Ammonia Decomposition over Alkali Metal (Li, K, Cs)-Promoted Bulk Mo2N Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Activity Tests
3. Results
3.1. Surface Characterization
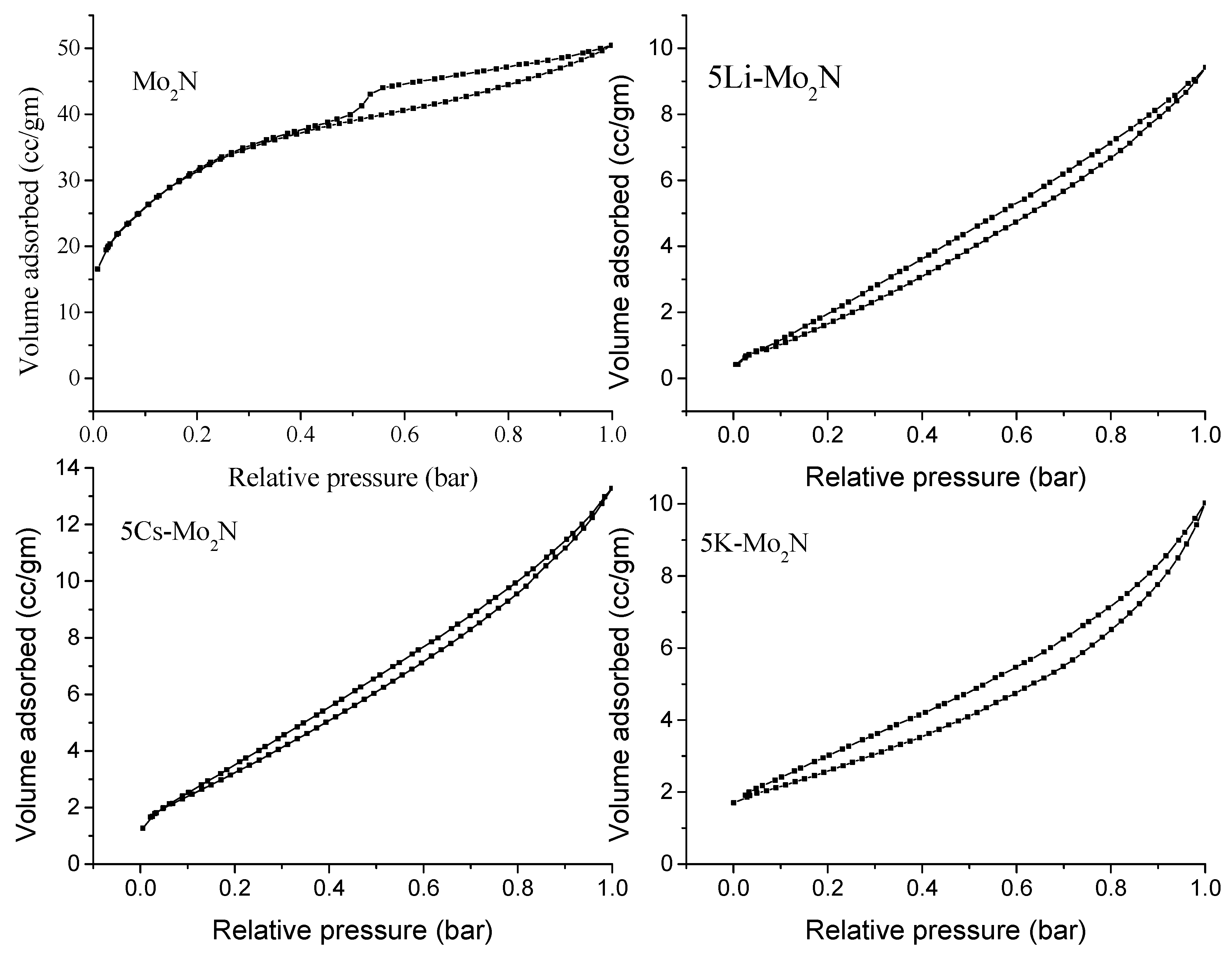
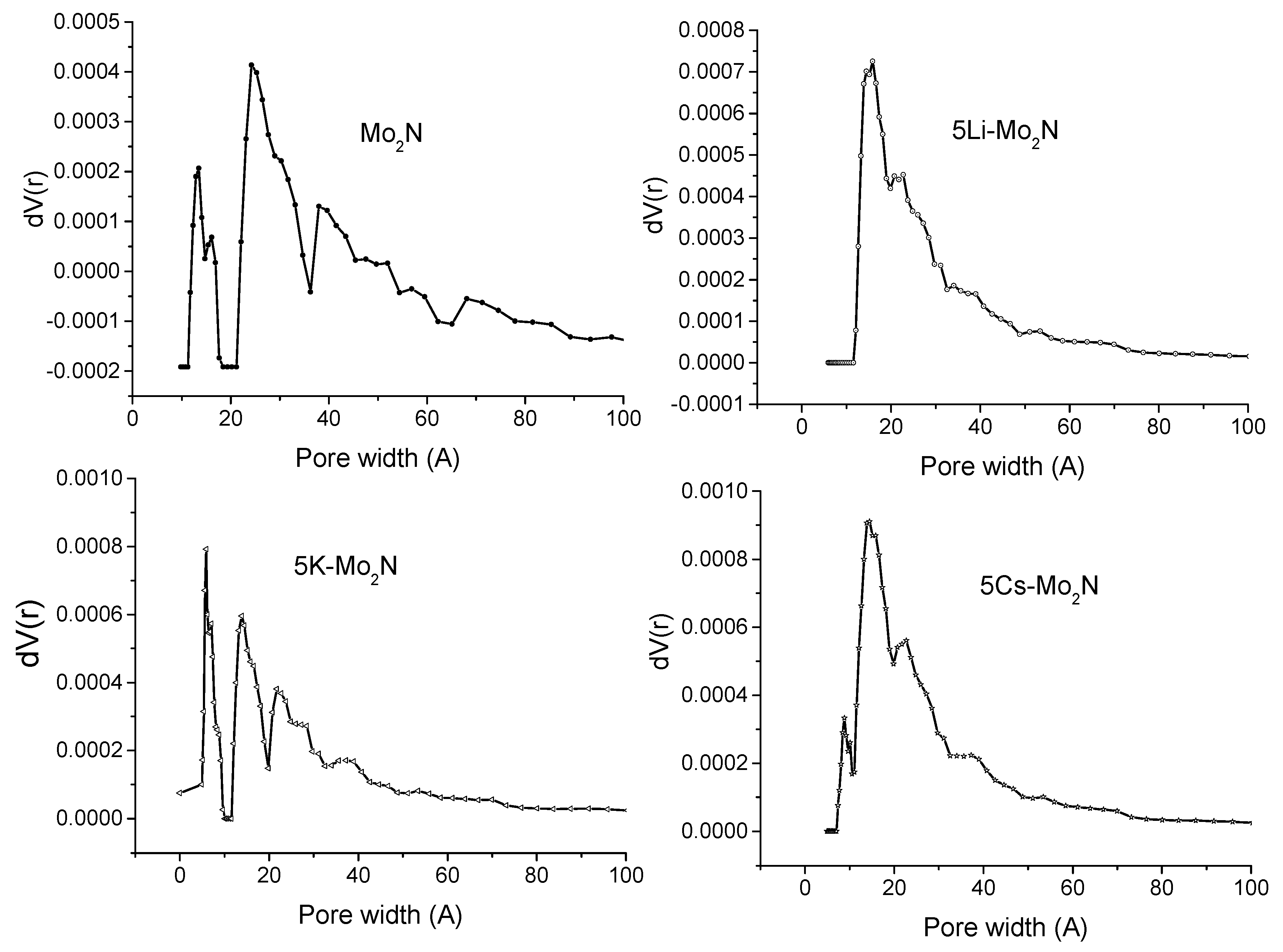
3.1.1. BET Surface Area and Pore Size Distribution
3.1.2. XRD
3.1.3. XPS Analysis of the Sample
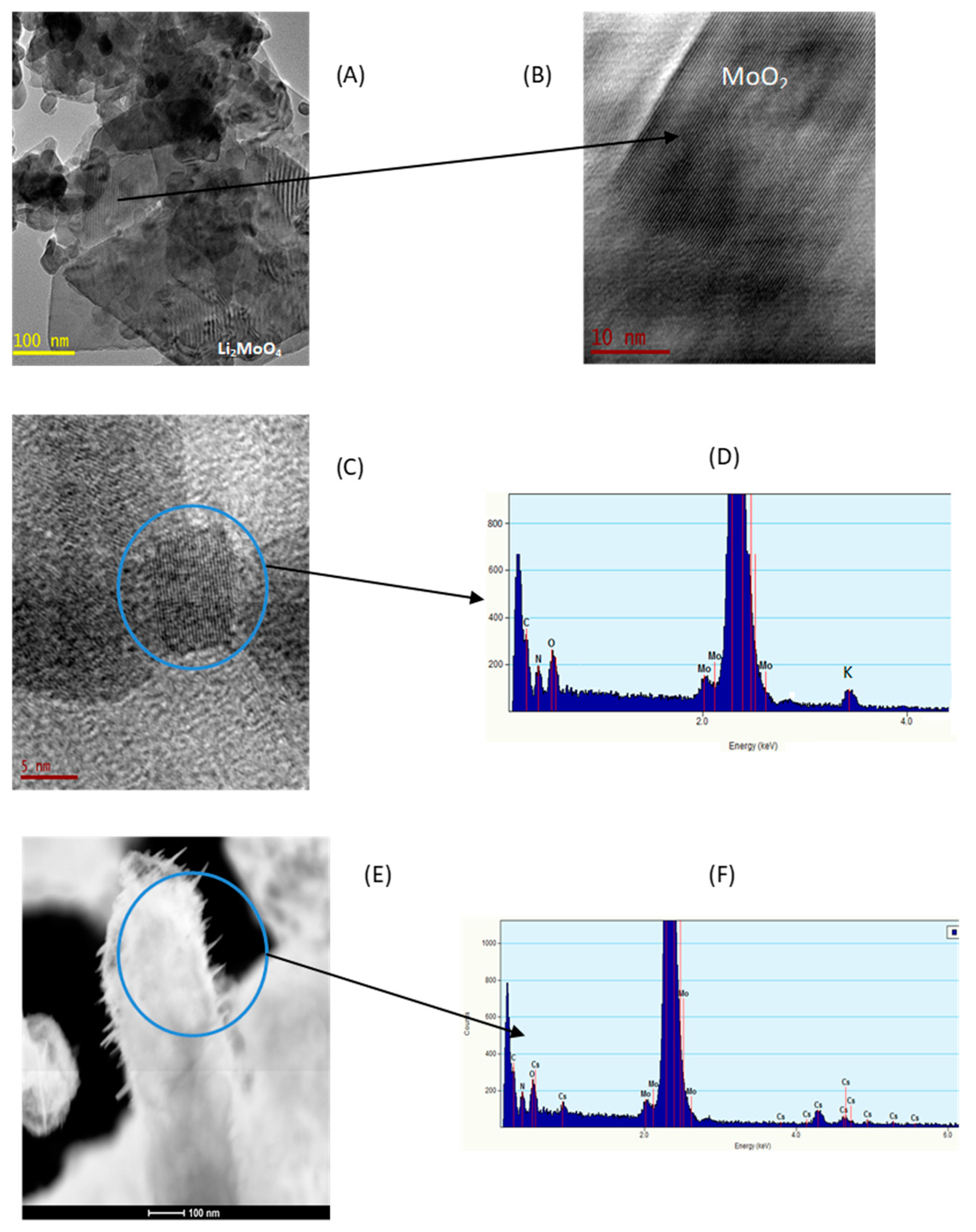
3.1.4. HRTEM and EDS Analysis
3.2. Results and Discussion
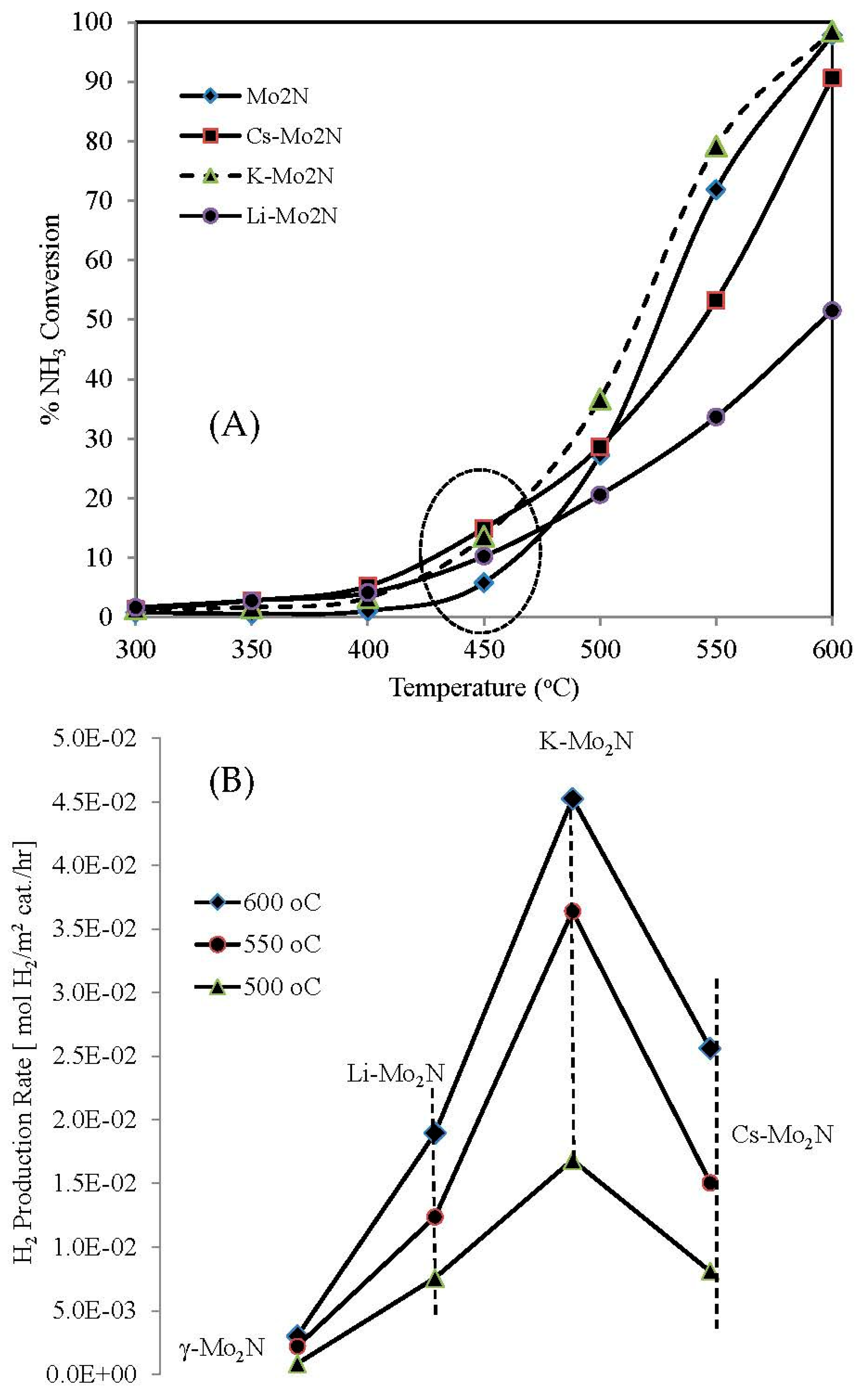
3.2.1. Activity Results
3.2.2. Long-Run Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lendzion-Bielun, Z.; Narkiewicz, U.; Arabczyk, W. Cobalt-based Catalysts for Ammonia Decomposition. Materials 2013, 6, 2400–2409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muroyama, H.; Saburi, C.; Matsui, T.; Eguchi, K. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen. Appl. Catal. A Gen. 2012, 443–444, 119–124. [Google Scholar] [CrossRef]
- Bell, T.E.; Torrent-Murciano, L. H2 production via ammonia decomposition using non-Noble metal catalysts: A review. Top. Catal. 2016, 59, 1438–1457. [Google Scholar] [CrossRef] [Green Version]
- Opeyemi, A.O.; Zaman, S.F. Ammonia decomposition for hydrogen production: A thermodynamic study. Chem. Pap. 2021, 75, 57–65. [Google Scholar]
- Lateef, J.; Zaman, S.F. Catalytic Ammonia Decomposition for Hydrogen Production: Utilization of ammonia in a fuel cell. In Sustainable Ammonia Production; Inamuddin, Boddula, R., Asiri, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 81–105. [Google Scholar]
- Podila, S.; Zaman, S.F.; Driss, H.; Alhamed, Y.; Al-Zahrani, A.A.; Petrov, L.A. Hydrogen production by ammonia decomposi-tion using high surface area Mo2N and Co3Mo3N catalysts. Catal. Sci. Technol. 2016, 6, 1496–1506. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini review on ammonia decomposition catalysts for on-site generation of hydro-gen for fuel cell applications. Appl. Catal. A 2004, 277, 1–9. [Google Scholar] [CrossRef]
- William, I.F.D.; Joshua, W.M.; Samantha, K.C.; Hazel, M.A.H.; James, D.T.; Thomas, J.W.; Martin, O.J. Hydrogen production from ammonia using sodium amide. J. Am. Chem. Soc. 2014, 136, 13082–13085. [Google Scholar]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Ge, Q.; Li, W. Highly efficient Ru/MgO catalysts for NH3 decomposition: Synthesis, characterization and promoter effect. Catal. Commun. 2006, 7, 148–152. [Google Scholar] [CrossRef]
- Weidenthaler, C.; Tseng, H.C. In situ diffraction studies during transition metal catalysis. In Proceedings of the 30th European Crystallographic Meeting, Congress Center, Basel, Switzerland, 28 August–1 September 2016. [Google Scholar]
- Liu, H. Ammonia Synthesis Catalysts: Innovation and Practice; World Scientific Publishing Co. Pvt. Ltd.: Singapore, 2013; pp. 1–896. [Google Scholar]
- Wang, S.; Yin, S.; Li, L.; Xu, B.; Ng, C.; Au, C. Investigation on modification of Ru/CNTs catalyst for the generation of COx-free hydrogen from ammonia. Appl. Catal. B: Environ. 2004, 52, 287–299. [Google Scholar] [CrossRef]
- Hill, A.K.; Torrente-Murciano, L. Low temperature H2 production from ammonia using ruthenium-based catalysts: Synergetic effect of promoter and support. Appl. Catal. B: Environ. 2015, 172–173, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, Z.; Sentek, J.; Jodzis, S.; Muhler, M.; Hinrichsen, O. Effect of Potassium on the Kinetics of Ammonia Synthesis and Decomposition over Fused Iron Catalyst at Atmospheric Pressure. J. Catal. 1997, 169, 407–414. [Google Scholar] [CrossRef]
- Aika, K.; Takano, T.; Murata, S. Preparation and characterization of chlorine-free ruthenium catalysts and the promoter effect in ammonia synthesis: 3. A magnesia-supported ruthenium catalyst. J. Catal. 1992, 136, 126–140. [Google Scholar] [CrossRef]
- Chang, F.; Guo, J.; Wu, G.; Wang, P.; Yu, P.; Chen, P. Influence of alkali metal amides on the catalytic activity of manganese nitride for ammonia decomposition. Catal. Today 2017, 286, 141–146. [Google Scholar] [CrossRef]
- Patterson, P.M.; Das, T.K.; Davis, B.H. Carbon monoxide hydrogenation over molybdenum and tungsten carbides. Appl. Catal. A: Gen. 2003, 251, 449–455. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, X. Supported cobalt molybdenum bimetallic nitrides for ammonia decomposition. Chin. J. Chem. Eng. 2005, 13, 696–700. [Google Scholar]
- Podila, S.; Zaman, S.F.; Driss, H.; Al-Zahrani, A.A.; Daous, M.A.; Petrov, L.A. High performance of bulk Mo2N and Co3Mo3 N catalysts for hydrogen production from ammonia: Role of citric acid to Mo molar ratio in preparation of high surface area nitride catalysts. Int. J. Hydrog. Energy 2017, 42, 8006–8020. [Google Scholar] [CrossRef]
- Srifa, A.; Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. COx-free hydrogen production via ammonia decom-position over molybdenum nitride-based catalysts. Catal. Sci. Technol. 2016, 6, 7495–7504. [Google Scholar] [CrossRef]
- Tagliazucca, V.; Schlichte, K.; Schüth, F.; Weidenthaler, C. Molybdenum-based catalysts for the decomposition of ammonia: In situ X-ray diffraction studies, microstructure, and catalytic properties. J. Catal. 2013, 305, 277–289. [Google Scholar] [CrossRef]
- Guo, J.; Wang, P.; Wu, G.; Wu, A.; Hu, D.; Xiong, Z.; Wang, J.; Yu, P.; Chang, F.; Chen, Z.; et al. Lithium Imide Synergy with 3d Transition-Metal Nitrides Leading to Unprecedented Catalytic Activities for Ammonia Decomposition. Angew. Chem. Int. Ed. 2015, 54, 2950–2954. [Google Scholar] [CrossRef]
- Joshua, W.M.; Thomas, J.W.; Phillip, L.M.; Ronald, I.S.; Claire, A.M.; William, I.F.D. Bulk phase behavior of lithium imide–metal nitride ammonia decomposition catalysts. Phys. Chem. Chem. Phys. 2018, 20, 22689–22697. [Google Scholar]
- Atthapon, S.; Kaname, O.; Takeou, O.; Hiroki, M.; Toshiaki, M.; Koichi, E. Hydrogen production by ammonia decomposition over Cs-modified Co3Mo3N catalysts. Appl. Catal. B Environ. 2017, 218, 1–8. [Google Scholar]
- Panda, R.N.; Kaskel, S. Synthesis and characterization of high surface area molybdenum nitride. J. Mater. Sci. 2006, 41, 2465–2470. [Google Scholar] [CrossRef]
- Andersson, R.; Boutonnet, M.; Järås, S. Correlation patterns and effect of syngas conversion level for product selectivity to alcohols and hydrocarbons over molybdenum sulfide based catalysts. Appl. Catal. A Gen. 2012, 417–418, 119–128. [Google Scholar] [CrossRef]
- Meimei, L.V.; Xie, W.; Sun, S.; Wu, G.; Zheng, L.; Chu, S.; Gaoa, C.; Bao, J. Activated-carbon-supported K–Co–Mo catalysts for synthesis of higher alcohols from syngas. Catal. Sci. Technol. 2015, 5, 2925–2934. [Google Scholar]
- Tanaka, S.; Taniguchi, M.; Tanigawa, H. XPS and UPS studies on electronic structure of Li2O. J. Nucl. Mater. 2000, 283–287, 1405–1408. [Google Scholar] [CrossRef]
- Moggia, J.M.; Milt, V.G.; Ulla, M.A.; Cornaglia, L.M. Surface characterization of Co,K/La2O3 catalysts used for the catalytic combustion of diesel soot. Surf. Interface Anal. 2003, 35, 216–225. [Google Scholar] [CrossRef]
- Sawyer, R.; Nesbitt, H.; Secco, R. High resolution X-ray Photoelectron Spectroscopy (XPS) study of K2O–SiO2 glasses: Evidence for three types of O and at least two types of Si. J. Non-Cryst. Solids 2012, 358, 290–302. [Google Scholar] [CrossRef]
- Nefedov, V.; Salyn, Y.; Leonhardt, G.; Scheibe, R. A comparison of different spectrometers and charge corrections used in X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1977, 10, 121–124. [Google Scholar] [CrossRef]
- Chukeaw, T.; Seubsai, A.; Phon-in, P.; Charoen, K.; Witoon, T.; Donphai, W.; Parpainainar, P.; Chareonpanich, M.; Noon, D.; Zohourd, B.; et al. Multimetallic catalysts of RuO2–CuO–Cs2O–TiO2/SiO2 for direct gas-phase epoxidation of propylene to propylene oxide. RSC Adv. 2016, 6, 56116–56126. [Google Scholar] [CrossRef]
- Podgornov, E.A.; Prosvirin, I.P.; Bukhtiyarov, V.I. XPS, TPD and TPR studies of Cs–O complexes on silver: Their role in eth-ylene epoxidation. J. Mol. Catal. A: Chem. 2000, 158, 337–343. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Waltham, MA, USA, 1979. [Google Scholar]
- Yin, S.; Xu, B.; Zhu, W.; Ng, C.; Zhou, X.; Au, C. Carbon nanotubes-supported Ru catalyst for the generation of COX-free hydrogen from ammonia. Catal. Today 2004, 93–95, 27–38. [Google Scholar] [CrossRef]
- Czekajło, Ł.; Lendzion-Bieluń, Z. Effect of preparation conditions and promoters on the structure and activity of the ammonia decomposition reaction catalyst based on nanocrystalline cobalt. Chem. Eng. J. 2016, 289, 254–260. [Google Scholar] [CrossRef]
- Okura, K.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Additive effect of alkaline earth metals on ammonia decomposi-tion reaction over Ni/Y2O3 catalysts. RSC Adv. 2016, 6, 85142–85148. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamufleh, H.S.; Zaman, S.F. Ammonia Decomposition over Alkali Metal (Li, K, Cs)-Promoted Bulk Mo2N Catalyst. Processes 2023, 11, 2287. https://doi.org/10.3390/pr11082287
Bamufleh HS, Zaman SF. Ammonia Decomposition over Alkali Metal (Li, K, Cs)-Promoted Bulk Mo2N Catalyst. Processes. 2023; 11(8):2287. https://doi.org/10.3390/pr11082287
Chicago/Turabian StyleBamufleh, Hisham S., and Sharif F. Zaman. 2023. "Ammonia Decomposition over Alkali Metal (Li, K, Cs)-Promoted Bulk Mo2N Catalyst" Processes 11, no. 8: 2287. https://doi.org/10.3390/pr11082287
APA StyleBamufleh, H. S., & Zaman, S. F. (2023). Ammonia Decomposition over Alkali Metal (Li, K, Cs)-Promoted Bulk Mo2N Catalyst. Processes, 11(8), 2287. https://doi.org/10.3390/pr11082287






