod. 2019, 207, 510–521. [Google Scholar] [CrossRef]
- Mohamed, M.; Saleh, Q.; Mohamed, S.A.; Yasser, F.S.; Ahmed, M.A. Insights into the adsorption performance and mechanism of Cr(VI) onto porous nanocomposite prepared from gossans and modified coal interface: Steric, energetic, and thermodynamic parameters interpretations. Chin. J. Chem. Eng. 2023, in press. [CrossRef]
- Dunn, S.C.; von der Heyden, B.P. Gold remobilization in gossans of the Amani area, southwestern Tanzania. Ore Geol. Rev. 2021, 131, 104033. [Google Scholar] [CrossRef]
- Santoro, L.; Putzolu, F.; Mondillo, N.; Boni, M.; Herrington, R. Influence of genetic processes on geochemistry of Fe-oxy-hydroxides in supergene Zn non-sulfide deposits. Minerals 2020, 10, 602. [Google Scholar] [CrossRef]
- Yang, J.L. Research on the Separation and Recovery of Zinc and Iron from Gossan Ores. Ph.D. Thesis, Guangxi University, Nanning, China, 2012. (In Chinese). [Google Scholar]
- Yang, J.; Li, Z.; Huo, x.; Li, H.; Liao, S.; Ma, S.; Li, H. Study on the Preparation of ZnFeO4 by Roasting Zinc-Containing Gossan Ore. Processes 2023, 11, 1991. [Google Scholar] [CrossRef]
- Pannaparayil, T.; Komarneni, S.; Marande, R.; Zadarko, M. Subdomain zinc ferrite particles: Synthesis and characterization. J. Appl. Phys. 1990, 67, 5509–5511. [Google Scholar] [CrossRef]
- Kazantseva, N.E.; Bespyatykh, Y.I.; Sapurina, I.; Stejskal, J.; Vilčáková, J.; Sáha, P. Magnetic materials based on manganese-zinc ferrite with surface-organized polyaniline coating. J. Magn. Magn. Mater. 2006, 301, 155–165. [Google Scholar] [CrossRef]
- Yang, J.; Xu, S.; Zhu, P. Comparative study on properties of zinc ferrite and its purified products. J. Phys. Conf. Ser. 2021, 2044, 012068. [Google Scholar] [CrossRef]
- Ismail, M.; Hao, A.; He, S.; Huang, W.; Qin, N.; Bao, D. Reversible transitions among four modes of nonpolar resistive switching characteristics in nano-crystalline zinc ferrite magnetic thin films. J. Alloys Compd. 2018, 753, 100–110. [Google Scholar] [CrossRef]
- Ehrhardt, H.; Campbell, S.J.; Hofmann, M. Magnetism of the nanostructured spinel zinc ferrite. Scr. Mater. 2003, 48, 1141–1146. [Google Scholar] [CrossRef]
- Hankare, P.P.; Patil, R.P.; Jadhav, A.V.; Garadkar, K.M.; Sasikala, R. Enhanced photocatalytic degradation of methyl red and thymol blue using titania-alumina-zinc ferrite nanocomposite. Appl. Catal. B 2011, 107, 333–339. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Jinka, K.M.; Bajaj, H.C. Nanosized sulfated zinc ferrite as catalyst for the synthesis of nopol and other fine chemicals. Catal. Today 2012, 198, 98–105. [Google Scholar] [CrossRef]
- Li, Q.; Zhuang, L.; Chen, S.; Xu, J.; Li, H. Preparation of carbon-supported zinc ferrite and its performance in the catalytic degradation of mercaptan. Energy Fuels 2012, 26, 7092–7098. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, P.; Ma, S. Study on properties of gossan ore from zinc sulfide deposit. J. Phys. Conf. Ser. 2021, 2044, 012081. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, H.; Li, Z.; Huo, X.; Liao, S.; Ma, S.; Li, H. Study on Sulfuric Acid Leaching and Purification of Zinc Ferrite by Roasting Zinc-Containing Gossan Ore. Processes 2023, 11, 2149. https://doi.org/10.3390/pr11072149
Yang J, Li H, Li Z, Huo X, Liao S, Ma S, Li H. Study on Sulfuric Acid Leaching and Purification of Zinc Ferrite by Roasting Zinc-Containing Gossan Ore. Processes. 2023; 11(7):2149. https://doi.org/10.3390/pr11072149
Chicago/Turabian StyleYang, Jinlin, Hangyu Li, Zongyu Li, Xingnan Huo, Shizhen Liao, Shaojian Ma, and Hengjun Li. 2023. "Study on Sulfuric Acid Leaching and Purification of Zinc Ferrite by Roasting Zinc-Containing Gossan Ore" Processes 11, no. 7: 2149. https://doi.org/10.3390/pr11072149
APA StyleYang, J., Li, H., Li, Z., Huo, X., Liao, S., Ma, S., & Li, H. (2023). Study on Sulfuric Acid Leaching and Purification of Zinc Ferrite by Roasting Zinc-Containing Gossan Ore. Processes, 11(7), 2149. https://doi.org/10.3390/pr11072149





