Application of High-Intensity Ultrasound on Acerola (Malpighia emarginata) Juice Supplemented with Fructooligosaccharides and Its Effects on Vitamins, Phenolics, Carotenoids, and Antioxidant Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Ultrasound Processing
2.3. Determination of Vitamins
2.4. Total Phenolics, Total Carotenoid Content, and Antioxidant Capacity
2.5. FOS Concentration and Degree of Polymerization
2.6. Hydrogen Peroxide Concentration
2.7. Statistical Analysis
3. Results and Discussion
3.1. Vitamins Content
3.2. Total Phenolic Content
3.3. Total Carotenoid Content
3.4. Antioxidant Capacity
3.5. Changes in the Fructooligosaccharide Degree of Polymerization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhang, S.; Tang, L.; Zhang, M.; Wang, P.; Sun, X.; Shang, L.; Wang, Q.; Zhao, Y.; Meng, D.; et al. Analysis of the Effects of Rosa Roxburghii Tratt Fruit Polyphenols on Immune Function in Mice through Gut Microbiota and Metabolomics: An in Vivo Preclinical Trial Study. J. Funct. Foods 2023, 102, 105464. [Google Scholar] [CrossRef]
- Stojanovska, L.; Ali, H.I.; Kamal-Eldin, A.; Souka, U.; Al Dhaheri, A.S.; Cheikh Ismail, L.; Hilary, S. Soluble and Insoluble Dietary Fibre in Date Fruit Varieties: An Evaluation of Methods and Their Implications for Human Health. Foods 2023, 12, 1231. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.J.; Chen, J.S.; Sheu, D.C. Kinetic Studies and Mathematical Model for Enzymatic Production of Fructooligosaccharides from Sucrose. Enzyme Microb. Technol. 1994, 16, 334–339. [Google Scholar] [CrossRef]
- Tan, D.; Chen, W.; Yang, Z.; Rong, M.; Huang, R.; Wu, X.; Bai, W.; Tian, L. Enzymatic Synthesis of Propionyl-Fructooligosaccharides and Their Evaluation as a Gut Microbiota Modulator. Food Hydrocoll. 2023, 142, 108782. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Health Benefits of Non-Digestible Oligosaccharides. In Dietary Fiber in Health and Disease. Advances in Experimental Medicine and Biology; Kritchevsky, D., Bonfield, C., Eds.; Springer: Boston, MA, USA, 1997; Volume 427, pp. 211–219. [Google Scholar]
- USDA. USDA National Nutrient Database for Standard Reference; Release 28; U.S. Department of Agriculture: Washington, DC, USA, 2015.
- Silva, N.L.; Crispim, J.M.S.; Vieira, R.P. Kinetic and Thermodynamic Analysis of Anthocyanin Thermal Degradation in Acerola (Malpighia Emarginata D.C.) Pulp. J. Food Process. Preserv. 2017, 41, e13053. [Google Scholar] [CrossRef]
- Wurlitzer, N.J.; Dionísio, A.P.; Lima, J.R.; dos Santos Garruti, D.; da Silva Araújo, I.M.; da Rocha, R.F.J.; Maia, J.L. Tropical Fruit Juice: Effect of Thermal Treatment and Storage Time on Sensory and Functional Properties. J. Food Sci. Technol. 2019, 56, 5184–5193. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Wurlitzer, N.J.; Fernandes, F.A.N.; Rabelo, M.C.; Fonteles, T.V.; Rodrigues, S. Evaluation of Thermal and Non-Thermal Processing Effect on Non-Prebiotic and Prebiotic Acerola Juices Using1H QNMR and GC–MS Coupled to Chemometrics. Food Chem. 2018, 265, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Piyasena, P.; Mohareb, E.; McKellar, R.C. Inactivation of Microbes Using Ultrasound: A Review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Montilla, A.; Soria, A.C.; Villamiel, M. Effects of Conventional and Ultrasound Blanching on Enzyme Inactivation and Carbohydrate Content of Carrots. Eur. Food Res. Technol. 2012, 234, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.-H.; Moon, K.-D. Inhibition of Polyphenol Oxidase and Peroxidase Activities on Fresh-Cut Apple by Simultaneous Treatment of Ultrasound and Ascorbic Acid. Food Chem. 2011, 124, 444–449. [Google Scholar] [CrossRef]
- Lee, H.; Zhou, B.; Feng, H.; Martin, S.E. Effect of PH on Inactivation of Escherichia Coli K12 by Sonication, Manosonication, Thermosonication, and Manothermosonication. J. Food Sci. 2009, 74, 191–198. [Google Scholar] [CrossRef]
- Ekonomou, S.I.; Boziaris, I.S. Non-Thermal Methods for Ensuring the Microbiological Quality and Safety of Seafood. Appl. Sci. 2021, 11, 833. [Google Scholar] [CrossRef]
- Jat, K.; Jayachandran, L.E.; Rao, P.S. Impact of Temperature Assisted Ultrasonication on the Quality Attributes of Beetroot (Beta vulgaris L.) Juice. J. Food Process. Eng. 2023, 2023, e14329. [Google Scholar] [CrossRef]
- Cruz, J.M.d.A.; Ramos, A.S.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; Kinupp, V.F.; Bezerra, J.d.A. Thermal Treatment and High-Intensity Ultrasound Processing to Evaluate the Chemical Profile and Antioxidant Activity of Amazon Fig Juices. Processes 2023, 11, 408. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E. Effect of Ultrasonic, Thermal and Enzymatic Treatment of Mash on Yield and Content of Bioactive Compounds in Strawberry Juice. Appl. Sci. 2023, 13, 4268. [Google Scholar] [CrossRef]
- Kesavan, R.K.; Gogoi, S.; Nayak, P.K. Influence of Thermosonication and Pasteurization on the Quality Attributes of Kutkura (Meyna spinosa) Juice. Appl. Food Res. 2023, 3, 100268. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Costa, M.G.M.; de Jesus, A.L.T.; Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. Power Ultrasound Processing of Cantaloupe Melon Juice: Effects on Quality Parameters. Food Res. Int. 2012, 48, 41–48. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Fonteles, T.V.; Jesus, A.L.T.; Almeida, F.D.L.; Miranda, M.R.A.; Fernandes, F.A.N.; Rodrigues, S. High-Intensity Ultrasound Processing of Pineapple Juice. Food Bioproc. Technol. 2011, 6, 997–1006. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of Ultrasound Processing on Anthocyanins and Color or Red Grape Juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef]
- Shaik, L.; Chakraborty, S. Ultrasound Processing of Sweet Lime Juice: Effect of Matrix PH on Microbial Inactivation, Enzyme Stability, and Bioactive Retention. J. Food Process. Eng. 2023, 46, 14231. [Google Scholar] [CrossRef]
- Kalsi, B.S.; Singh, S.; Alam, M.S. Influence of Ultrasound Processing on the Quality of Guava Juice. J. Food Process. Eng. 2023, 46, 14163. [Google Scholar] [CrossRef]
- Cheng, L.; Soh, C.; Liew, S.; Teh, F. Effects of Sonication and Carbonation on Guava Juice Quality. Food Chem. 2007, 104, 1396–1401. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Effects of Sonication on the Kinetics of Orange Juice Quality Parameters. J. Agric. Food Chem. 2008, 56, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.N.; Rodrigues, S. Ultrasound Applications in Minimal Processing. Stewart Postharvest Rev. 2009, 5, 1–7. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Application of Ultrasound and Ultrasound-Assisted Osmotic Dehydration in Drying of Fruits. Dry. Technol. 2008, 26, 1509–1516. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Ultrasound Applications in Drying of Fruits from a Sustainable Development Goals Perspective. Ultrason. Sonochem. 2023, 96, 106430. [Google Scholar] [CrossRef]
- Santos, V.; Rodrigues, S.; Fernandes, F. Improvements on the Stability and Vitamin Content of Acerola Juice Obtained by Ultrasonic Processing. Foods 2018, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Alves Filho, E.G.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Tiwari, B.K.; Brito, E.S.; Rodrigues, S.; Fernandes, F.A.N. Evaluation of Plasma, High-Pressure and Ultrasound Processing on the Stability of Fructooligosaccharides. Int. J. Food Sci. Technol. 2016, 51, 2034–2040. [Google Scholar] [CrossRef]
- Rizzolo, A.; Polesello, S. Chromatographic Determination of Vitamins in Foods. J. Chromatogr. 1992, 624, 103–152. [Google Scholar] [CrossRef]
- Jedlicka, A.; Klimes, J. Determination of Water- and Fat-Soluble Vitamins in Different Matrices Using High-Performance Liquid Chromatography. Chem. Pap. 2005, 59, 202–222. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Santos, V.O.; Rodrigues, S. Effects of Glow Plasma Technology on Some Bioactive Compounds of Acerola Juice. Food Res. Int. 2019, 115, 16–22. [Google Scholar] [CrossRef]
- Selimović, A.; Salkić, M.; Selimović, A. Direct Spectrophotometric Determination of L-Ascorbic Acid in Pharmaceutical Preparations Using Sodium Oxalate as a Stabilizer. Int. J. Basic Appl. Sci. 2011, 11, 106–109. [Google Scholar]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Amaya, D. A Guide to Carotenoids Analysis in Food; International Life Sciences Institute Press: Washington, DC, USA, 2001. [Google Scholar]
- Shiomi, N.; Onodera, S.; Sakai, H. Fructo-Oligosaccharide Content and Fructosyltransferase Activity during Growth of Onion Bulbs. New Phytol. 1997, 136, 105–113. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Gomes, W.F.; Cavalcante, R.S.; Tiwari, B.K.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Fructooligosaccharides Integrity after Atmospheric Cold Plasma and High-Pressure Processing of a Functional Orange Juice. Food Res. Int. 2017, 102, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ovenston, T.C.J.; Rees, W.T. The Spectrophotometric Determination of Small Amounts of Hydrogen Peroxide in Aqueous Solutions. Analyst 1950, 75, 204–208. [Google Scholar] [CrossRef]
- Ball, G.F.M. Vitamins in Foods: Analysis, Bioavailability, and Stability; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ghosh, H.P.; Sarkar, P.K.; Guha, B.C. Distribution of the Bound Form of Nicotinic Acid in Natural Materials. J. Nutr. 1963, 79, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.S.; Carpenter, K.J. Variation in Availability of Niacin in Grain Products. Food Technol. 1988, 42, 198–204. [Google Scholar]
- Plesovsky-Vig, N. Pantothenic Acid. In Modern Nutrition in Health and Disease; Shils, M.E., Olson, J.A., Shike, M., Ross, A.C., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1999; p. 423. [Google Scholar]
- Fernandes, F.A.N.; Rodrigues, S.; Cárcel, J.A.; García-Pérez, J.V. Ultrasound-Assisted Air-Drying of Apple (Malus domestica L.) and Its Effects on the Vitamin of the Dried Product. Food Bioproc. Technol. 2015, 8, 1503–1511. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Cold Plasma Processing on Fruits and Fruit Juices: A Review on the Effects of Plasma on Nutritional Quality. Processes 2021, 9, 2098. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.N.; Oliveira, V.S.; Gomes, W.F.; Rodrigues, S. Degradation Kinetics of Vitamin E during Ultrasound Application and the Adjustment in Avocado Puree by Tocopherol Acetate Addition. LWT Food Sci. Technol. 2016, 69, 342–347. [Google Scholar] [CrossRef]
- Mason, T.J. Power Ultrasound in Food Processing—The Way Forward. In Ultrasounds in Food Processing; Povey, M.J.W., Mason, T.J., Eds.; Blackie Academic and Professional: Glasgow, UK, 1998. [Google Scholar]
- Ozhogina, O.A.; Kasaikina, O.T. B-Carotene as an Interceptor of Free Radicals. Free Radic. Biol. Med. 1995, 19, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Health Protective Effects of Carotenoids and Their Interactions with Other Biological Antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef]
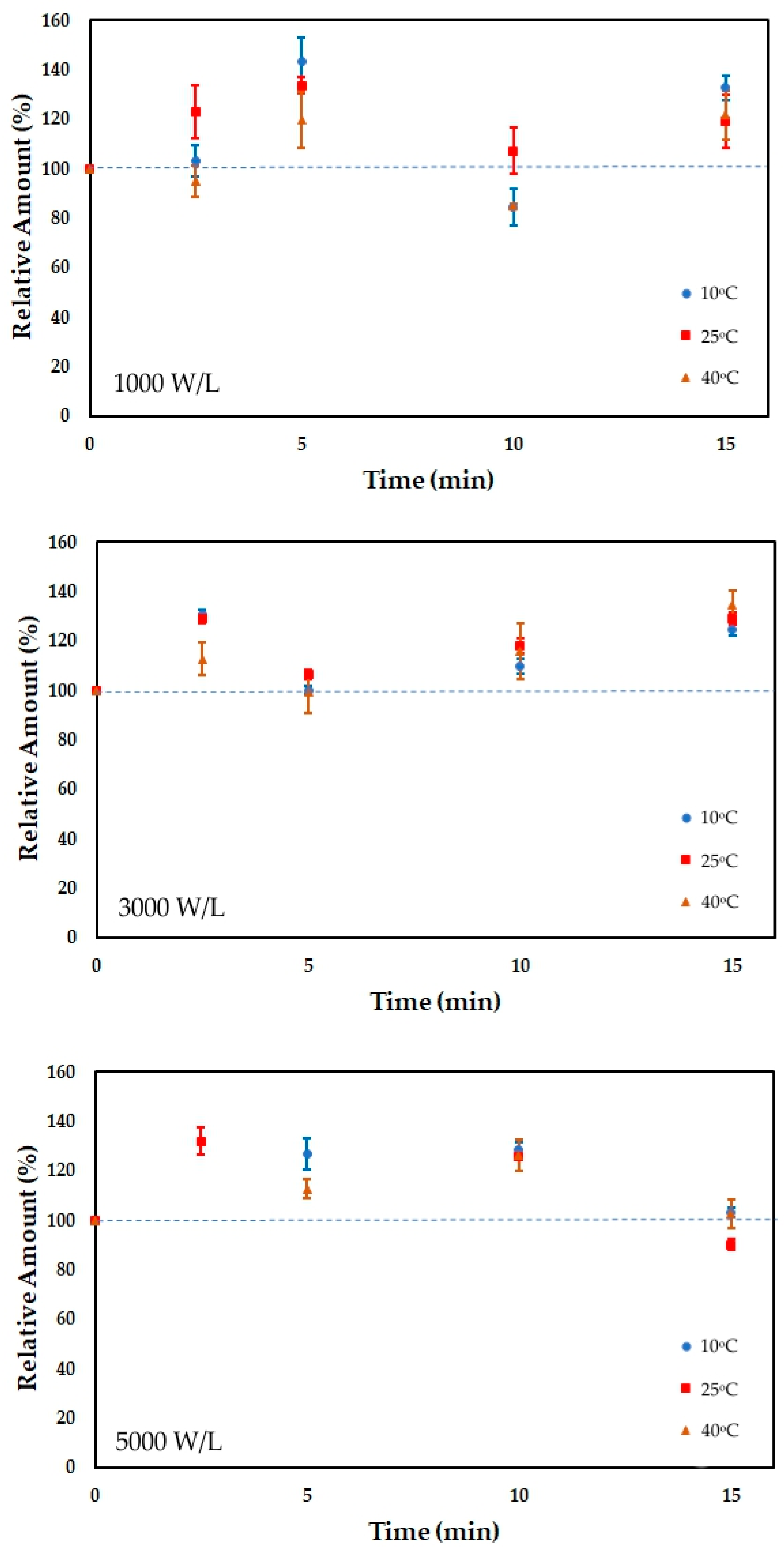


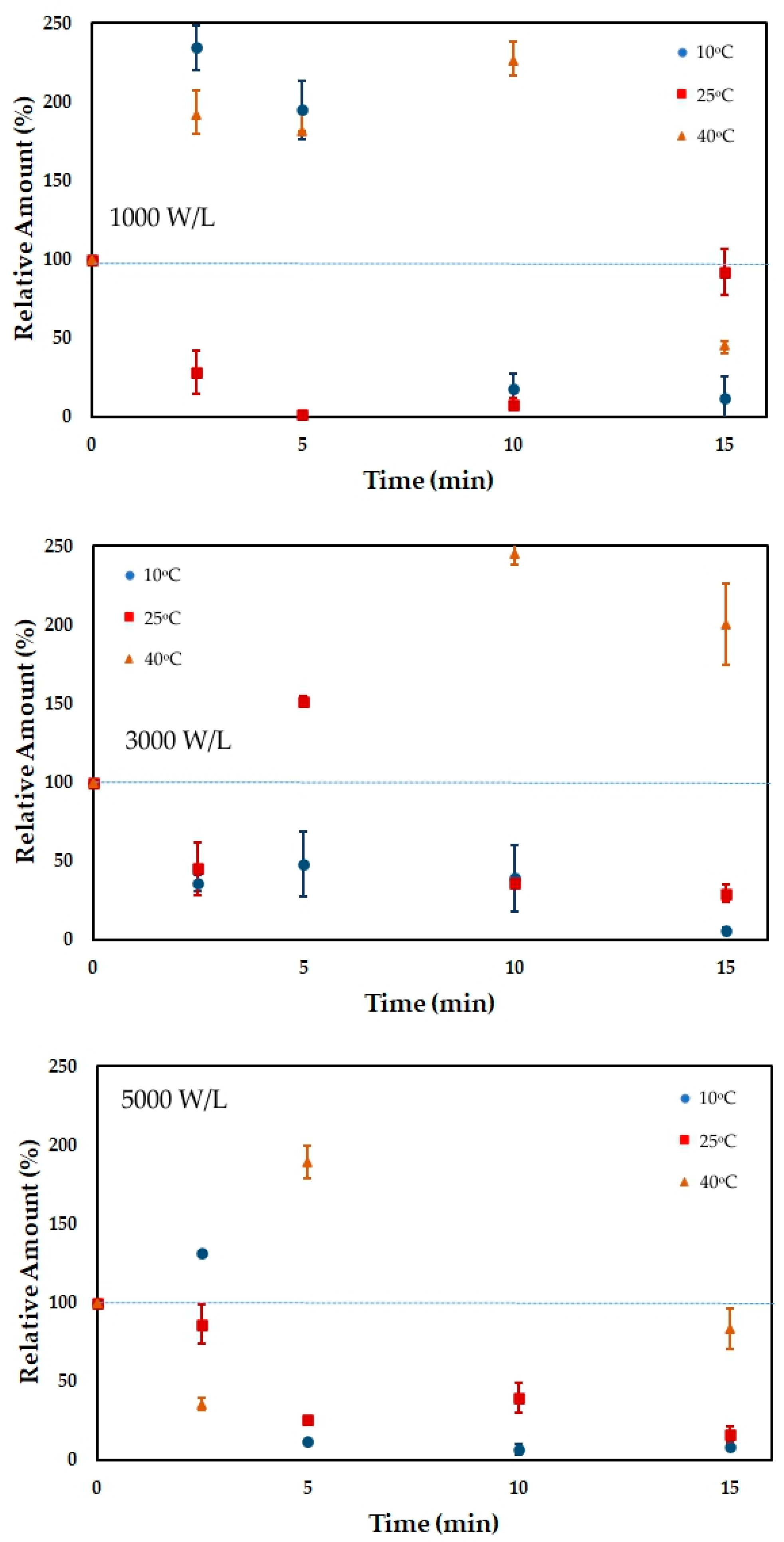
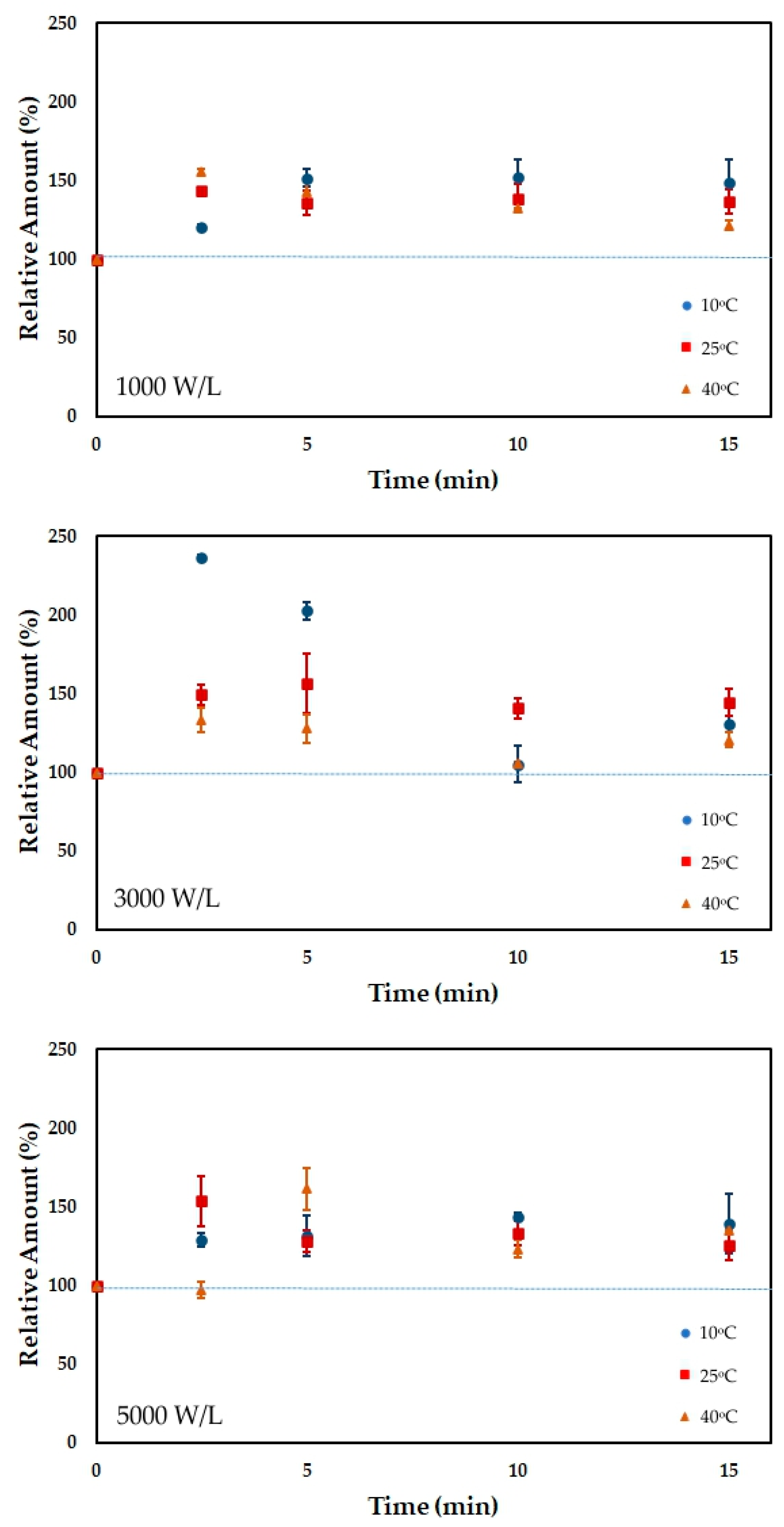
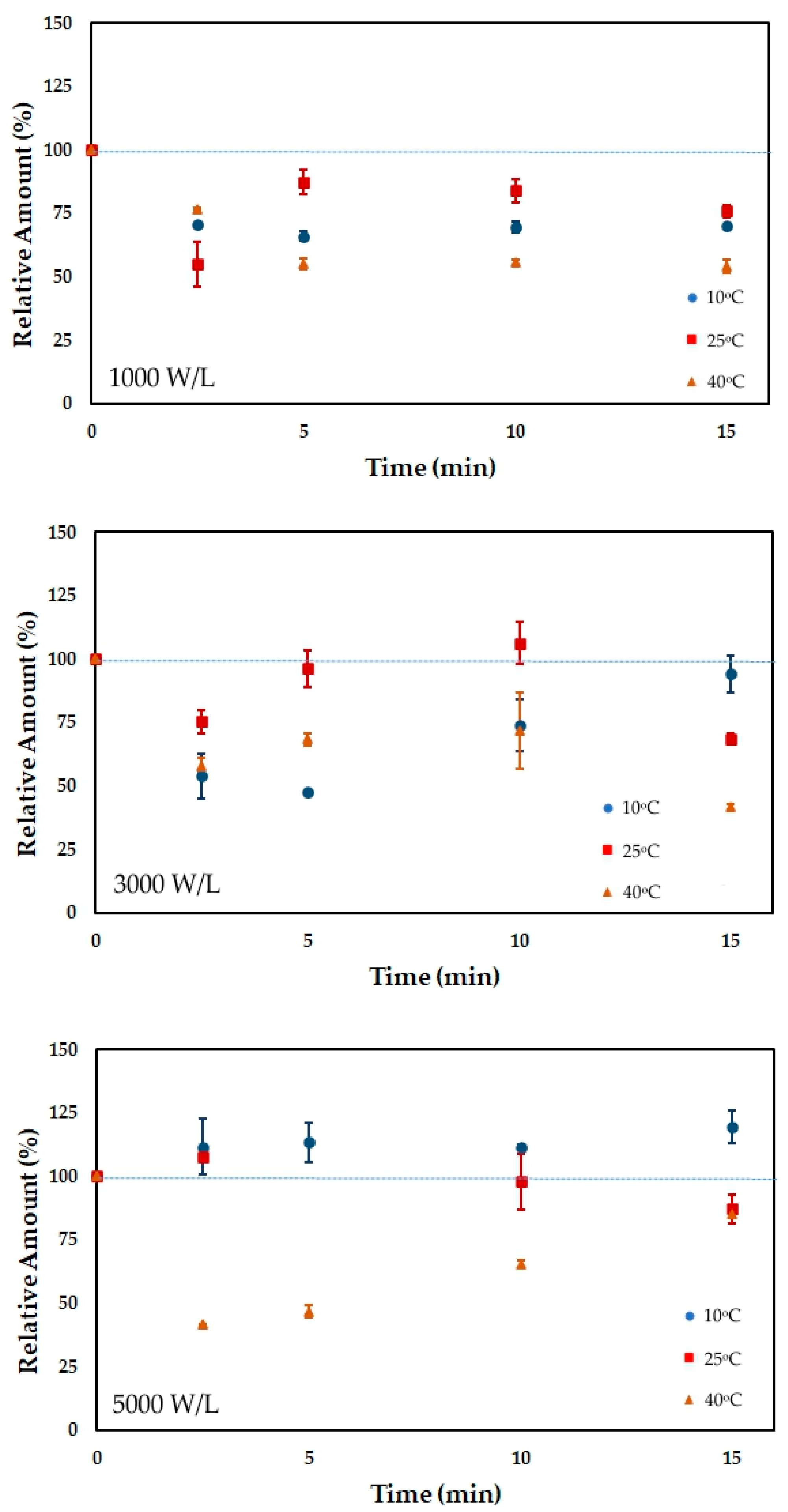

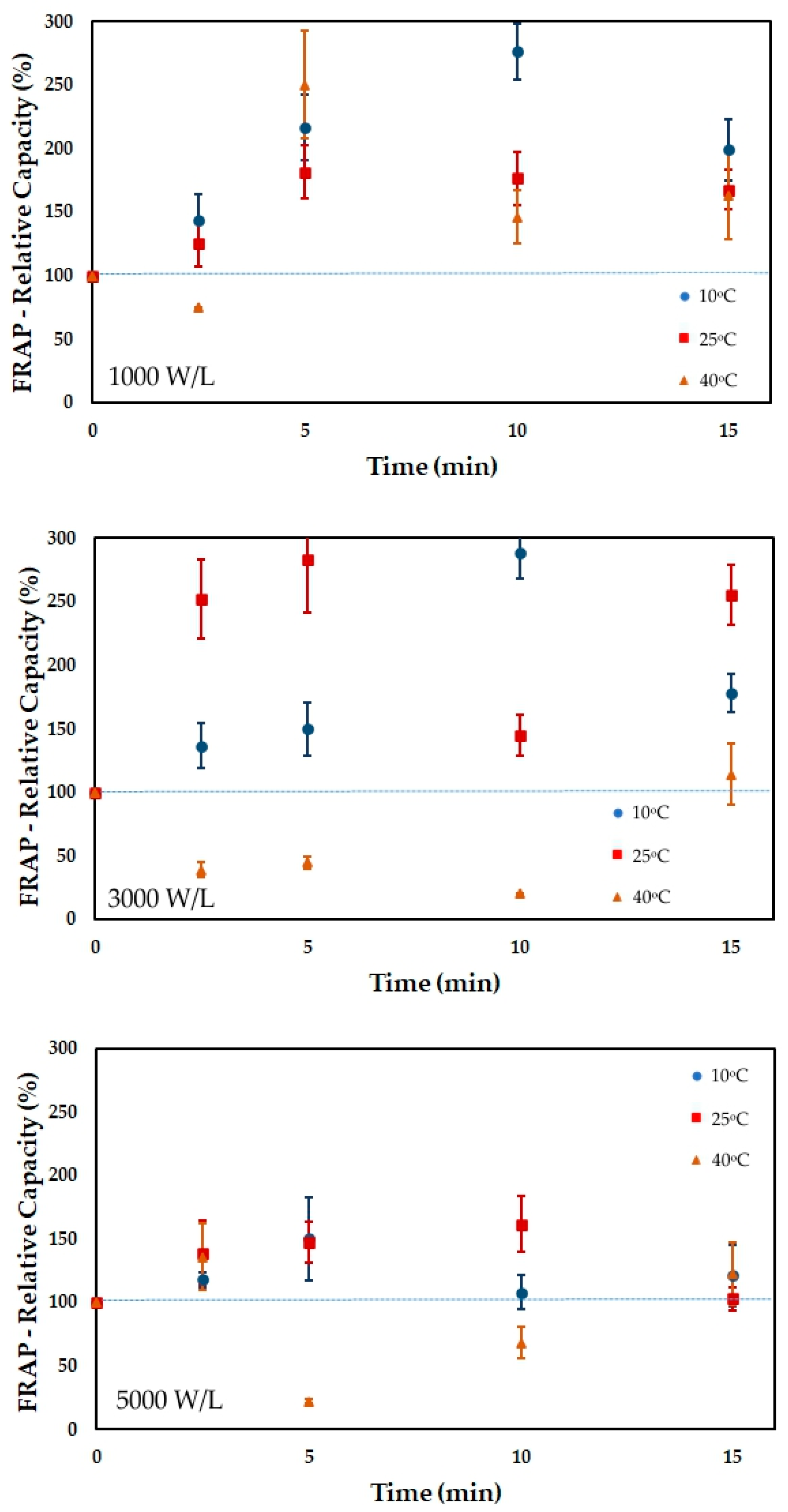

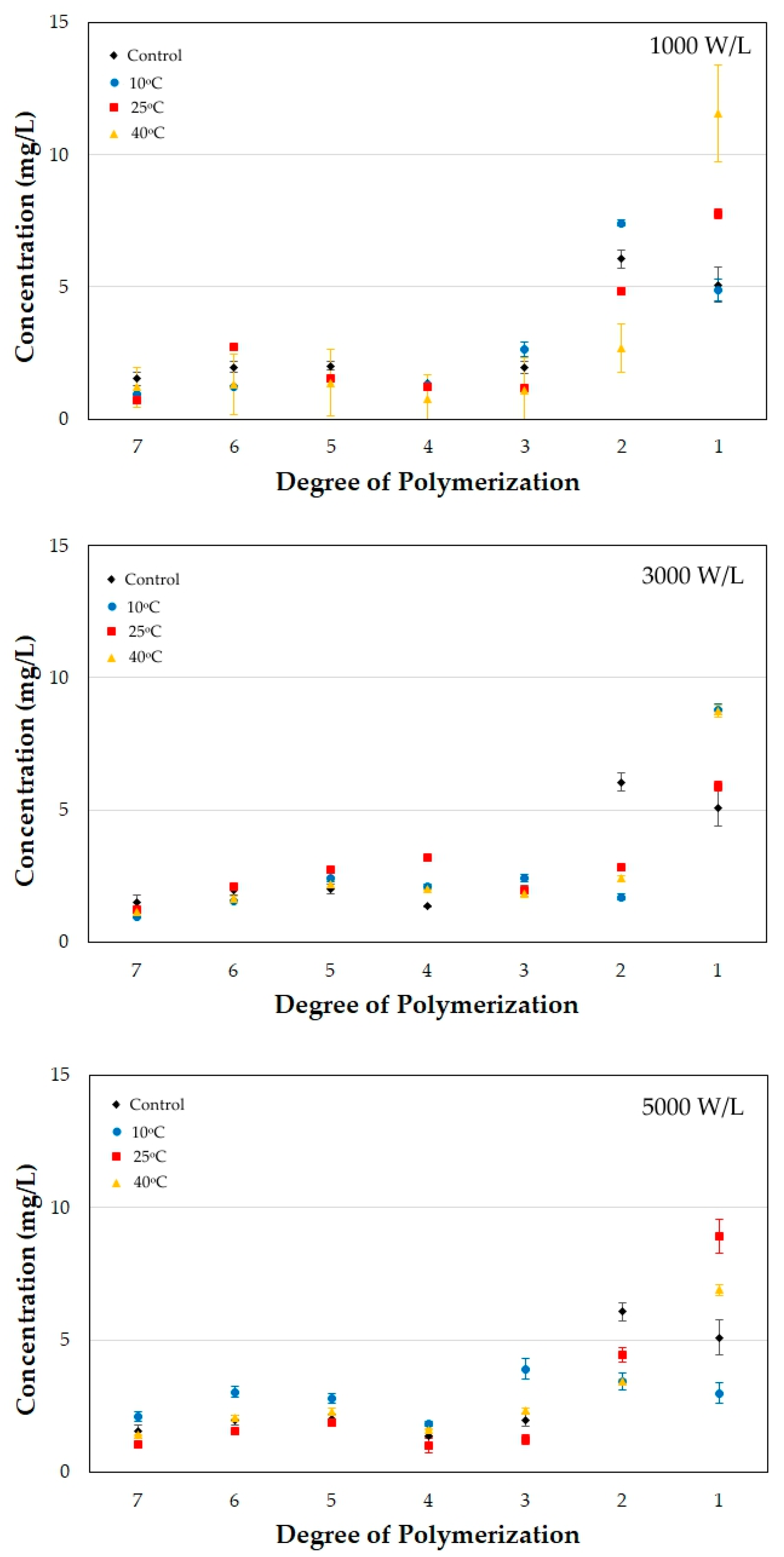
| Time | 10 °C | 10 °C | 10 °C | 25 °C | 25 °C | 25 °C | 40 °C | 40 °C | 40 °C |
|---|---|---|---|---|---|---|---|---|---|
| (min) | 1000 W/L | 3000 W/L | 5000 W/L | 1000 W/L | 3000 W/L | 5000 W/L | 1000 W/L | 3000 W/L | 5000 W/L |
| 0 | 197 ± 20 | 197 ± 20 | 197 ± 20 | 197 ± 20 | 197 ± 20 | 197 ± 20 | 197 ± 20 | 197 ± 20 | 197 ± 20 |
| 2, 5 | 262 ± 21 | 221 ± 30 | 313 ± 6 | 375 ± 9 | 377 ± 5 | 348 ± 10 | 241 ± 15 | 248 ± 35 | 300 ± 4 |
| 5 | 347 ± 20 | 199 ± 6 | 331 ± 5 | 360 ± 10 | 372 ± 2 | 315 ± 8 | 213 ± 10 | 242 ± 10 | 287 ± 3 |
| 10 | 299 ± 5 | 213 ± 6 | 331 ± 6 | 428 ± 7 | 355 ± 6 | 347 ± 7 | 277 ± 25 | 304 ± 25 | 301 ± 9 |
| 15 | 333 ± 2 | 239 ± 25 | 340 ± 5 | 437 ± 2 | 384 ± 2 | 357 ± 9 | 233 ± 8 | 303 ± 9 | 294 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, F.A.N.; Santos, V.O.; Gomes, W.F.; Rodrigues, S. Application of High-Intensity Ultrasound on Acerola (Malpighia emarginata) Juice Supplemented with Fructooligosaccharides and Its Effects on Vitamins, Phenolics, Carotenoids, and Antioxidant Capacity. Processes 2023, 11, 2243. https://doi.org/10.3390/pr11082243
Fernandes FAN, Santos VO, Gomes WF, Rodrigues S. Application of High-Intensity Ultrasound on Acerola (Malpighia emarginata) Juice Supplemented with Fructooligosaccharides and Its Effects on Vitamins, Phenolics, Carotenoids, and Antioxidant Capacity. Processes. 2023; 11(8):2243. https://doi.org/10.3390/pr11082243
Chicago/Turabian StyleFernandes, Fabiano A. N., Valéria O. Santos, Wesley F. Gomes, and Sueli Rodrigues. 2023. "Application of High-Intensity Ultrasound on Acerola (Malpighia emarginata) Juice Supplemented with Fructooligosaccharides and Its Effects on Vitamins, Phenolics, Carotenoids, and Antioxidant Capacity" Processes 11, no. 8: 2243. https://doi.org/10.3390/pr11082243
APA StyleFernandes, F. A. N., Santos, V. O., Gomes, W. F., & Rodrigues, S. (2023). Application of High-Intensity Ultrasound on Acerola (Malpighia emarginata) Juice Supplemented with Fructooligosaccharides and Its Effects on Vitamins, Phenolics, Carotenoids, and Antioxidant Capacity. Processes, 11(8), 2243. https://doi.org/10.3390/pr11082243








