Investigation of Oxygen Evolution Performance of Highly Efficient Water Electrolysis Catalyst: NiFe LDH/BPene
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of BPene
2.3. Preparation of NiFe LDH
2.4. Fabrication of NiFe LDH/BPene
2.5. Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
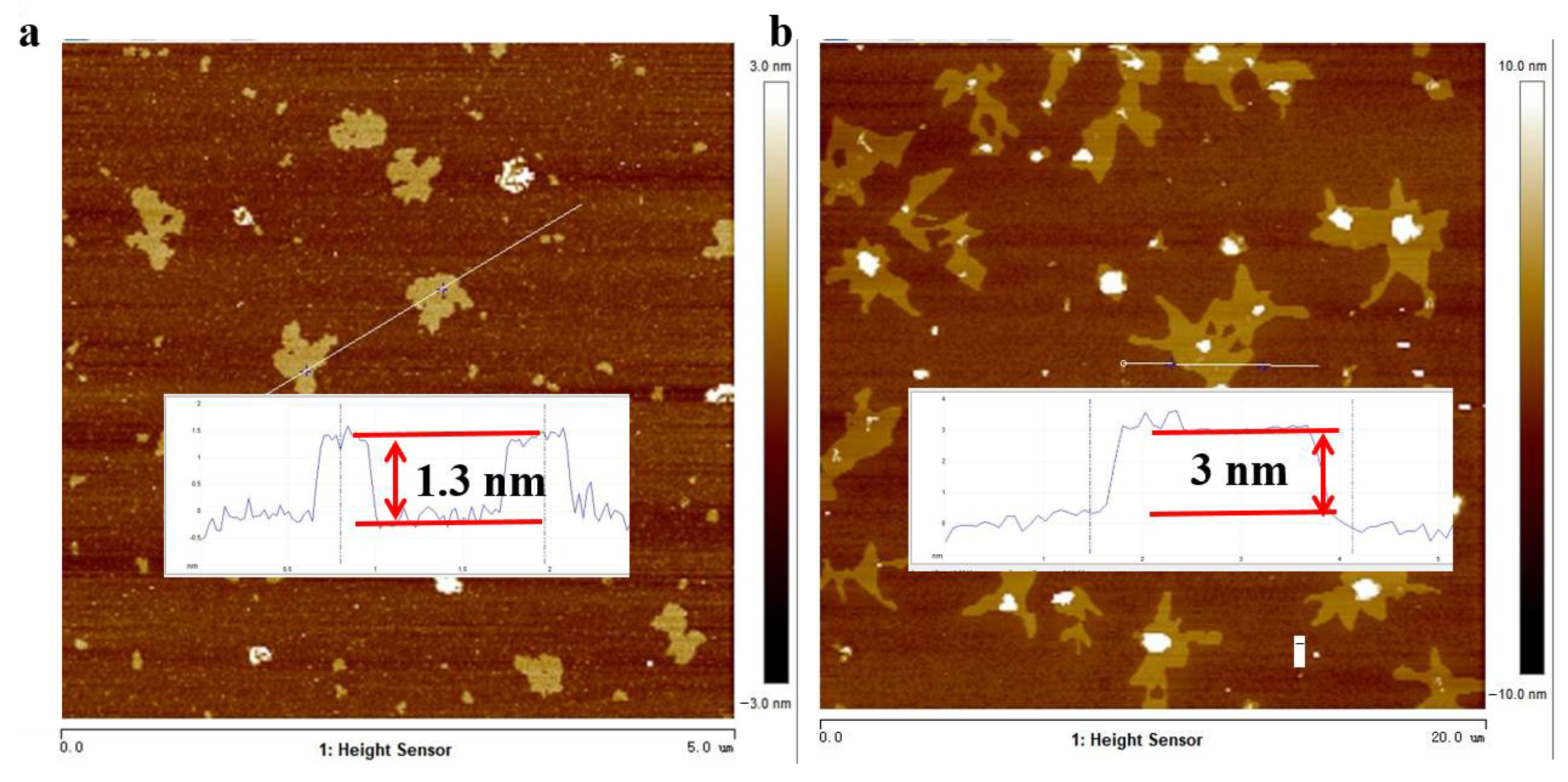
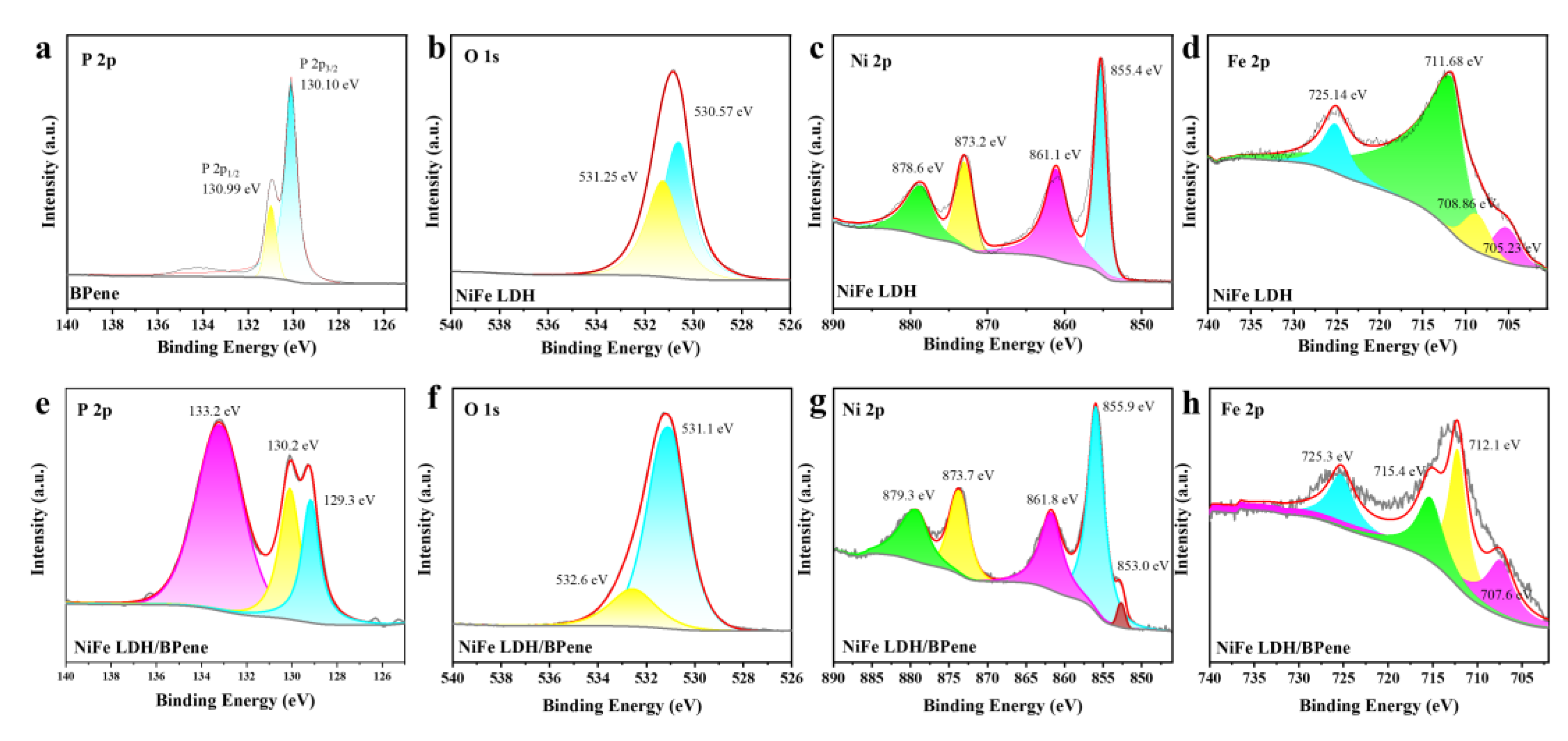
3.1. Characterization of NiFe LDH/BPene
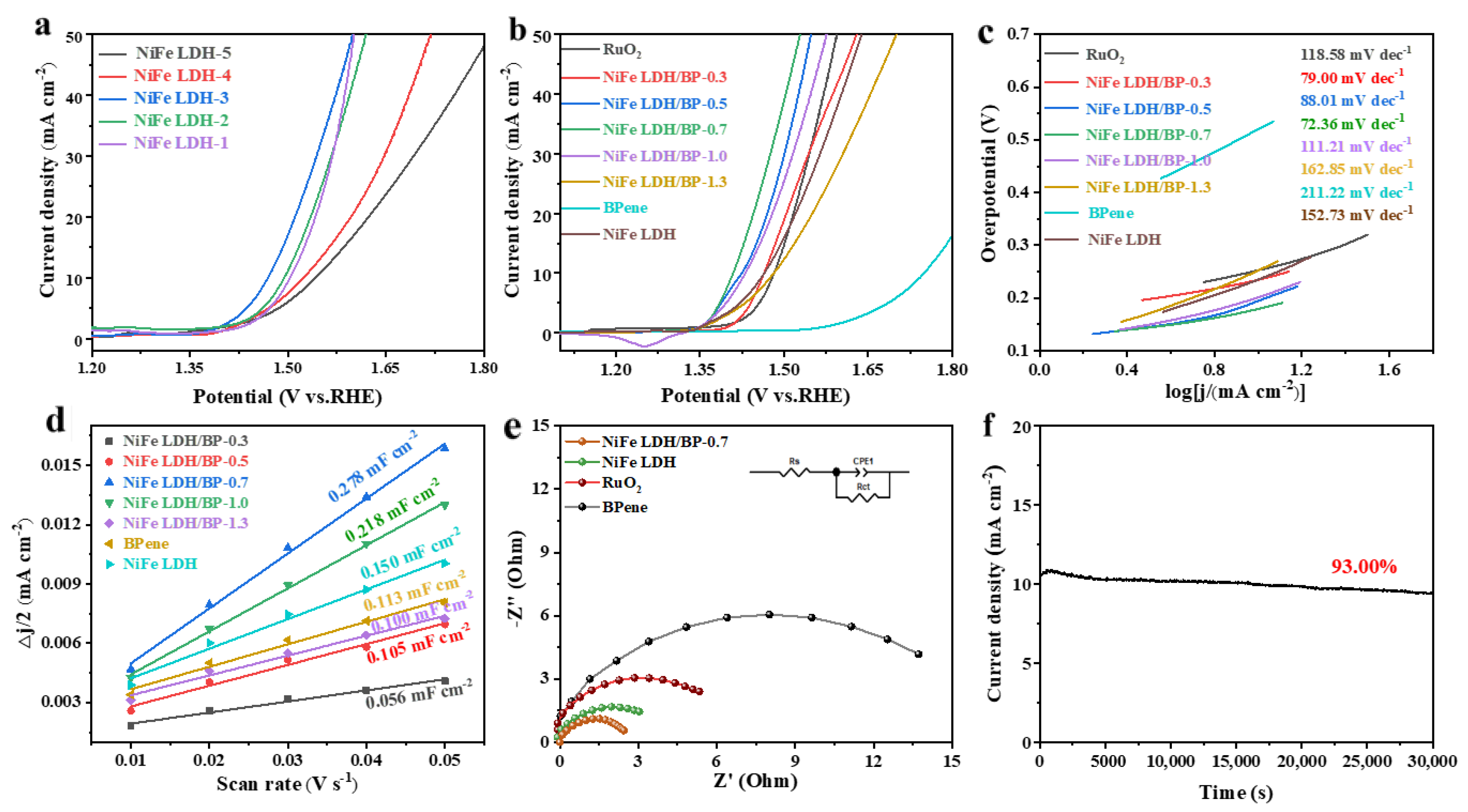
3.2. OER Performance Testing of NiFe LDH/BPene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.; Cheng, B.; Fan, J.; Yu, J.; Liu, G. Near-infrared absorbing 2D/3D ZnIn2S4/N-doped graphene photocatalyst for highly efficient CO2 capture and photocatalytic reduction. Sci. China Mater. 2020, 63, 552–565. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.; Xing, T.; Chen, Y.; Zheng, L.; Peng, J.; Wu, C.; Chang, B.; Luo, Z.; Wang, X. Hierarchically structured spherical nickel cobalt layered double hydroxides particles grown on biomass porous carbon as an advanced electrode for high specific energy asymmetric supercapacitor. J. Energy Storage 2020, 30, 101454. [Google Scholar]
- Shao, L.; Sun, H.; Miao, L.; Chen, X.; Han, M.; Sun, J.; Liu, S.; Li, L.; Cheng, F.; Chen, J. Facile preparation of NH2-functionalized black phosphorene for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 2494–2499. [Google Scholar] [CrossRef]
- Song, X.; Li, B.; Peng, W.; Wang, C.; Li, K.; Zhu, Y.; Mei, Y. A palladium doped 1T-phase molybdenum disulfide-black phosphorene two-dimensional van der Waals heterostructure for visible-light enhanced electrocatalytic hydrogen evolution. Nanoscale 2021, 13, 5892–5900. [Google Scholar]
- Sun, S.; Zhou, X.; Cong, B.; Hong, W.; Chen, G. Tailoring the d-Band Centers Endows (NixFe1−x)2P Nanosheets with Efficient Oxygen Evolution Catalysis. ACS Catal. 2020, 10, 9086–9097. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Liu, D.; Zhang, Y.; Chu, P.K.; Zhou, Z.-Y.; Yu, X.-F. Insight into the overpotentials of electrocatalytic hydrogen evolution on black phosphorus decorated with metal clusters. Electrochim. Acta 2020, 358, 136902. [Google Scholar]
- Xu, Q.; Zhu, Y.; Xie, T.; Shi, C.; Zhang, N. Simultaneous Preparation and Functionalization of Ultrathin Few−layer Black Phosphorus Nanosheets and Their Electrocatalytic OER and HER Performance. Chem. Cat. Chem. 2020, 13, 592–602. [Google Scholar]
- Takashima, Y.; Suwaki, Y.; Tsuruoka, T.; Akamatsu, K. Cooperative catalysis in a metal-organic framework via post-synthetic immobilisation. Dalton Trans. 2022, 51, 9229–9232. [Google Scholar]
- Yang, R.; Fan, Y.; Zhang, Y.; Mei, L.; Zhu, R.; Qin, J.; Hu, J.; Chen, Z.; Hau Ng, Y.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem.-Int. Ed. 2023, 62, e202218016. [Google Scholar]
- Pakhira, S.; Upadhyay, S.N. Efficient electrocatalytic H2 evolution mediated by 2D Janus MoSSe transition metal dichalcogenide. Sustain. Energy Fuels 2022, 6, 1733–1752. [Google Scholar]
- Zhang, L.; Jang, H.; Liu, H.; Kim, M.G.; Yang, D.; Liu, S.; Liu, X.; Cho, J. Sodium-Decorated Amorphous/Crystalline RuO2 with Rich Oxygen Vacancies: A Robust Ph-Universal Oxygen Evolution Electrocatalyst. Angew. Chem. Int. Ed. 2021, 60, 18821–18829. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chang, Y.; He, N.; Zhu, S.; Wang, L.; Liu, X. Competition between Lattice Oxygen and Adsorbate Evolving Mechanisms in Rutile Ru-Based Oxide for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2023, 15, 20563–20570. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, C.; Xiang, Y.; Dong, B.; Ding, S.; Tang, Y. Nitrogen-Doped Graphene Quantum Dots Anchored on Thermally Reduced Graphene Oxide as an Electrocatalyst for the Oxygen Reduction Reaction. Chem. Electro. Chem. 2016, 3, 864–870. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Yuan, H.; Liang, Z.; Huang, Z.; Zhou, Y.; Zhang, W.; Zheng, H.; Cao, R. Inherent mass transfer engineering of a Co, N co-doped carbon material towards oxygen reduction reaction. J. Energy Chem. 2021, 58, 391–396. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, L.; Chen, N.; Zhang, H.; Dai, L.; Wang, S. Facile Synthesis of Black Phosphorus: An Efficient Electrocatalyst for the Oxygen Evolving Reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 13849–13853. [Google Scholar] [CrossRef] [PubMed]
- Rabiei Baboukani, A.; Khakpour, I.; Drozd, V.; Wang, C. Liquid-Based Exfoliation of Black Phosphorus into Phosphorene and Its Application for Energy Storage Devices. Small Struct. 2021, 2, 2000148. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, W.; Gong, S.; He, J.; Li, Z.; Li, Y. Optical properties of ZnO/Black Phosphorus/ZnO sandwich structures. Phys. B Condens. Matter 2020, 579, 411903. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, X.; Liu, Y.; Yang, X.; Wang, J.; Liu, Q.; Luo, Q.; Jing, C.; Yu, X.-F.; Qu, G.; et al. Surface and interface control of black phosphorus. Chem 2022, 8, 632–662. [Google Scholar] [CrossRef]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Allagui, A.; Wang, C. Single-step exfoliation of black phosphorus and deposition of phosphorene via bipolar electrochemistry for capacitive energy storage application. J. Mater. Chem. A 2019, 7, 25548–25556. [Google Scholar] [CrossRef]
- Sanchez-Padilla, N.; Benavides, R.; Gallardo, C.; Fernandez, S.; De-Casas, E.; Morales-Acosta, D. Influence of doping level on the electrocatalytic properties for oxygen reduction reaction of N-doped reduced graphene oxide. Int. J. Hydrogen Energy 2021, 46, 26040–26052. [Google Scholar] [CrossRef]
- Liu, X.; Ryder, C.R.; Wells, S.A.; Hersam, M.C. Resolving the In-Plane Anisotropic Properties of Black Phosphorus. Small Methods 2017, 1, 1700143. [Google Scholar] [CrossRef]
- Ejigu, A.; Miller, B.; Kinloch, I.A.; Dryfe, R.A. Optimisation of electrolytic solvents for simultaneous electrochemical exfoliation and functionalisation of graphene with metal nanostructures. Carbon 2018, 128, 257–266. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Yang, J.; Wang, L. Electrodeposited Bi dendrites/2D black phosphorus nanosheets composite used for boosting formic acid production from CO2 electroreduction. J. CO2 Util. 2020, 38, 32–38. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, X.; Xiao, H.; Gao, J.; Xue, S.; Wang, X.; Wu, H.; Jia, J.; Yang, N. Cobalt-iron oxide/black phosphorus nanosheet heterostructure: Electrosynthesis and performance of (photo-)electrocatalytic oxygen evolution. Nano Res. 2023, 16, 6057–6066. [Google Scholar] [CrossRef]
- Chen, X.; Ponraj, J.S.; Fan, D.; Zhang, H. An overview of the optical properties and applications of black phosphorus. Nanoscale 2020, 12, 3513–3534. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Xue, X.-X.; Jiang, X.-X.; Xu, Z.; Chen, K.; Yu, J.-F.; Feng, Y. Chemically modified phosphorene as efficient catalyst for hydrogen evolution reaction. J. Phys. Condens. Matter 2020, 32, 025202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Q.; Liu, H.-L.; Liu, Z.; Zhang, Z.; Cheng, G.-G.; Wang, X.-D.; Ding, J.-N. Anisotropic interfacial properties between monolayered black phosphorus and water. Appl. Surf. Sci. 2019, 475, 857–862. [Google Scholar] [CrossRef]
- Su, S.; Wang, X.; Xue, J. Nanopores in two-dimensional materials: Accurate fabrication. Mater. Horiz. 2021, 8, 1390–1408. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Peng, C.K.; Bu, L.; Chiang, C.L.; Tian, K.; Zhao, Y.; Zhao, J.; Lin, Y.G.; Lee, J.M.; et al. Co-Induced Electronic Optimization of Hierarchical NiFe LDH for Oxygen Evolution. Small 2020, 16, e2002426. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Wang, C.; Zhuang, C.; Yao, Y.; Li, Z.; Wu, C.; Feng, J.; Zou, Z. 2D High-Entropy Hydrotalcites. Small 2021, 17, e2103412. [Google Scholar] [CrossRef]
- Wang, F.-G.; Liu, B.; Wang, H.-Y.; Lin, Z.-Y.; Dong, Y.-W.; Yu, N.; Luan, R.-N.; Chai, Y.-M.; Dong, B. Motivating borate doped FeNi layered double hydroxides by molten salt method toward efficient oxygen evolution. J. Colloid. Interface Sci. 2022, 610, 73–181. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sakthivel, A.; Michelraj, S.; Muthurasu, A.; Ganesh, V. Surfactant Intercalated Mono-metallic Cobalt Hydrotalcite: Preparation, Characterization, and its Bi-functional Electrocatalytic Application. Chemistryselect 2020, 5, 9615–9622. [Google Scholar] [CrossRef]
- Que, R.; Liu, S.; Yang, Y.; Pan, Y. High catalytic performance of core-shell structure ZnCo2O4@NiFe LDH for oxygen evolution reaction. Mater. Lett. 2021, 298, 129982. [Google Scholar] [CrossRef]
- Wang, J.; Lv, G.; Wang, C. A highly efficient and robust hybrid structure of CoNiN@NiFe LDH for overall water splitting by accelerating hydrogen evolution kinetics on NiFe LDH. Appl. Surf. Sci. 2021, 570, 151182. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, W.; Zhu, W.; Yang, Q.; Lei, X.; Liu, J.; Li, Y.; Sun, X.; Duan, X. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 2014, 50, 6479–6482. [Google Scholar] [CrossRef] [PubMed]
- Louie, M.W.; Bell, A.T. An investigation of thin-film Ni–Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liao, X.; Pan, X.; Yan, M.; He, L.; Wu, P.; Zhao, Y.; Luo, W.; Mai, L. Unveiling the Role of Surface P–O Group in P-doped Co3O4 for Electrocatalytic Oxygen Evolution by On-chip Micro-device. Nano Energy 2023, 83, 105748. [Google Scholar]
- Lei, H.; Ma, L.; Wan, Q.; Tan, S.; Yang, B.; Wang, Z.; Mai, W.; Fan, H.J. Promoting Surface Reconstruction of NiFe Layered Double Hydroxide for Enhanced Oxygen Evolution. Adv. Energy Mater. 2022, 12, 2202522. [Google Scholar] [CrossRef]
- Li, Y.-F.; Selloni, A. Mechanism and Activity of Water Oxidation on Selected Surfaces of Pure and Fe-Doped NiOx. ACS Catal. 2014, 4, 1148–1153. [Google Scholar] [CrossRef]
- Kosmala, T.; Bardini, L.; Caporali, M.; Serrano-Ruiz, M.; Sedona, F.; Agnoli, S.; Peruzzini, M.; Granozzi, G. Interfacial chemistry and electroactivity of black phosphorus decorated with transition metals. Inorg. Chem. Front. 2021, 8, 684–692. [Google Scholar] [CrossRef]
- Zhai, Y.; Ren, X.; Sun, Y.; Li, D.; Wang, B.; Liu, S.F. Synergistic effect of multiple vacancies to induce lattice oxygen redox in NiFe-layered double hydroxide OER catalysts. Appl. Catal. B Environ. 2023, 323, 122091. [Google Scholar] [CrossRef]
- Chang, Y.; Nie, A.; Yuan, S.; Wang, B.; Mu, C.; Xiang, J.; Yang, B.; Li, L.; Wen, F.; Liu, Z. Liquid-exfoliation of S-doped black phosphorus nanosheets for enhanced oxygen evolution catalysis. Nanotechnology 2019, 30, 035701. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Du, X.; Zhao, M.; Li, Y.; Hu, T.; Wu, H.; Jia, J.; Yang, N. Structural dependence of electrosynthesized cobalt phosphide/black phosphorus pre-catalyst for oxygen evolution in alkaline media. Nanoscale 2021, 13, 7381–7388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Khurram, M.; Sun, Z.; Yan, Q. Uniform Tellurium Doping in Black Phosphorus Single Crystals by Chemical Vapor Transport. Inorg. Chem. 2018, 57, 4098–4103. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Qiao, H.; Huang, Z.; Liu, F.; Duan, C.; Zhou, Y.; Liao, G.; Qi, X. Photo-assisted electrocatalysis of black phosphorus quantum dots/molybdenum disulfide heterostructure for oxygen evolution reaction. Appl. Surf. Sci. 2021, 562, 150213. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, S.; Bu, K.; Zhao, W.; Bi, Q.; Lin, T.; Huang, J.; Li, Y.; Huang, F. Nickel nitride–black phosphorus heterostructure nanosheets for boosting the electrocatalytic activity towards the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 22063–22069. [Google Scholar] [CrossRef]
- He, M.M.; Wang, D.; Shiigi, H.; Liu, C.H.; Wang, W.C.; Shan, X.L.; Chen, Z.D. Black phosphorous dots phosphatized bio-based carbon nanofibers/bimetallic organic framework as catalysts for oxygen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 17194–17203. [Google Scholar] [CrossRef]
- Liang, T.; Lenus, S.; Liu, Y.; Chen, Y.; Sakthivel, T.; Chen, F.; Ma, F.; Dai, Z. Interface and M3+/M2+ Valence Dual-Engineering on Nickel Cobalt Sulfoselenide/Black Phosphorus Heterostructure for Efficient Water Splitting Electrocatalysis. Energy Environ. Mater. 2022, 6, e12332. [Google Scholar]
- Zhai, W.; Chen, Y.; Liu, Y.; Sakthivel, T.; Ma, Y.; Guo, S.; Qu, Y.; Dai, Z. Bimetal-Incorporated Black Phosphorene with Surface Electron Deficiency for Efficient Anti-Reconstruction Water Electrolysis. Adv. Funct. Mater. 2023, 33, 202301565. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Yang, M.; Fang, Z.; Jian, J.; Yu, D.; Chen, X.; Dai, L. Ultrathin Black Phosphorus-on-Nitrogen Doped Graphene for Efficient Overall Water Splitting: Dual Modulation Roles of Directional Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 4972–4979. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, X.; Min, Y.; Li, Q.; Xu, Q. Investigation of Oxygen Evolution Performance of Highly Efficient Water Electrolysis Catalyst: NiFe LDH/BPene. Processes 2023, 11, 2179. https://doi.org/10.3390/pr11072179
Wang Y, Wang X, Min Y, Li Q, Xu Q. Investigation of Oxygen Evolution Performance of Highly Efficient Water Electrolysis Catalyst: NiFe LDH/BPene. Processes. 2023; 11(7):2179. https://doi.org/10.3390/pr11072179
Chicago/Turabian StyleWang, Yaru, Xiao Wang, Yulin Min, Qiaoxia Li, and Qunjie Xu. 2023. "Investigation of Oxygen Evolution Performance of Highly Efficient Water Electrolysis Catalyst: NiFe LDH/BPene" Processes 11, no. 7: 2179. https://doi.org/10.3390/pr11072179




