An Experimental Study on the Influence of the Fractal Characteristics of X80 Steel Surface Morphology on Water Ring Stability
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Construction of Morphology on X80 Steel
- (1)
- Specimen cleaning: cut the X80 steel sheet into several test specimens with the size of 15 × 10 × 2 mm by the JMQ-60Z automatic precision cutting machine (Shanghai Metallurgical Equipment Company Ltd., Shanghai, China), place the test specimens in a mixed solution of acetone and anhydrous ethanol (volume ratio of 1:1) to ultrasonic cleaning for 10 min and dried at room temperature.
- (2)
- Etching solution preparation: prepare 50 mL of a 0.3 mol/L zinc acetate solution, 50 mL of a 0.1 mol/L zinc nitrate solution, and 50 mL of a 0.1 mol/L hexamethyltetramine solution, respectively. Then, uniformly mix the zinc nitrate solution and the hexamethyltetramine solution.
- (3)
- Surface etching: place the X80 steel specimens pretreated by step (1) in the zinc acetate solution and take them out after standing for 15 min, 20 min, 25 min, 30 min, 35 min, 40 min, 45 min, and 50 min, respectively. Then, place all specimens in a vacuum drying oven at 100 °C for 30 min. After that, calcine them in a resistance furnace at 400 °C for 2 h. Finally, take them out and cool them to room temperature.
- (4)
- Surface hydrophilic underwater oleophobic modification: pour the mixed solution of zinc nitrate and hexamethyltetramine and place the pretreated X80 steel specimens successively into the crucible of the resistance furnace. React together under a closed condition of 105 °C for 3 h. After the reaction, take out the test specimens, wash the floating matter on the surface with deionized water, and dry it with nitrogen.
- (5)
- Surface morphology collection: paste the specimen on the sample table of the sample room with conductive adhesive, observe from the low multiple, and gradually increase the multiple so that the surface morphology can be clearly and intuitively observed. Then, take photos of the surface morphology for preservation.
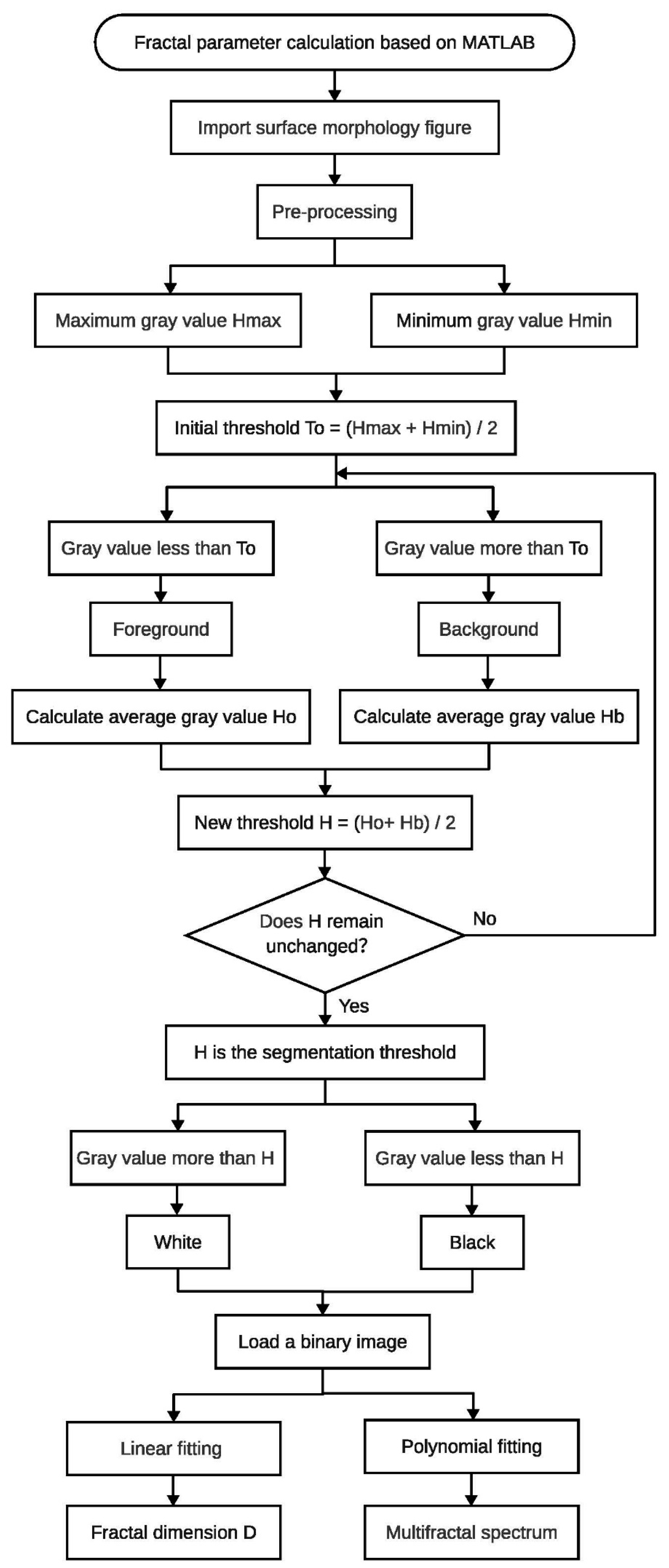
2.2. Fractal Characteristics of Morphology
2.3. Characterization of Water Ring Stability
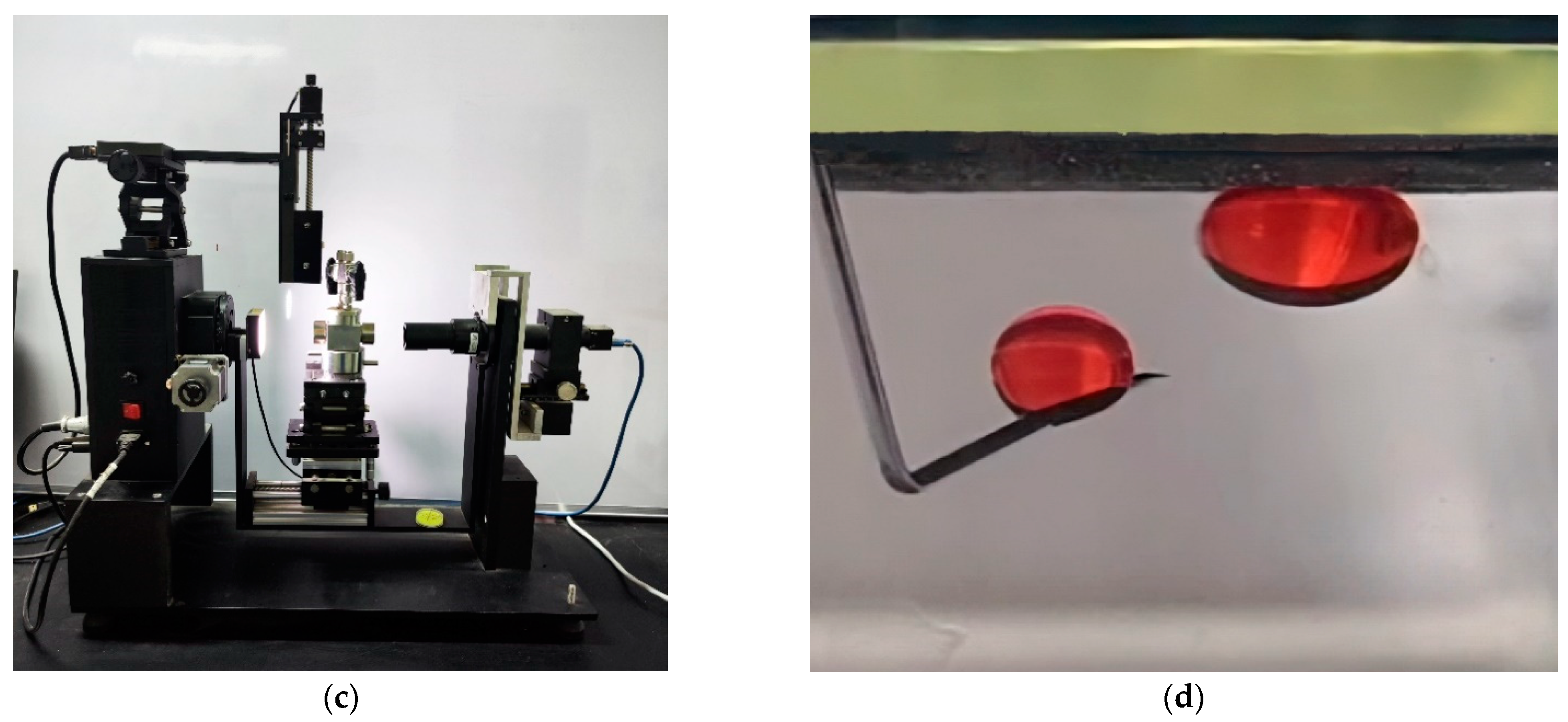
2.3.1. Contact Angle
2.3.2. Rolling Angle
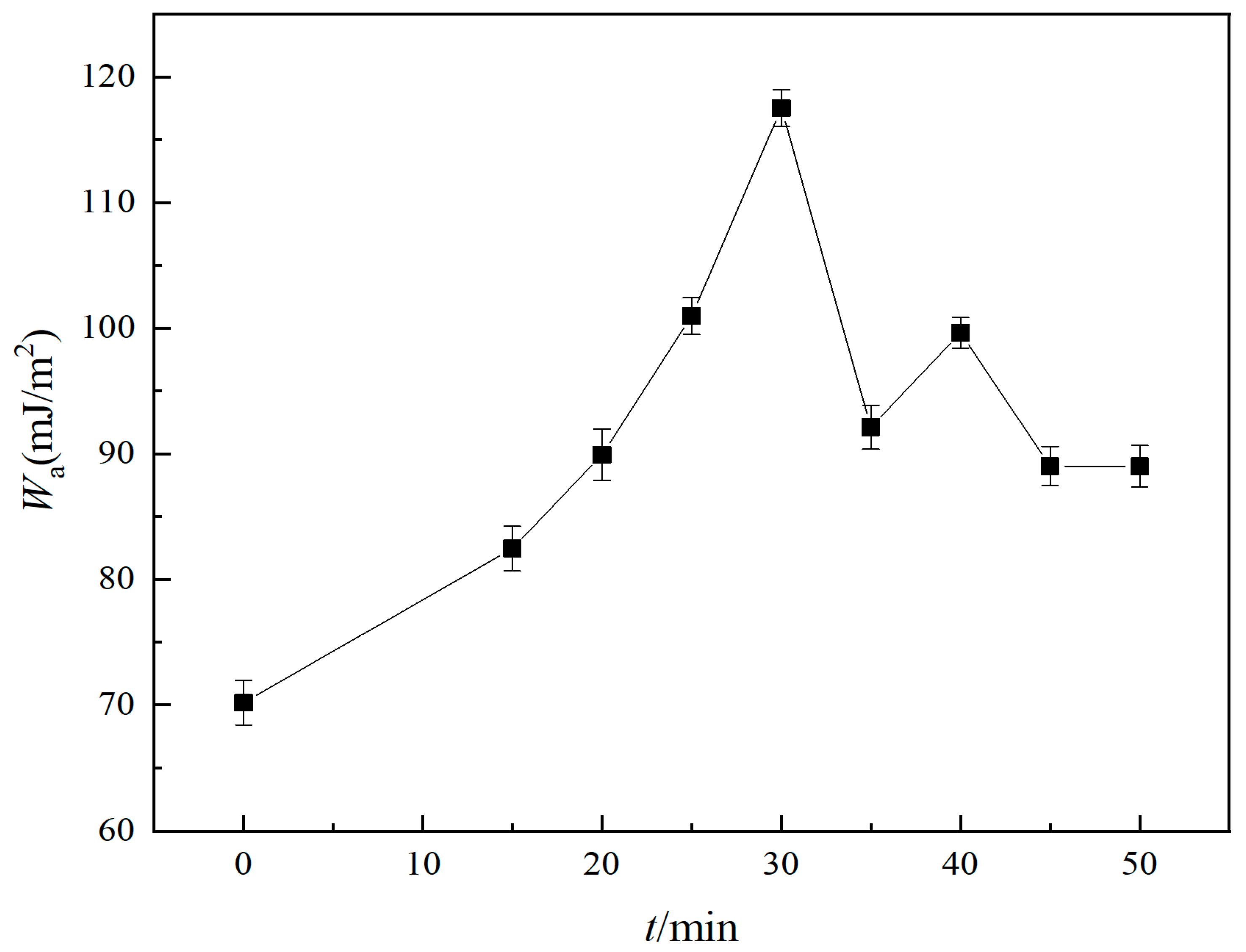
2.3.3. Adhesion Work
3. Results and Discussion
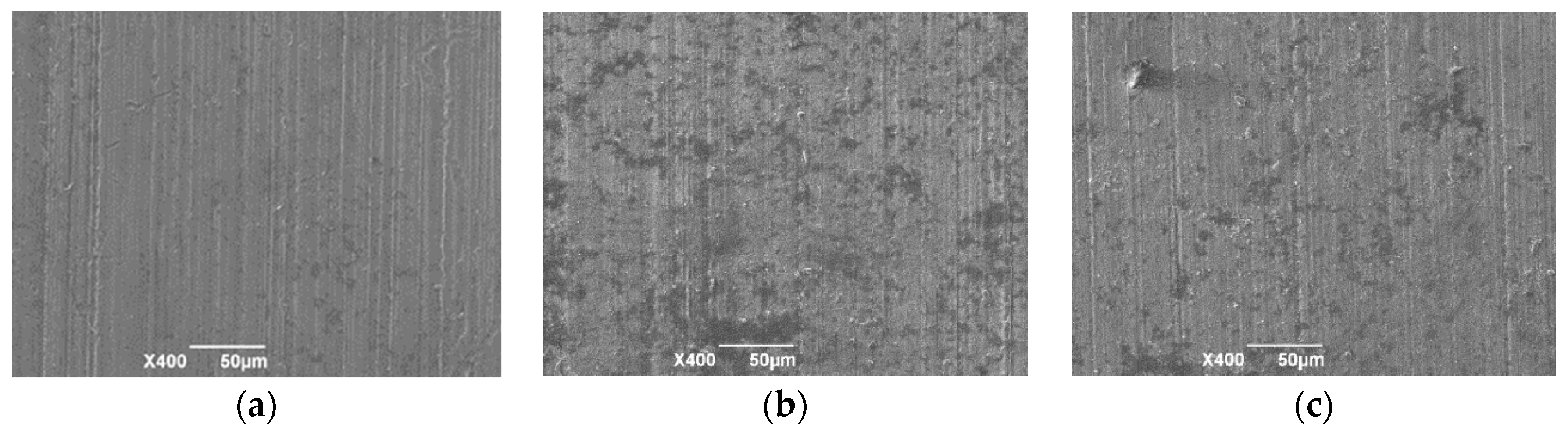
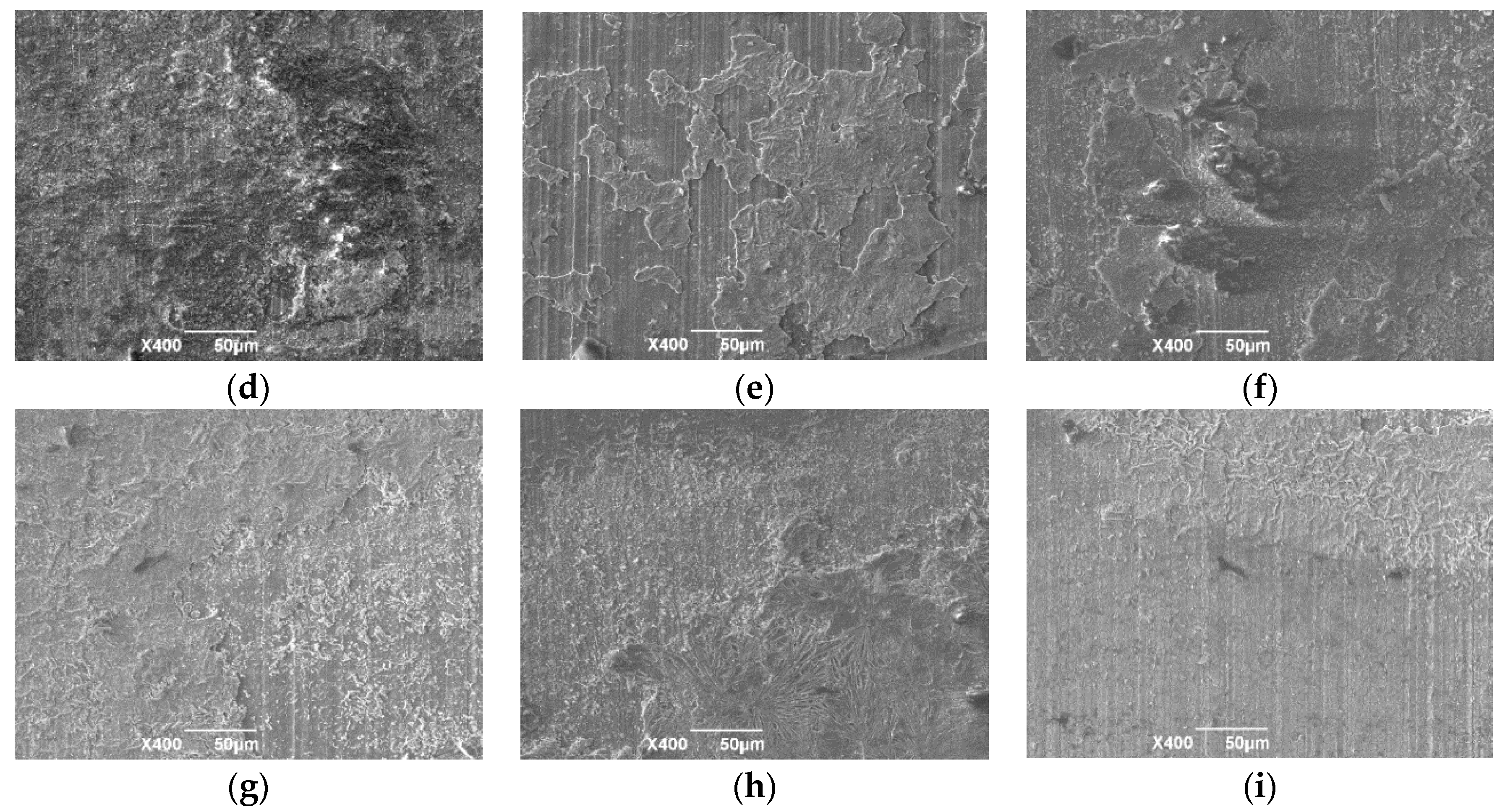
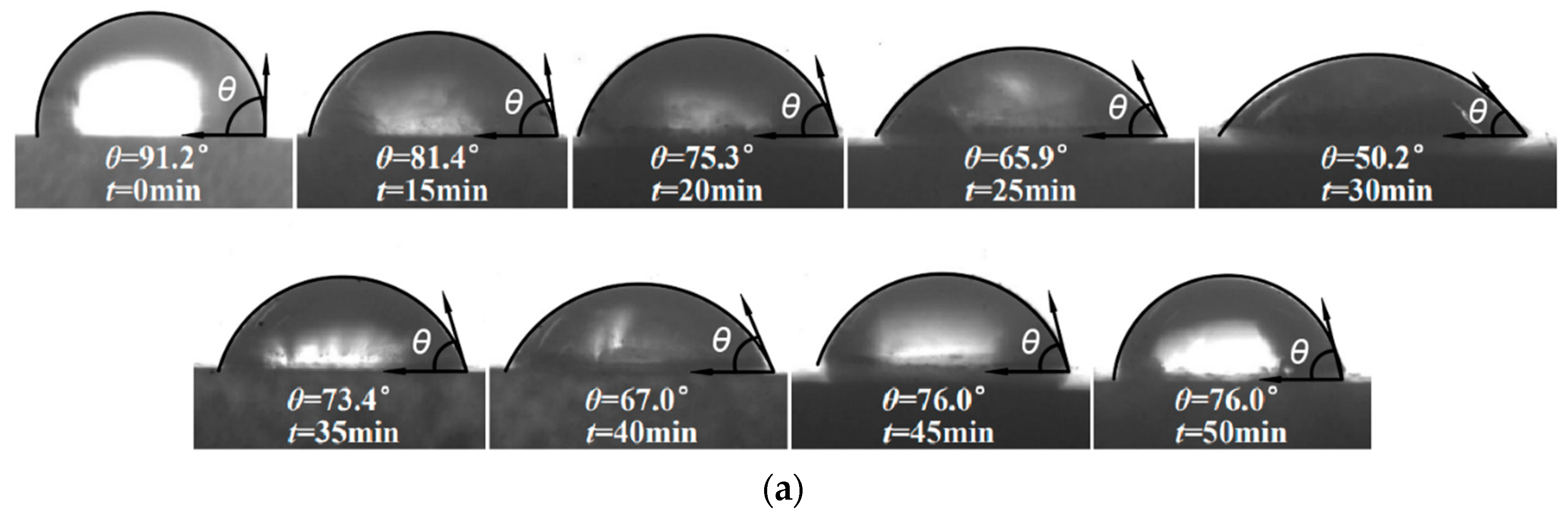
3.1. Surface Morphology at Different Reaction Times
3.2. Fractal Parameters under Different Reaction Time
3.3. Effect of Fractal Parameters on the Stability of Water Ring
3.3.1. Effect of Fractal Parameters on the Contact Angle
3.3.2. Effect of Fractal Parameters on the Rolling Angle
3.3.3. Effect of Fractal Parameters on the Adhesion Work
3.4. Discussion
4. Conclusions
- (1)
- The fractal dimension of the X80 steel specimens first increased and then decreased with the increase of reaction time and was greater than 2, indicating that the surface had distinct fractal characteristics. In addition, the spectral difference Δf(α) was greater than 0, demonstrating that the proportion of the small-scale morphology on the surface of the X80 specimens was large. The homogeneity characterized by the spectral width Δα was in overall agreement with the SEM observations. Therefore, the combination of multifractal spectrum parameters provided a quantitative and more comprehensive characterization of surface morphology compared to a single fractal dimension.
- (2)
- The contact angle of distilled water on the surface of the X80 steel specimens decreased with the increase of fractal dimension, while the contact angle of underwater oil droplets increased with the increase of the fractal dimension. When the fractal dimension reached the maximum value of 2.0808, the measured contact angle of distilled water on the surface of the X80 steel specimens reached the minimum value of 50.2°, and the contact angle of underwater oil droplets was 166.4° at maximum. Furthermore, both the rolling angle and the adhesion work increased with the increase of the fractal dimension.
- (3)
- For the core annular flow, the larger the fractal dimension of the pipe surface, the stronger the hydrophilic underwater oleophobic properties of the pipe wall, the more stable the water ring would be. Consequently, increasing the fractal dimension of the pipe surface was beneficial to enhancing the stability of the water ring, and the efficiency of the core annular flow to transport heavy oil would be improved.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Santos, R.G.; Loh, W.; Bannwart, A.C.; Trevisan, O.V. An Overview of Heavy Oil Properties and Its Recovery and Transportation Methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef] [Green Version]
- Vegad, G.D.; Jana, A.K. Viscosity Reduction of Indian Heavy Crude Oil by Emulsification to O/W Emulsion Using Polysorbate-81. J. Surfactants Deterg. 2020, 24, 301–311. [Google Scholar] [CrossRef]
- Soleimani, A.S. Mohammad Amin, Movahedirad, Salman, An Investigation on the Viscosity Reduction of Iranian Heavy Crude Oil through Dilution Method. Iran. J. Chem. Chem. Eng. 2021, 40, 934–944. [Google Scholar]
- Dove, I.J.; Buckner, S.J. Method of Piping Fluids. U.S. Patent No.0759374, 1 November 1904. [Google Scholar]
- Du, M.; Jing, J.; Xiong, X.; Lang, B.; Wang, X.; Shi, S. Experimental Study on Heavy Oil Drag Reduction in Horizontal Pipelines by Water Annular Conveying. Fluid Dyn. Mater. Process. 2022, 18, 81–91. [Google Scholar] [CrossRef]
- Jing, J.; Du, M.; Yin, R.; Wang, Y.; Teng, Y. Numerical study on two-phase flow characteristics of heavy oil-water ring transport boundary layer. J. Pet. Sci. Eng. 2020, 191, 107173. [Google Scholar] [CrossRef]
- Silva, R.; Mohamed, R.S.; Bannwart, A.C. Wettability alteration of internal surfaces of pipelines for use in the transportation of heavy oil via core-flow. J. Pet. Sci. Eng. 2006, 51, 17–25. [Google Scholar] [CrossRef]
- Santos, R.; Mohamed, R.S.; Bannwart, A.C.; Loh, W. Contact angle measurements and wetting behavior of inner surfaces of pipelines exposed to heavy crude oil and water. J. Pet. Sci. Eng. 2006, 51, 9–16. [Google Scholar] [CrossRef]
- Shi, J.; Gourma, M.; Yeung, H. A CFD study on horizontal oil-water flow with high viscosity ratio. Chem. Eng. Sci. 2021, 229, 116097. [Google Scholar] [CrossRef]
- Ding, R. Study on Preparation of Micro-Nano Structured 304 Stainless Steel with Extreme Wettability by Using Femtosecond Laser; University of Electronic Science and Technology of China: Chengdu, China, 2018. [Google Scholar]
- Buczko, Z.; Olkowicz, K.; Krasucki, J.; Grabowiecki, K.; Osuchowska, E.; Tomassi, P. Superhydrophobic properties of aluminium produced by surface abrasive blasting, anodic oxidation and fatty acid impregnation. Trans. IMF 2021, 99, 73–79. [Google Scholar] [CrossRef]
- Li, J.; Du, F.; Li, Q.; Xu, J.K.; Yu, H.D. Wettability of Alumina Substrates with Micro-Morphology and Microstructure. Adv. Mater. Res. 2013, 838–841, 166–169. [Google Scholar] [CrossRef]
- Tong, J.; Liu, S.; Peng, R.; Sun, H.; Jiang, S. Development of a micro/nano composite super-hydrophobic silicon surface with nail-shaped texture/dual self-assembly monolayers and its wetting behavior. Appl. Surf. Sci. 2021, 544, 148803. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Hang, T.; Li, M. Morphologies and wetting properties of copper film with 3D porous micro-nano hierarchical structure prepared by electrochemical deposition. Appl. Surf. Sci. 2016, 372, 7–12. [Google Scholar] [CrossRef]
- Sajid, H.U.; Kiran, R. Influence of corrosion and surface roughness on wettability of ASTM A36 steels. J. Constr. Steel Res. 2018, 144, 310–326. [Google Scholar] [CrossRef]
- Sajid, H.U.; Kiran, R. Improving the wettability of structural steels by employing ionic liquids. J. Mol. Liquids 2021, 324, 115137. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, M.; Qi, H.; Liang, A.; Wang, Y.; Sun, N.; Wu, Z. Fractal Characteristics of the Microstructures of Three Hydrophobic Surfaces with Steel Substrate and Their Effects on Wettability. Chem. J. Chin. Univ. 2020, 41, 1313–1319. [Google Scholar]
- Huang, W.; Samanta, A.; Chen, Y.; Baek, S.; Shaw, S.K.; Ding, H. Machine learning model for understanding laser superhydrophobic surface functionalization. J. Manuf. Process. 2021, 69, 491–502. [Google Scholar] [CrossRef]
- Karl, C.W.; Krauklis, A.E.; Lang, A.; Giese, U. Characterization of Rough PTFE Surfaces by the Modified Wilhelmy Balance Technique. Polymers 2020, 12, 1528. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, Z.; Wu, J. Direct image-based fractal characterization of micromorphology of calcium carbonate fouling crystals. Chin. J. Chem. Eng. 2020, 28, 466–476. [Google Scholar] [CrossRef]
- Das, A.; Chawla, V.; Matos, R.S.; da Fonseca Filho, H.D.; Yadav, R.P.; Ţălu, Ş.; Kumar, S. Surface microtexture and wettability analysis of quasi two-dimensional (Ti, Al)N thin films using fractal geometry. Surf. Coat. Technol. 2021, 421, 127420. [Google Scholar] [CrossRef]
- Mwema, F.M.; Akinlabi, E.T.; Oladijo, O.P. Micromorphology of sputtered aluminum thin films: A fractal analysis. Mater. Today Proc. 2019, 18, 2430–2439. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Jackson, R.L. A mixed lubrication analysis of a thrust bearing with fractal rough surfaces. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2019, 234, 608–621. [Google Scholar] [CrossRef] [Green Version]
- Ţălu, Ş.; Morozov, I.A.; Yadav, R.P. Multifractal analysis of sputtered indium tin oxide thin film surfaces. Appl. Surf. Sci. 2019, 484, 892–898. [Google Scholar] [CrossRef]
- Modabberasl, A.; Sharifi, M.; Shahbazi, F.; Kameli, P. Multifractal analysis of DLC thin films deposited by pulsed laser deposition. Appl. Surf. Sci. 2019, 479, 639–645. [Google Scholar] [CrossRef]
- Talu, S.; Astinchap, B.; Abdolghaderi, S.; Shafiekhani, A.; Morozov, I.A. Multifractal investigation of Ag/DLC nanocomposite thin films. Sci. Rep. 2020, 10, 22266. [Google Scholar] [CrossRef]
- Shakoury, R.; Grayeli Korpi, A.; Ghosh, K.; Ţălu, Ş.; Rezaee, S.; Mwema, F.; Mardani, M.; Arman, A. Stereometric and scaling law analysis of surface morphology of stainless steel type AISI 304 coated with Mn: A conventional and fractal evaluation. Mater. Res. Express 2019, 6, 116436. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Li, C.; Jing, W.; Lin, Q.; Jiang, Z.; Zhang, Y. Morphology of Al2O3 Film Fabricated by Atomic Layer Deposition. Rare Met. Mater. Eng. 2015, 44, 5. [Google Scholar]
- Lyu, Y.; Huang, Q.; Li, R.; Zhang, F.; Liu, L.; Zhang, H.; Zhang, Y.; Cui, Y.; Wang, Q. Effect of temperature on wall sticking of heavy oil in low-temperature transportation. J. Pet. Sci. Eng. 2021, 206, 108944. [Google Scholar] [CrossRef]
- Xu, C.; Wang, W.; Song, P.; Gao, H.; Chen, Y. Corrosion Behavior of X80 Pipeline Steel in Wetting-Drying Alternating and Water Saturated Hami Soil. Surf. Technol. 2020, 49, 10. [Google Scholar]
- Guo, S. Preparation and Estimation of Superhydrophobic CuO Surfaces and Research of Water-Droplet Evaporation; Dalian University of Technology: Dalian, China, 2012. [Google Scholar]
- Lin, Q.; Meng, Q.; Wang, C.; Zhang, Q.; Zhao, M.; Jiang, Z. The influence of fractal dimension in the microcontact of three-dimensional elastic-plastic fractal surfaces. Int. J. Adv. Manuf. Technol. 2019, 104, 17–25. [Google Scholar] [CrossRef]
- Qi, H. Study on Inner Wall Wettability and Flow Property in Liquid Pipe; Southwest Petroleum University: Chengdu, China, 2017. [Google Scholar]







| t/min | D | Δf(α) | Δα |
|---|---|---|---|
| 0 | 2.0228 | 0.0117 | 0.1665 |
| 15 | 2.0397 | 0.0552 | 0.2294 |
| 20 | 2.0508 | 0.0391 | 0.1833 |
| 25 | 2.0624 | 0.0272 | 0.2149 |
| 30 | 2.0808 | 0.0227 | 0.2585 |
| 35 | 2.0765 | 0.0184 | 0.2633 |
| 40 | 2.0668 | 0.0221 | 0.2054 |
| 45 | 2.0697 | 0.0383 | 0.2289 |
| 50 | 2.0613 | 0.025 | 0.1818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Hu, J.; Ju, Y.; Jiang, H.; Liu, M. An Experimental Study on the Influence of the Fractal Characteristics of X80 Steel Surface Morphology on Water Ring Stability. Processes 2023, 11, 2150. https://doi.org/10.3390/pr11072150
Qi H, Hu J, Ju Y, Jiang H, Liu M. An Experimental Study on the Influence of the Fractal Characteristics of X80 Steel Surface Morphology on Water Ring Stability. Processes. 2023; 11(7):2150. https://doi.org/10.3390/pr11072150
Chicago/Turabian StyleQi, Hongyuan, Juan Hu, Yiyi Ju, Huayi Jiang, and Mei Liu. 2023. "An Experimental Study on the Influence of the Fractal Characteristics of X80 Steel Surface Morphology on Water Ring Stability" Processes 11, no. 7: 2150. https://doi.org/10.3390/pr11072150




