Improving the Accuracy of Silicon Transfer Prediction in Submerged Arc Welding: A Multi-Reaction-Zone Analysis
Abstract
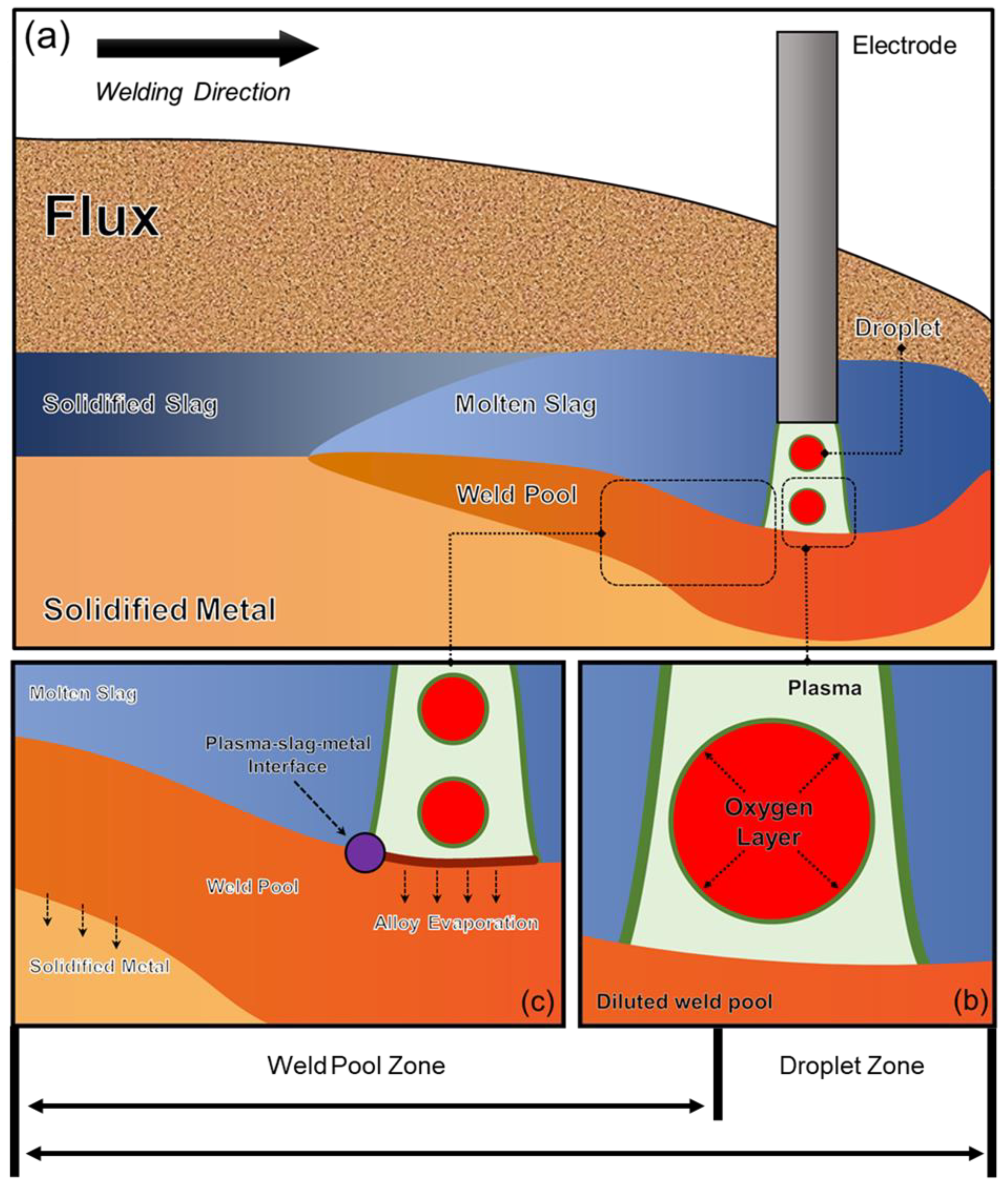
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation and Welding Experiment
2.2. Elemental Transfer Calculation
3. Thermodynamic Modeling and Simulation
3.1. Droplet Reactions
- Significant improvement in O content in the droplet.
- Potential loss of alloy elements induced by the arc plasma.
- The FToxid, Fstel, and FactPS databases were selected for utilization. The solution phases of ASlag-liq all oxides, S (FToxid-SLAGH), and LIQUID (FStel-Liqu) were opted for simulating the molten slag and steel phases.
- The equilibrium temperature for the SAW process was designated as 2500 °C, which corresponds to the temperature of the arc plasma. The input metal chemistries were derived from the BM compositions.
- In order to predict the O concentration in the droplet, an equilibrium calculation was performed using iron (Fe) and O as the input metal constituents. The PO2 value provided in Table 3 was utilized in this calculation. Table 3 summarizes the simulated PO2 and O concentrations in the droplet obtained as the output.
3.2. Weld Pool Reactions
- The Equilib module is employed to conduct the modeling regarding the weld pool zone reactions.
- To simulate the molten slag and steel phases, the FToxid, Fstel, and FactPS databases were utilized. The selected solution phases included ASlag-liq for all oxides, S (FToxid-SLAGH), and LIQUID (FStel-Liqu).
- A modeling temperature of 2000 °C was established.
3.3. Quantification of Elemental Transfer
4. Results and Discussion
4.1. Transfer Behavior of Si
4.2. Transfer Behavior of O
- Chai et al. [16] conducted a study where they designed CaF2-based binary fluxes and measured the O content in the weld metal (WM). Their findings revealed that even stable oxides have the tendency to decompose, resulting in the release of O2 and subsequently increasing the O content in the submerged arc-welded metal.
- Indacochea et al. [8] made an assumption that the equilibrium PO2 in the arc plasma regulates the extent to which the flux can chemically enhance the O level in submerged arc-welded metal.
4.3. Predicted ΔSi vs. Real ΔSi
- In this study, a new approach was suggested for predicting the Si content and Si transfer behavior in SAW when using SiO2-bearing fluxes. By comparing the prediction results with those obtained from the traditional equilibrium model, it was found that solely considering the chemical interactions within the weld pool zone is inadequate for assessing the overall Si transfer behavior in the SAW process.
- The ΔSi value plays a crucial role in determining the compatibility of base metal (BM), electrode, and flux. It governs the extent to which the flux can chemically impact the Si content in SAW. Nevertheless, conventional SAW engineering and scientific research solely focus on the changes in Si content caused by the nominal Si content and the chemical reaction within the weld pool zone, resulting in notable inaccuracies. Hence, this study offers a novel quantitative perspective on elemental transfer in SAW engineering and scientific research.
5. Conclusions
- The thermodynamic equilibrium models do not account for the loss of Si during the droplet zone and the enhancement of O content in the molten droplet. As a result, these models tend to overestimate the level of Si transfer (ΔSi).
- The study provides a more precise depiction of the trend in Si and O transfer behavior during the SAW process, surpassing the accuracy of the thermodynamic equilibrium model.
- Traditional quantitative methods for predicting ΔSi values may lead to errors due to their focus solely on chemical reactions in the weld pool zone. However, considering the physical and chemical phenomena of submerged arc welding, it is crucial to separately consider the droplet zone and the weld pool zone to achieve more accurate predictions of Si transfer behavior, particularly regarding Si evaporation induced by arc plasma and Si oxidation by O in the droplet.
- Physical interactions can also affect Si content, especially the physical involvement of inclusions.
- The reduction of Si at the slag–metal interface due to the oxides becoming more stable at lower temperatures during the solidification process of the molten pool.
Author Contributions
Funding
Conflicts of Interest
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding-A Review. Weld. J. 2019, 98, 283–313. [Google Scholar] [CrossRef]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding. In ASM Handbook; ASM International: Almere, The Netherlands, 1993; Volume 6, pp. 55–63. [Google Scholar] [CrossRef]
- Chai, C.-S. Slag-Metal Reactions during Flux Shielded Arc Welding. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1980. [Google Scholar]
- Zhang, J.; Peng, L.; Zhou, L.; Chen, Y. On the Si Content Prediction for Submerged Arc Welded Metal via Calphad Technique: A Brief Discussion. J. Mater. Res. Technol. 2022, 21, 1856–1862. [Google Scholar] [CrossRef]
- Lau, T.; Weatherly, G.; McLean, A. Gas/metal/slag Reactions in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1986, 65, 31–38. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding using CaO-Al2O3 Based Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions During Submerged Arc Welding with FeO-MnO-SiO2 Fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Prediction of Weld-metal Composition during Flux-shielded Welding. J. Mater. Energy Syst. 1983, 5, 160–164. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag-metal Equilibrium during Submerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, G.; Guo, Y.; Xu, Q.; Liu, Z. Facilitating Flux Design Process Geared Towards Submerged Arc Welding via Thermodynamic Approach: Case Study into CaF2–SiO2–Na2O–Al2O3–TiO2 Agglomerated Flux. Calphad 2022, 79, 102483. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D. Thermodynamic Simulation of O Content Variation Roadmap in Submerged Arc Welding Process: From Droplet to Weld Metal. Processes 2023, 11, 784. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Zhang, D. Advancing Manganese Content Prediction in Submerged Arc Welded Metal: Development of a Multi-Zone Model via the Calphad Technique. Processes 2023, 11, 1265. [Google Scholar] [CrossRef]
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80. [Google Scholar]
- Shao, G.; Liu, Z.; Fan, J.; Guo, Y.; Xu, Q.; Zhang, J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals 2022, 12, 1530. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag Metal Reactions in Binary CaF2-Metal Oxide Welding Fluxes. Weld. J. 1982, 61, 229–232. [Google Scholar]
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld Metal. Weld. Met. Fabr. 1969, 37, 327–339. [Google Scholar]
- Zhang, J.; Shao, G.; Fan, J.; Wang, L.; Zhang, D. A Review on Parallel Development of Flux Design and Thermodynamics Subject to Submerged Arc Welding. Processes 2022, 10, 2305. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L. Upgrading the Prediction Model for Mn Content in Submerged Arc Welded Metal via CALPHAD Technology: Case Study into Typical Acidic and Basic Fluxes. Ceram. Int. 2022, 49, 6573–6579. [Google Scholar] [CrossRef]
- Jung, I.-H. Overview of the Applications of Thermodynamic Databases to Steelmaking Processes. Calphad 2010, 34, 332–362. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Yang, S.; Shao, G.; Liu, Z. Element Transfer Behavior for CaF2-Na2O-SiO2 Agglomerated Flux Subject in Submerged Arc Welding Process. Processes 2022, 10, 1847. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part I. Evaluation and Reassessment of Existing Theories. Metall. Trans. B 1991, 22, 65–71. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions During Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part III. Verification of the Theory. Metall. Trans. B 1991, 22, 83–100. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Shi, H.; Huang, L.; Mao, Z. Element Loss Behavior and Compensation in Additive Manufacturing of Memory Alloys. Trans. China Weld. Inst. 2022 43, 50–55. [CrossRef]
- Zhang, D.; Shao, G.; Zhang, J.; Liu, Z. On the Moving of Neutral Point for Mn Subject to Submerged Arc Welding under Various Heat Inputs: Case Study into CaF2-SiO2-Na2O-MnO Agglomerated Fluxes. Processes 2022, 10, 1888. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 3rd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Dallam, C.; Liu, S.; Olson, D. Flux Composition Dependence of Microstructure and Toughness of Submerged Arc HSLA Weldments. Weld. J. 1985, 64, 140–151. [Google Scholar]





| WM | Flux | CaF2 | Na2O | SiO2 |
|---|---|---|---|---|
| WM-1 | F-1 | 88.77 | 1.21 | 10.02 |
| WM-2 | F-2 | 78.87 | 1.24 | 19.89 |
| WM-3 | F-3 | 68.67 | 1.23 | 30.10 |
| WM-4 | F-4 | 58.62 | 1.19 | 40.19 |
| WM-5 | F-5 | 48.84 | 1.22 | 49.94 |
| C | Si | Mn | Ti | Cr | O | |
|---|---|---|---|---|---|---|
| Base metal | 0.112 | 0.142 | 1.54 | 0.015 | 0.018 | 0.003 |
| Electrode | 0.127 | 0.049 | 1.65 | 0.015 | 0.015 | 0.003 |
| WM | PO2 (atm.) | Droplet O Content (wt pct) |
|---|---|---|
| WM-1 | 5.03 × 10-06 | 0.295 |
| WM-2 | 7.32 × 10-06 | 0.356 |
| WM-3 | 1.13 × 10-05 | 0.443 |
| WM-4 | 1.28 × 10-05 | 0.472 |
| WM-5 | 1.36 × 10-05 | 0.487 |
| WM | C | Si | Mn | Ti | Cr | O | Fe |
|---|---|---|---|---|---|---|---|
| WM-1 | 0.12 | 0.055 | 0.829 | 0.015 | 0.017 | 0.148 | Balanced |
| WM-2 | 0.12 | 0.055 | 0.829 | 0.015 | 0.017 | 0.178 | Balanced |
| WM-3 | 0.12 | 0.059 | 0.829 | 0.015 | 0.017 | 0.221 | Balanced |
| WM-4 | 0.12 | 0.060 | 0.829 | 0.015 | 0.017 | 0.236 | Balanced |
| WM-5 | 0.12 | 0.063 | 0.829 | 0.015 | 0.017 | 0.243 | Balanced |
| WM | ΔDSi | ΔWSi | ΔDO | ΔWO |
|---|---|---|---|---|
| WM-1 | −0.033 | 0.375 | 0.147 | −0.099 |
| WM-2 | −0.034 | 0.586 | 0.178 | −0.135 |
| WM-3 | −0.036 | 0.652 | 0.221 | −0.182 |
| WM-4 | −0.037 | 0.917 | 0.236 | −0.200 |
| WM-5 | −0.038 | 1.052 | 0.243 | −0.209 |
| WM | ΔESi | ΔTSi | ΔEO | ΔTO |
|---|---|---|---|---|
| WM-1 | 0.444 | 0.342 | 0.012 | 0.048 |
| WM-2 | 0.618 | 0.552 | 0.015 | 0.043 |
| WM-3 | 0.721 | 0.616 | 0.018 | 0.039 |
| WM-4 | 1.093 | 0.880 | 0.021 | 0.036 |
| WM-5 | 1.429 | 1.014 | 0.024 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fan, J.; Zhang, D. Improving the Accuracy of Silicon Transfer Prediction in Submerged Arc Welding: A Multi-Reaction-Zone Analysis. Processes 2023, 11, 2285. https://doi.org/10.3390/pr11082285
Zhang J, Fan J, Zhang D. Improving the Accuracy of Silicon Transfer Prediction in Submerged Arc Welding: A Multi-Reaction-Zone Analysis. Processes. 2023; 11(8):2285. https://doi.org/10.3390/pr11082285
Chicago/Turabian StyleZhang, Jin, Jun Fan, and Dan Zhang. 2023. "Improving the Accuracy of Silicon Transfer Prediction in Submerged Arc Welding: A Multi-Reaction-Zone Analysis" Processes 11, no. 8: 2285. https://doi.org/10.3390/pr11082285
APA StyleZhang, J., Fan, J., & Zhang, D. (2023). Improving the Accuracy of Silicon Transfer Prediction in Submerged Arc Welding: A Multi-Reaction-Zone Analysis. Processes, 11(8), 2285. https://doi.org/10.3390/pr11082285






