Doxycycline Removal by Solar Photo-Fenton on a Pilot-Scale Composite Parabolic Collector (CPC) Reactor
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Chemicals
2.2. Experimental Set-Up CPC Reactor
2.3. Analytical Procedures
2.4. Phytotoxicity Assay
3. Results and Discussion
3.1. Influence of the Operating Parameters
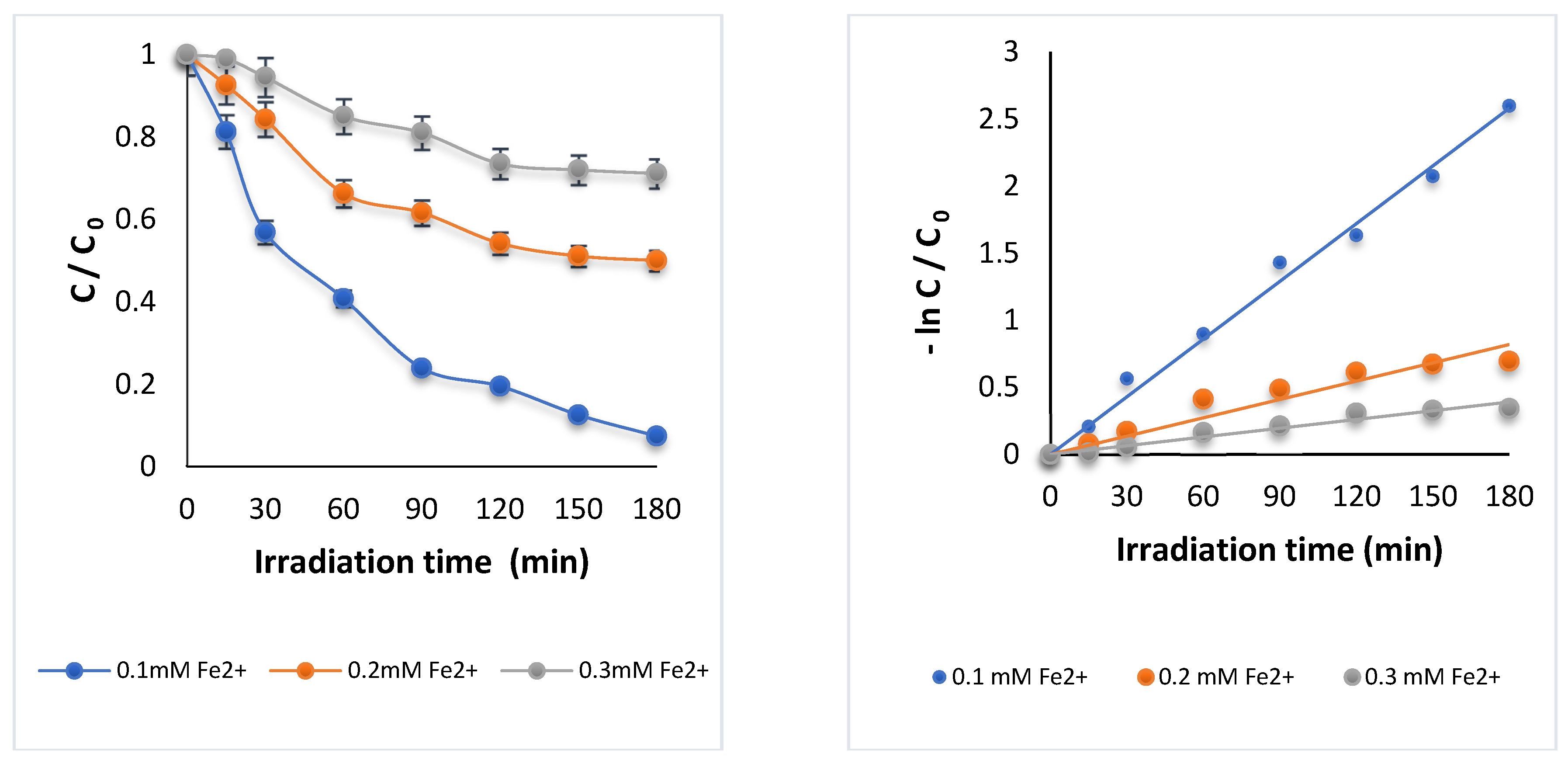
3.1.1. Effect of Iron Dosage Fe2+
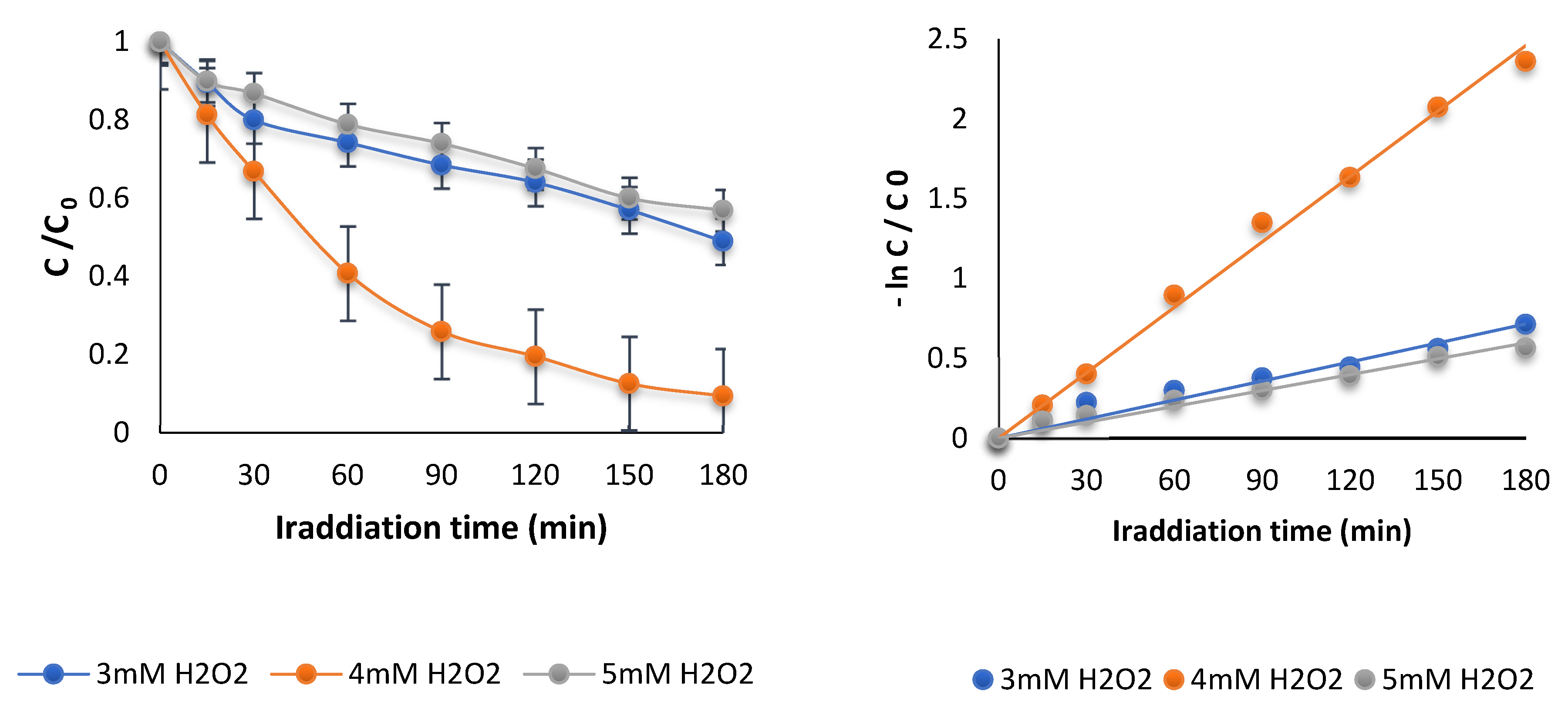
3.1.2. Effect of Initial Concentration of Hydrogen Peroxide Oxidant
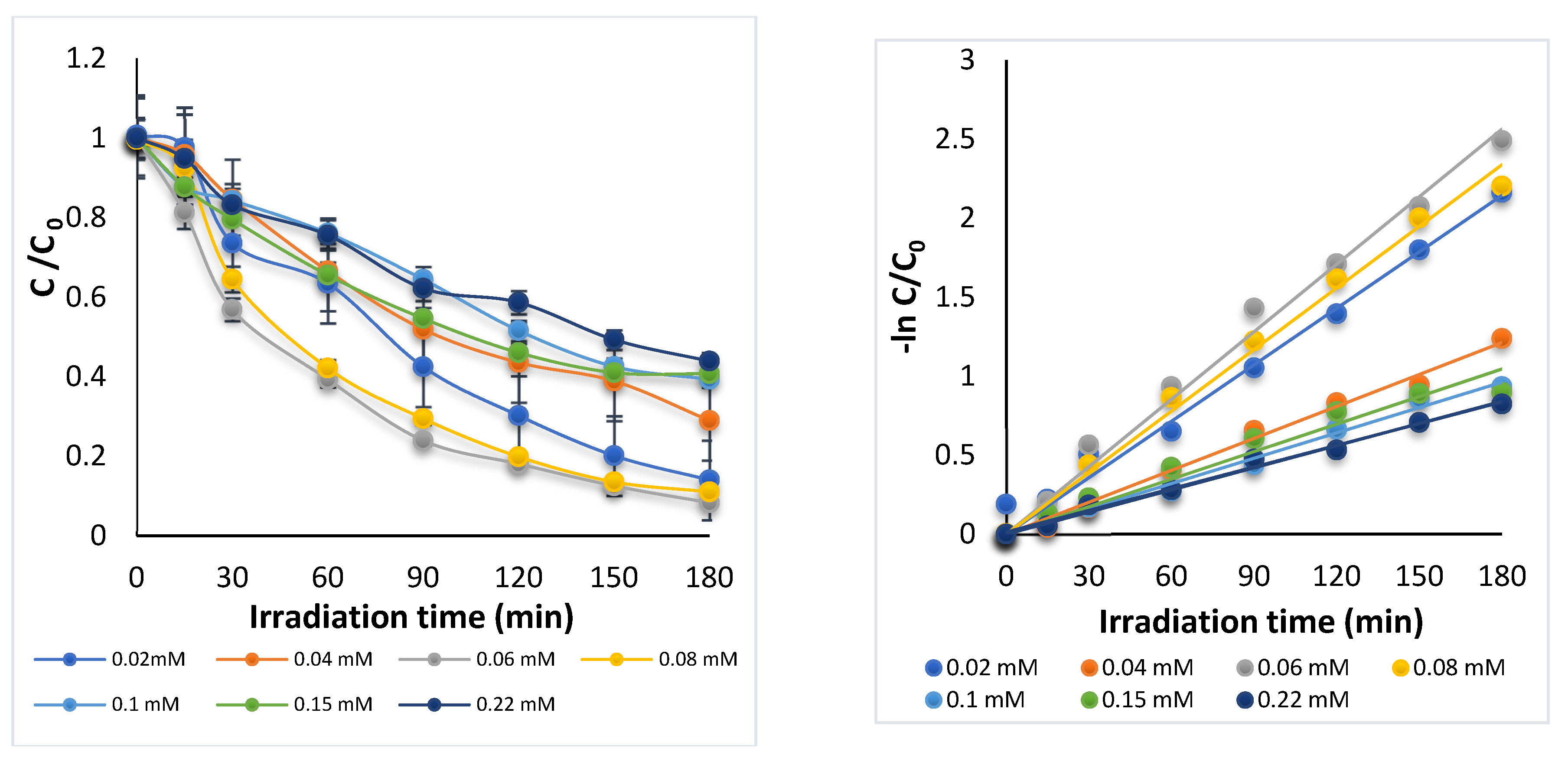
3.1.3. Effect of Initial DOX Concentration
3.2. Solar Photo-Fenton (SPF) at Optimum Conditions
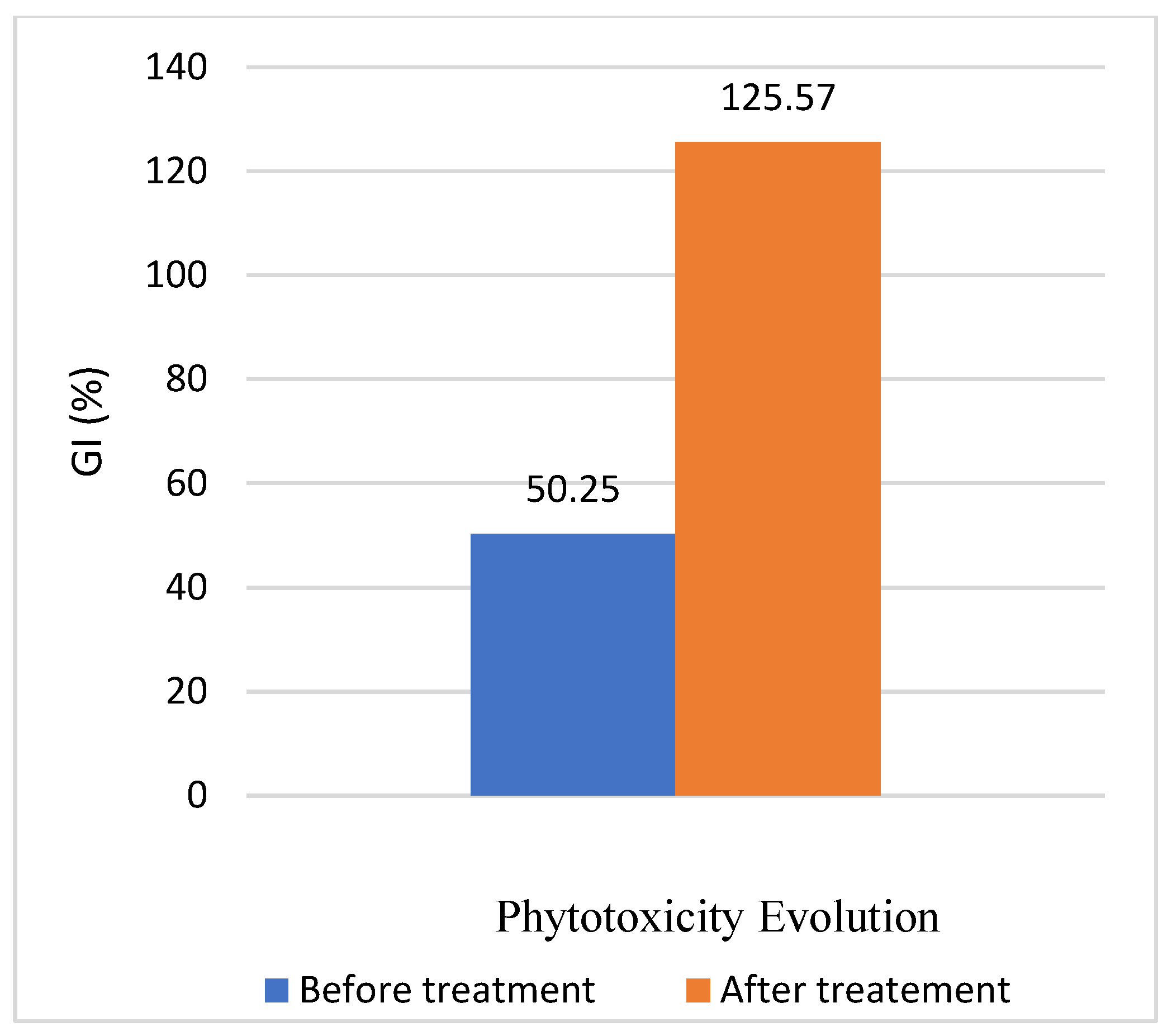
3.3. Phytotoxicity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kümmerer, K. Antibiotics in the aquatic environment—A review–Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Begum, T.; Rahman, N.; Khan, R.A. A review on antibiotic resistance and way of combating antimicrobial resistance. GSC Biol. Pharm. Sci. 2021, 14, 87–97. [Google Scholar] [CrossRef]
- Rizzo, L. Addressing main challenges in the tertiary treatment of urban wastewater: Are homogeneous photodriven AOPs the answer? Environ. Sci. Water Res. Technol. 2022, 8, 2145–2169. [Google Scholar] [CrossRef]
- Jodh, R.; Tawar, M.; Gomkale, K.; Jari, S.; Toply, J.; Faisal, N. An Updated Review on Doxycycline. Res. J. Pharmacol. Pharmacodyn. 2022, 14, 253–256. [Google Scholar] [CrossRef]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Nam, S.W.; Jo, B.I.; Yoon, Y.; Zoh, K.D. Occurrence and removal of selected micropollutants in a water treatment plant. Chemosphere 2014, 95, 156–165. [Google Scholar] [CrossRef]
- Yanovych, D.; Zasadna, Z.; Rydchuk, M.; Plotycya, S.; Kislova, S.; Pazderska, O.; Ivach, S. Doxycycline dertermination in animal tissues and blood plasma samples using screening and confirmatory methods. Sci. Tech. Bull. State Sci. Res. Control Inst. Vet. Med. Prod. Fodd. Addit. Inst. Anim. Biol. 2021, 22, 432–446. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Ma, B.; Zou, Y.; Wang, Y.; Liao, X.; Liang, J.; Mi, J.; Wu, Y. Different Concentrations of Doxycycline in Swine Manure Affect the Microbiome and Degradation of Doxycycline Residue in Soil. Front. Microbiol. 2018, 9, 3129. [Google Scholar] [CrossRef]
- Deng, W.; Li, N.; Zheng, H.; Lin, H. Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotoxicol. Environ. Saf. 2016, 125, 121–127. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Kanama, K.M.; Daso, A.P.; Mpenyana-Monyatsi, L.; Coetzee, M.A.A. Assessment of Pharmaceuticals, Personal Care Products, and Hormones in Wastewater Treatment Plants Receiving Inflows from Health Facilities in North West Province, South Africa. J. Toxicol. 2018, 2018, 3751930. [Google Scholar] [CrossRef]
- Aga, D.S.; O’Connor, S.; Ensley, S.; Payero, J.O.; Snow, D.; Tarkalson, D. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2005, 53, 7165–7171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ran, J.; Mao, K.; Yang, X.; Zhong, L.; Yang, C.; Feng, X.; Zhang, H. Recent progress in Fenton/Fenton-like reactions for the removal of antibiotics in aqueous environments. Ecotoxicol. Environ. Saf. 2022, 236, 113464. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Pineda, M.; Peyot, M.L.; Yargeau, V. Degradation of oxytetracycline and doxycycline by ozonation: Degradation pathways and toxicity assessment. Sci. Total Environ. 2023, 856, 159076. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Ma, R.; Wang, L.; Bai, L.; Yang, S.; Qian, J. Activation of peroxydisulfate (PDS) by Bi5O7I@MIL-100(Fe) for catalytic degradation of aqueous doxycycline (DOX) under UV light irradiation: Characteristic, performance and mechanism. J. Water Process Eng. 2022, 48, 102903. [Google Scholar] [CrossRef]
- Aboudalle, A.; Djelal, H.; Domergue, L.; Fourcade, F.; Amrane, A. A novel system coupling an electro-Fenton process and an advanced biological process to remove a pharmaceutical compound, metronidazole. J. Hazard. Mater. 2021, 415, 125705. [Google Scholar] [CrossRef]
- Rodrigues-Silva, F.; Lemos, C.R.; Naico, A.A.; Fachi, M.M.; do Amaral, B.; de Paula, V.C.S.; Rampon, D.S.; Beraldi-Magalhães, F.; Prola, L.D.T.; Pontarolo, R.; et al. Study of isoniazid degradation by Fenton and photo-Fenton processes, by-products analysis and toxicity evaluation. J. Photochem. Photobiol. A Chem. 2022, 425, 113671. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Mohamed Saheed, M.S.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2007, 36, 1–84. [Google Scholar] [CrossRef]
- Han, C.; Park, H.; Kim, S.; Yargeau, V.; Choi, J.; Lee, S.; Park, J. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yang, B.; Gao, F.Z.; Xiong, Q.; Feng, Y.; Li, Y.; Zhang, J.N.; Ying, G.G. Performance and mechanism in degradation of typical antibiotics and antibiotic resistance genes by magnetic resin-mediated UV-Fenton process. Ecotoxicol. Environ. Saf. 2021, 227, 112908. [Google Scholar] [CrossRef] [PubMed]
- Belalcázar-Saldarriaga, A.; Prato-Garcia, D.; Vasquez-Medrano, R. Photo-Fenton processes in raceway reactors: Technical, economic, and environmental implications during treatment of colored wastewaters. J. Clean. Prod. 2018, 182, 818–829. [Google Scholar] [CrossRef]
- Periša, M.; Babić, S.; Škorić, I.; Frömel, T.; Knepper, T.P. Photodegradation of sulfonamides and their N (4)-acetylated metabolites in water by simulated sunlight irradiation: Kinetics and identification of photoproducts. Environ. Sci. Pollut. Res. Int. 2013, 20, 8934–8946. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; Rieradevall, J.; Torrades, F.; Peral, J.; Domènech, X. Environmental assessment of different solar driven advanced oxidation processes. Sol. Energy 2005, 79, 369–375. [Google Scholar] [CrossRef]
- Rocha, E.M.R.; Vilar, V.J.P.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.R. Landfill leachate treatment by solar-driven AOPs. Sol. Energy 2011, 85, 46–56. [Google Scholar] [CrossRef]
- Nascimento, C.A.O.; Teixeira, A.C.S.C.; Guardani, R.; Quina, F.H.; Chiavone-Filho, O.; Braun, A.M. Industrial wastewater treatment by photochemical processes based on solar energy. J. Sol. Energy Eng. Trans. ASME 2007, 129, 45–52. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Bolobajev, J.; Trapido, M.; Goi, A. Effect of iron ion on doxycycline photocatalytic and Fenton-based autocatatalytic decomposition. Chemosphere 2016, 153, 220–226. [Google Scholar] [CrossRef]
- Sunarić, S.M.; Denić, M.S.; Bojanić, Z.Ž.; Bojanić, V.V. HPLC method development for determination of doxycycline in human seminal fluid. J. Chromatogr. B 2013, 939, 17–22. [Google Scholar] [CrossRef]
- Zeghioud, H.; Khellaf, N.; Amrane, A.; Djelal, H.; Bouhelassa, M.; Assadi, A.A.; Rtimi, S. Combining photocatalytic process and biological treatment for Reactive Green 12 degradation: Optimization, mineralization, and phytotoxicity with seed germination. Environ. Sci. Pollut. Res. 2021, 28, 12490–12499. [Google Scholar] [CrossRef] [PubMed]
- Cherif, S.; Djelal, H.; Firmin, S.; Bonnet, P.; Frezet, L.; Kane, A.; Amine Assadi, A.; Trari, M.; Yazid, H. The impact of material design on the photocatalytic removal efficiency and toxicity of two textile dyes. Environ. Sci. Pollut. Res. 2022, 29, 66640–66658. [Google Scholar] [CrossRef] [PubMed]
- Rahim Pouran, S.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Pagilla, K. Treatment of malathion pesticide wastewater with nanofiltration and photo-Fenton oxidation. Desalination 2010, 263, 36–44. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A. Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dye. Pigment. 2006, 71, 236–244. [Google Scholar] [CrossRef]
- Loaiza-Ambuludi, S.; Panizza, M.; Oturan, N.; Oturan, M.A. Removal of the anti-inflammatory drug ibuprofen from water using homogeneous photocatalysis. Catal. Today 2014, 224, 29–33. [Google Scholar] [CrossRef]
- Cahino, A.M.; de Andrade, M.M.A.; de Araújo, E.S.; Silva, E.L.; Cunha, C.D.O.; Rocha, E.M.R. Degradation of tetracycline by solar photo-Fenton: Optimization and application in pilot photoreactor. Environ. Qual. Manag. 2018, 28, 101–106. [Google Scholar] [CrossRef]
- Caianelo, M.; Rodrigues-Silva, C.; Maniero, M.G.; Guimarães, J.R. Antimicrobial activity against Gram-positive and Gram-negative bacteria during gatifloxacin degradation by hydroxyl radicals. Environ. Sci. Pollut. Res. 2017, 24, 6288–6298. [Google Scholar] [CrossRef]
- Giannakis, S.; Gamarra Vives, F.A.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Degradation of four pharmaceuticals by solar photo-Fenton process: Kinetics and costs estimation. J. Environ. Chem. Eng. 2015, 3, 46–51. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Alexander, J.; Schwartz, T.; Fatta-Kassinos, D. Investigation of the potential of a Membrane BioReactor followed by solar Fenton oxidation to remove antibiotic-related microcontaminants. Chem. Eng. J. 2017, 310, 491–502. [Google Scholar] [CrossRef]
- Cárdenas Sierra, R.S.; Zúñiga-Benítez, H.; Peñuela, G.A. Photo-assisted removal of doxycycline using H2O2 and simulated sunlight: Operational parameters optimization and ecotoxicity assessment. J. Photochem. Photobiol. A Chem. 2022, 425. [Google Scholar] [CrossRef]
- Annabi, C.; Abou Dalle, A.; Fourcade, F.; Assadi, A.A.; Soutrel, I.; Bellakhal, N.; Amrane, A. Enoxacin degradation by photo-Fenton process combined with a biological treatment: Optimization and improvement of by-products biodegradability. Int. J. Environ. Sci. Technol. 2019, 16, 655–666. [Google Scholar] [CrossRef]
- Li, S.; He, Y.; Kong, F.; Sun, W.; Hu, J. Photolytic Degradation of Tetracycline in the Presence of Ca(II) and/or Humic Acid. Water 2020, 12, 2078. [Google Scholar] [CrossRef]
- Chen, W.R.; Huang, C.H. Transformation of tetracyclines mediated by Mn(II) and Cu(II) ions in the presence of oxygen. Environ. Sci. Technol. 2009, 43, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Aye, T.T.; Low, T.Y.; Sze, S.K. Nanosecond laser-induced photochemical oxidation method for protein surface mapping with mass spectrometry. Anal. Chem. 2005, 77, 5814–5822. [Google Scholar] [CrossRef]
- Mota, A.L.N.; Muranaka, C.T.; Moraes, J.E.F.; Nascimento, C.A.O. Aplicação do Processo Foto-Fenton na Fotodegradação do Fenol em Meio Aquoso Utilizando l’Ampadas de Luz Negra como Fonte de Radiaçao; Federation de Ingenieros Quimicos del Peru: Peru, Lima, 2005. [Google Scholar]
- Álvarez-Esmorís, C.; Rodríguez-López, L.; Fernández-Calviño, D.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Degradation of Doxycycline, Enrofloxacin, and Sulfamethoxypyridazine under Simulated Sunlight at Different pH Values and Chemical Environments. Agronomy 2022, 12, 260. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Raj, S.; Prakash, H.; Fatta-Kassinos, D. Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effluents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- da Costa, E.P.; Bottrel, S.E.C.; Starling, M.C.V.M.; Leão, M.M.D.; Amorim, C.C. Degradation of carbendazim in water via photo-Fenton in Raceway Pond Reactor: Assessment of acute toxicity and transformation products. Environ. Sci. Pollut. Res. 2019, 26, 4324–4336. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Michael, C.; Varela, A.R.; Kyriakou, S.; Manaia, C.M.; Fatta-Kassinos, D. Solar photo-Fenton process on the abatement of antibiotics at a pilot scale: Degradation kinetics, ecotoxicity and phytotoxicity assessment and removal of antibiotic resistant enterococci. Water Res. 2012, 46, 5621–5634. [Google Scholar] [CrossRef]
- Sirtori, C.; Zapata, A.; Oller, I.; Gernjak, W.; Agüera, A.; Malato, S. Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res. 2009, 43, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.A.; Silva, M.F.; Al Arni, S.; Converti, A.; Palma, M.S.A. Doxycycline degradation by the oxidative Fenton process. J. Chem. 2015, 2015, 492030. [Google Scholar] [CrossRef]


| Scientific Nomenclature | Molecular Formula | Molecular Structure | Molecular Weight (g·mol−1) | pKa | |
|---|---|---|---|---|---|
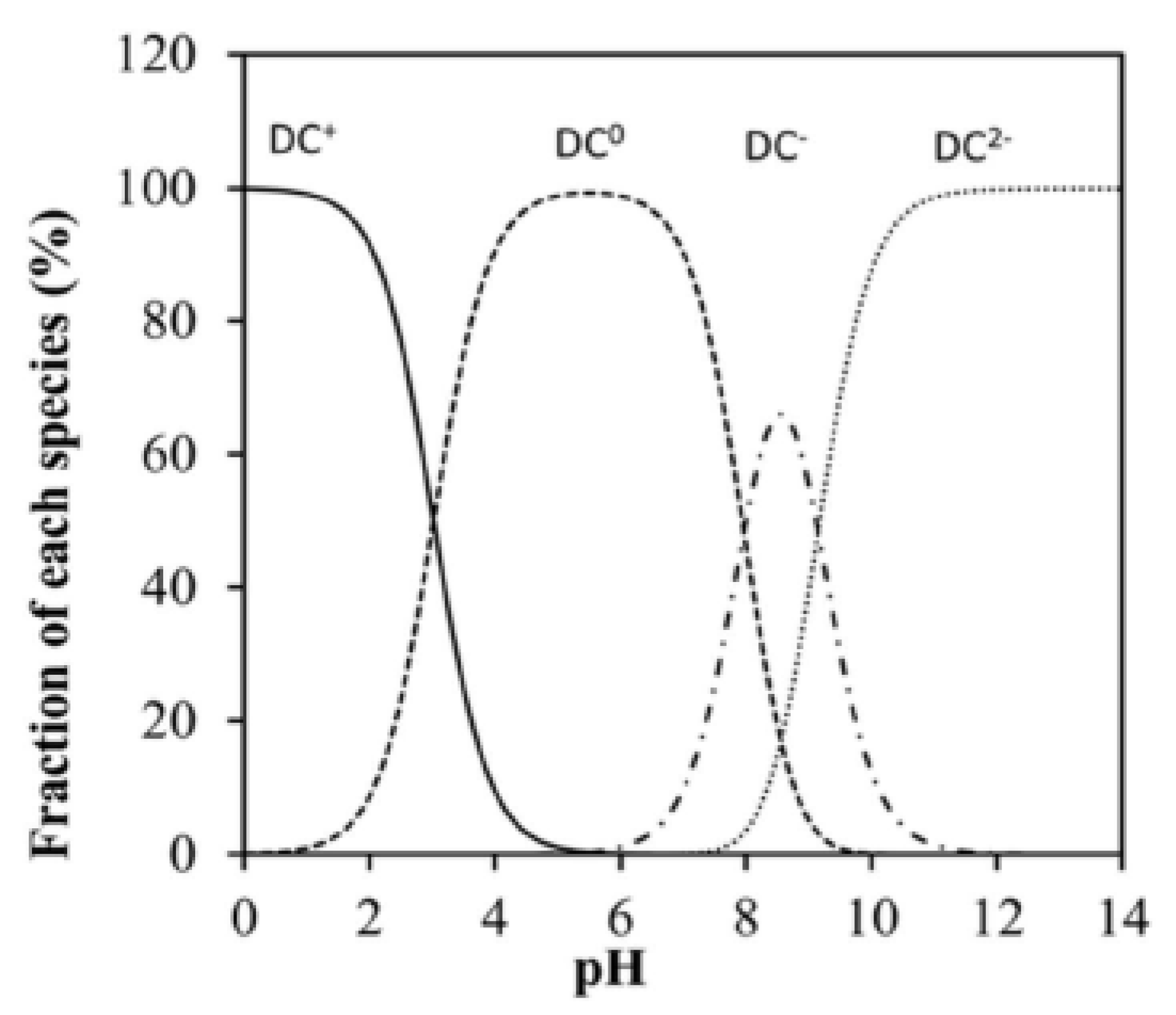
| Doxycycline | C22H24N2O8 | 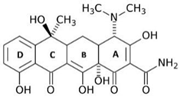 | 444.4 | = 278 | pKa1 = 3.50 pKa2 = 7.07 pKa3 = 9.13 |
| Fe2+ (mM) | K (min−1) | R2 | DOX Removal (%) |
|---|---|---|---|
| 0.1 | 0.0143 | 0.99 | 92.5 |
| 0.2 | 0.0045 | 0.91 | 50.0 |
| 0.3 | 0.0022 | 0.95 | 28.0 |
| H2O2 (mM) | K (min−1) × 10−1 | R2 | DOX Removal (%) |
|---|---|---|---|
| 3 | 0.04 | 0.94 | 51.00 |
| 4 | 0.13 | 0.99 | 90.00 |
| 5 | 0.03 | 0.97 | 43.10 |
| DOX (mM) | K (min−1) | R2 | DOX Removal (%) |
|---|---|---|---|
| 0.02 | 0.0119 | 0.99 | 87.4 |
| 0.04 | 0.0067 | 0.99 | 71.0 |
| 0.06 | 0.0142 | 0.99 | 91.7 |
| 0.08 | 0.0130 | 0.99 | 88.0 |
| 0.100 | 0.0053 | 0.98 | 60.7 |
| 0.150 | 0.0058 | 0.94 | 59.2 |
| 0.220 | 0.0047 | 0.99 | 56.1 |
| TOC | COD | |
|---|---|---|
| Before treatment | 17.67 ± 1.14 mg/L | 53.33 ± 4.79 mg O2/L |
| After treatment | 4.75 ± 0.04 mg/L | 9.9 ± 4.63 mg O2/L |
| Removal rate (%) | 73.05 | 81.43 |
| Pharmaceutical Pollutant | Ratio | Type and Time of the Process | Pollutant Removal (%) | COD Removal (%) | References |
|---|---|---|---|---|---|
| ofloxacin (OFX) and trimethoprim (TMP). | 15.0 | SPF 180 min | 100% | 21% | [51] |
| sulfamethoxazole (SMX) and erythromycin (ERY) | 10.0 | SPF 120 min | 100% | 53% | [41] |
| Ampicillin (AMP) | 10.0 | SPF 180 min | 95% | 24% | [49] |
| Isoniazid (INH) | 12.5 | SPF 120 min | 99.99% | 70% | [17] |
| Amoxicillin, Ampicillin, Diclofenac, and Paracetamol | 3 | SPF 120 min | 100% | - | [40] |
| nalidixic acid | 15 | SPF 190 min | 99.99% | 33% | [52] |
| Tetracycline | 0.67 | SPF 120 min | 88.7% | - | [37] |
| Doxycycline (DOX) | 40.0 | SPF 180 min | 95.07% | 81% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensaibi, F.; Chabani, M.; Bouafia, S.; Djelal, H. Doxycycline Removal by Solar Photo-Fenton on a Pilot-Scale Composite Parabolic Collector (CPC) Reactor. Processes 2023, 11, 2363. https://doi.org/10.3390/pr11082363
Bensaibi F, Chabani M, Bouafia S, Djelal H. Doxycycline Removal by Solar Photo-Fenton on a Pilot-Scale Composite Parabolic Collector (CPC) Reactor. Processes. 2023; 11(8):2363. https://doi.org/10.3390/pr11082363
Chicago/Turabian StyleBensaibi, Faiza, Malika Chabani, Souad Bouafia, and Hayet Djelal. 2023. "Doxycycline Removal by Solar Photo-Fenton on a Pilot-Scale Composite Parabolic Collector (CPC) Reactor" Processes 11, no. 8: 2363. https://doi.org/10.3390/pr11082363
APA StyleBensaibi, F., Chabani, M., Bouafia, S., & Djelal, H. (2023). Doxycycline Removal by Solar Photo-Fenton on a Pilot-Scale Composite Parabolic Collector (CPC) Reactor. Processes, 11(8), 2363. https://doi.org/10.3390/pr11082363








