Modelling the Effect of Water Removal by Reverse Osmosis on the Distillation of Mixtures of Short-Chain Organic Acids from Anaerobic Fermentation
Abstract
1. Introduction
2. Methodology
2.1. Streams and Process Scheme
2.2. Distillation Simulations
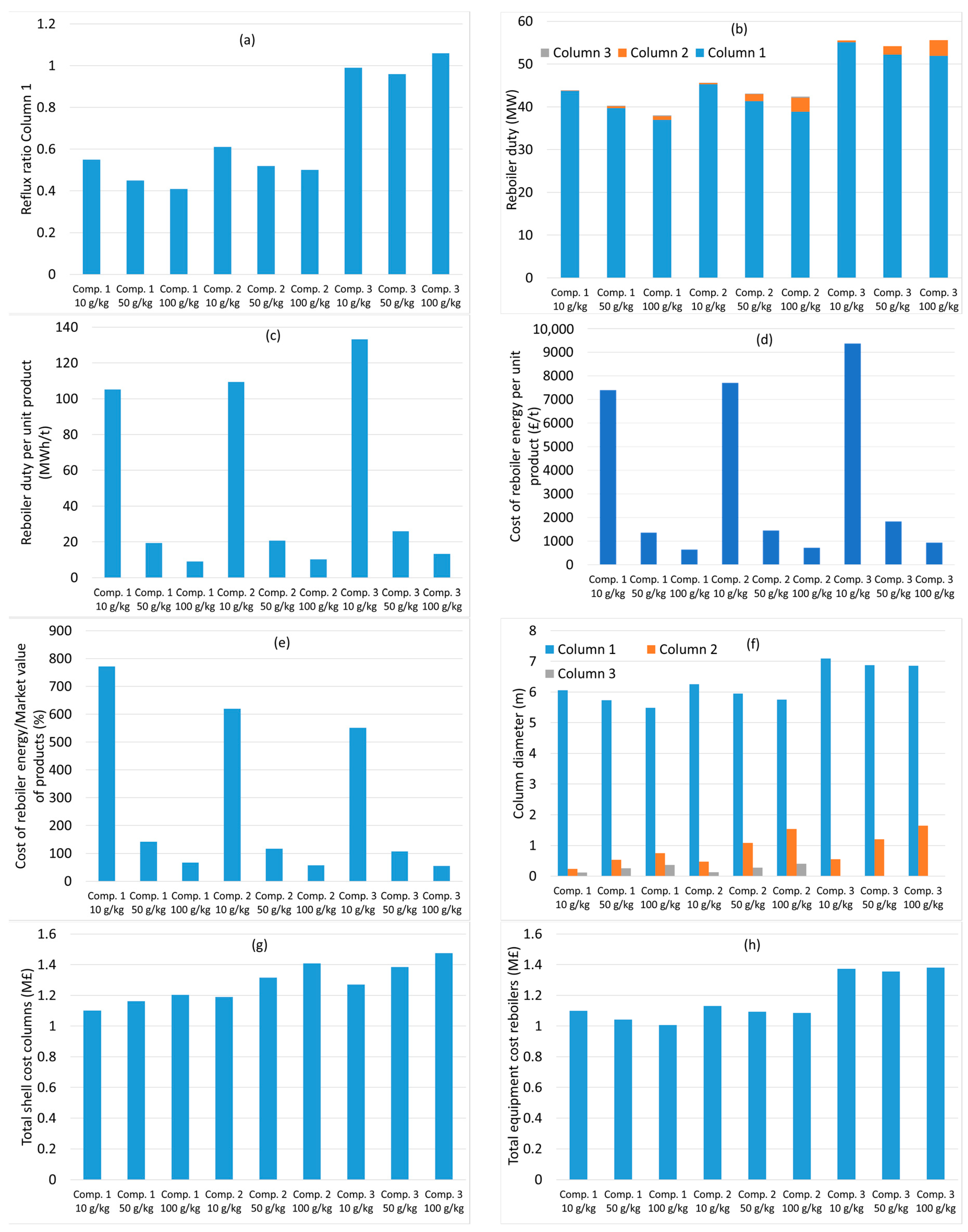
- In each column, the distillate flow rate was set equal to the mass flow rate entering the distillation process of the component to be purified as distillate, i.e., water in column 1, acetic acid in column 2 and propionic acid in column 3.
- The purity of each of the acids was set as 99.00 (% w/w) or higher.
- The reflux ratio in each column was varied manually to find the minimum value that ensured the required purity of all acids.
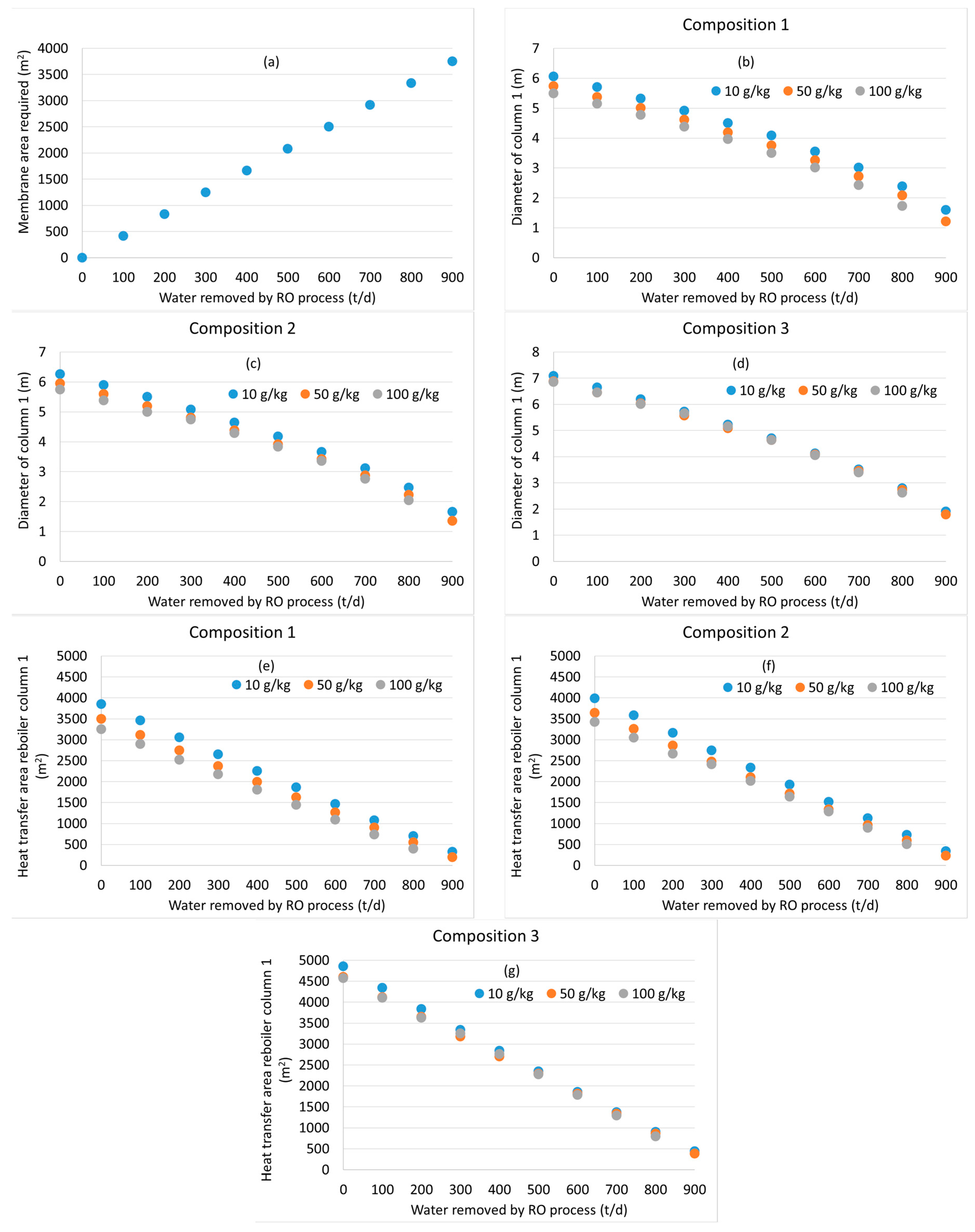
2.3. Equipment Sizing
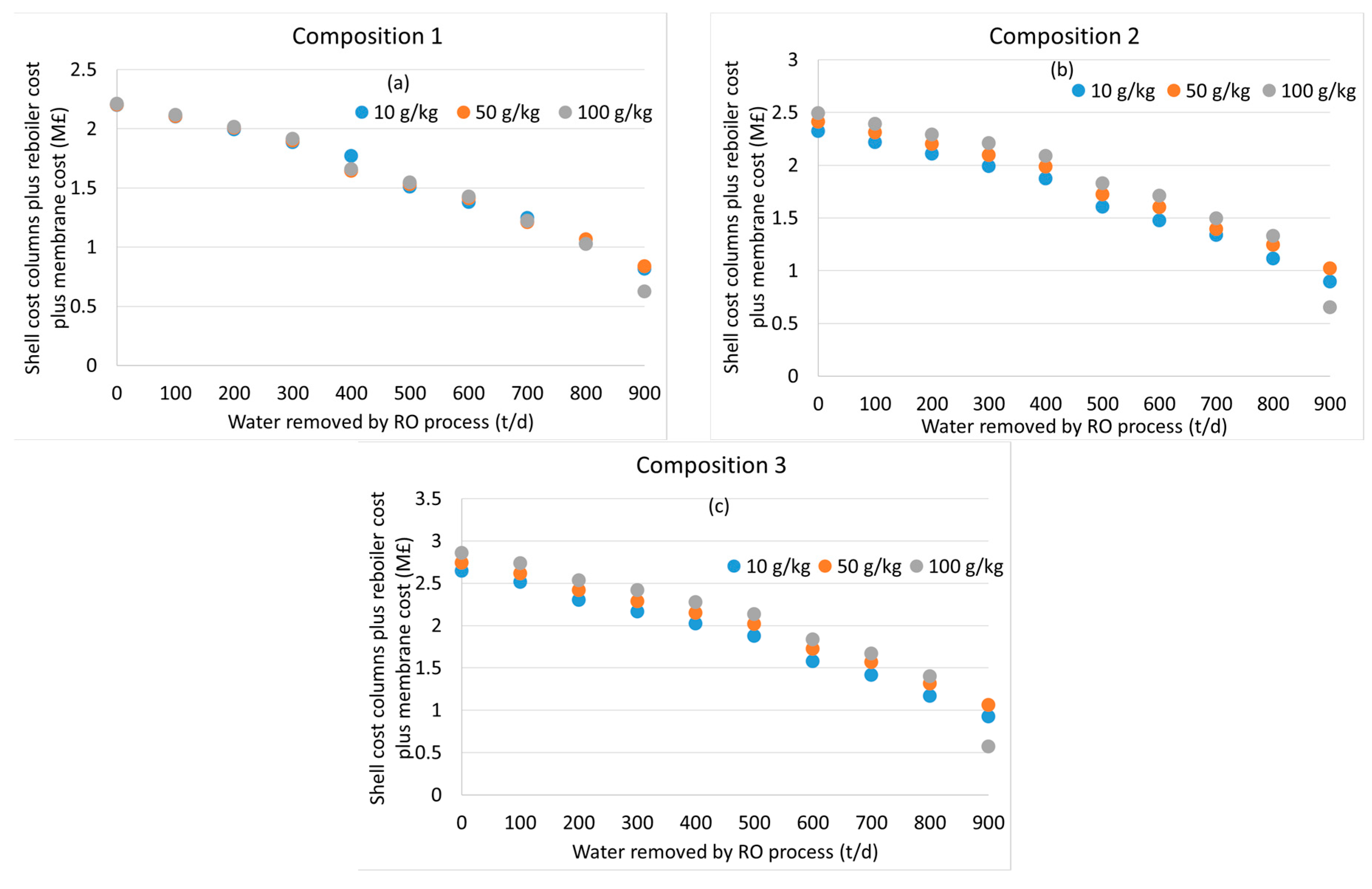
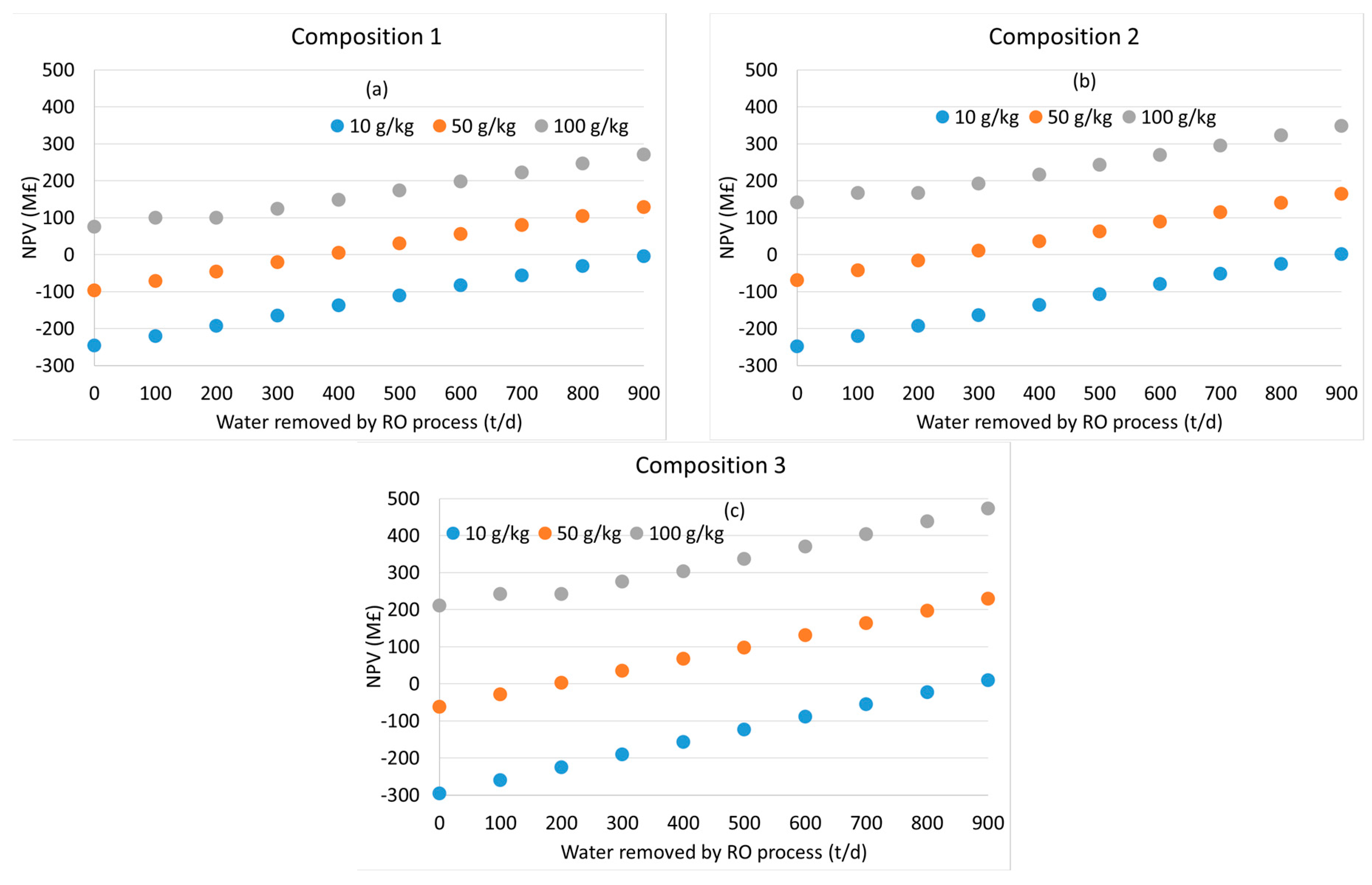
2.4. Economic Calculations
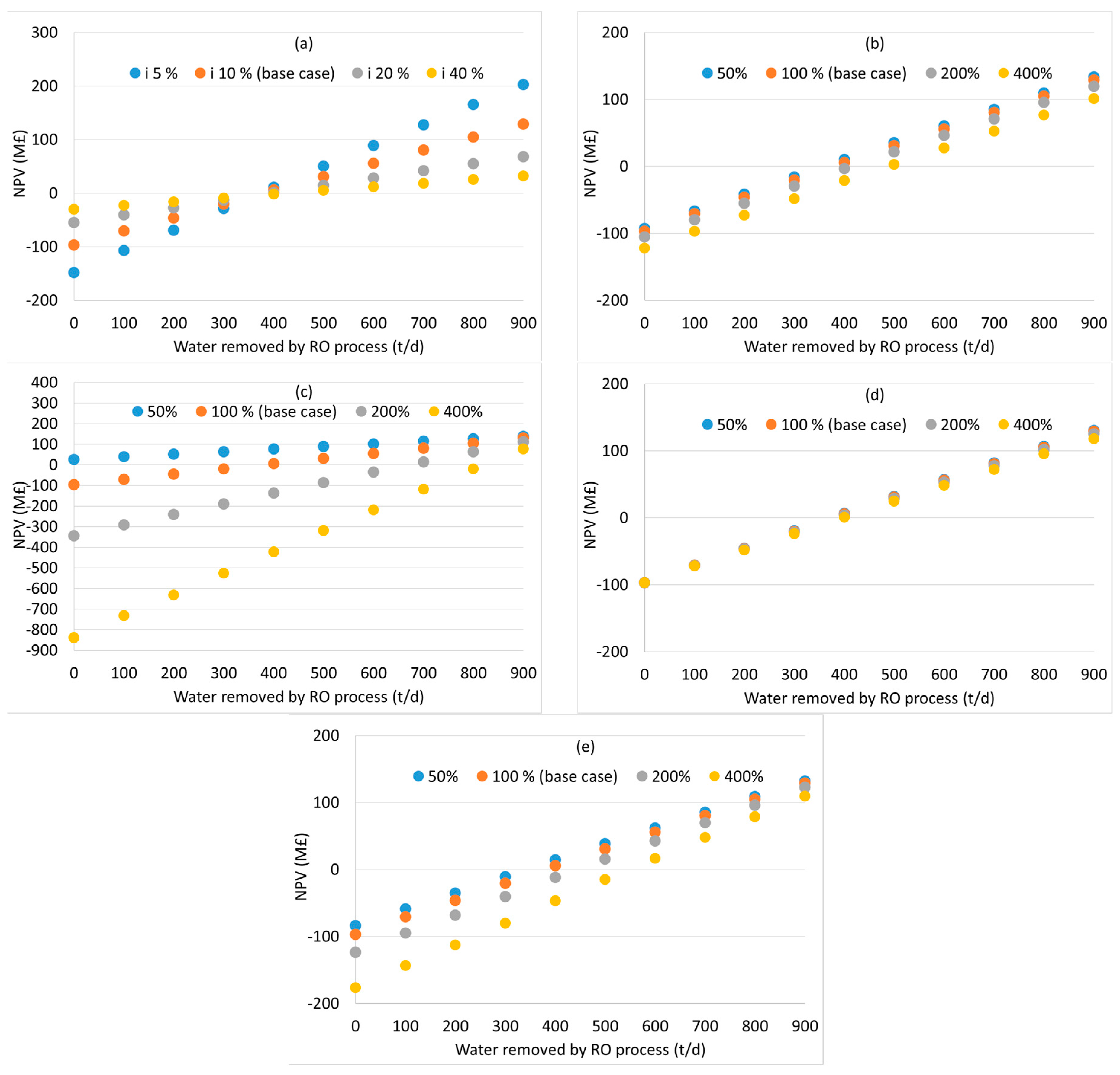
3. Results and Discussion
3.1. Distillation without Membrane Concentration
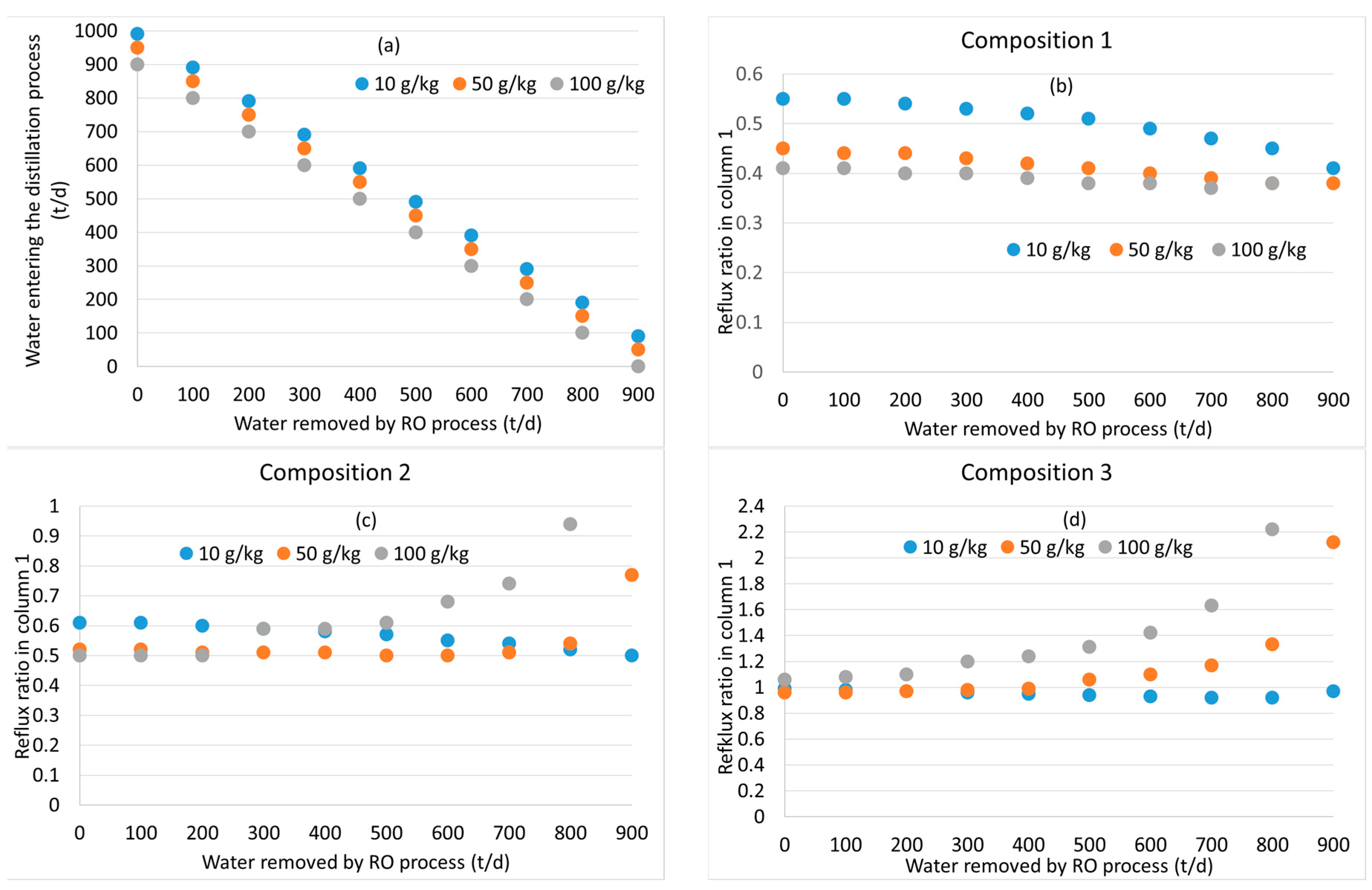
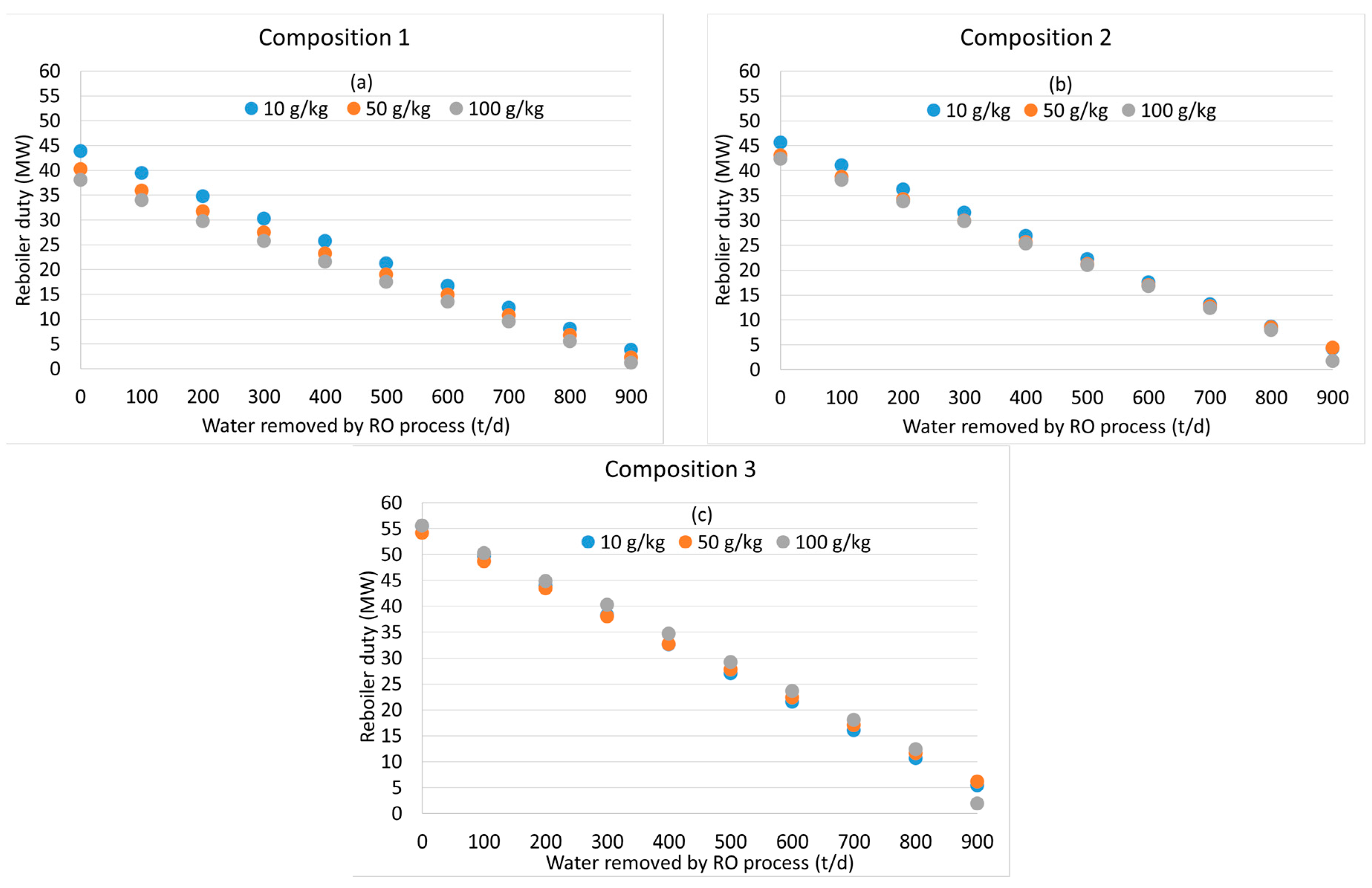
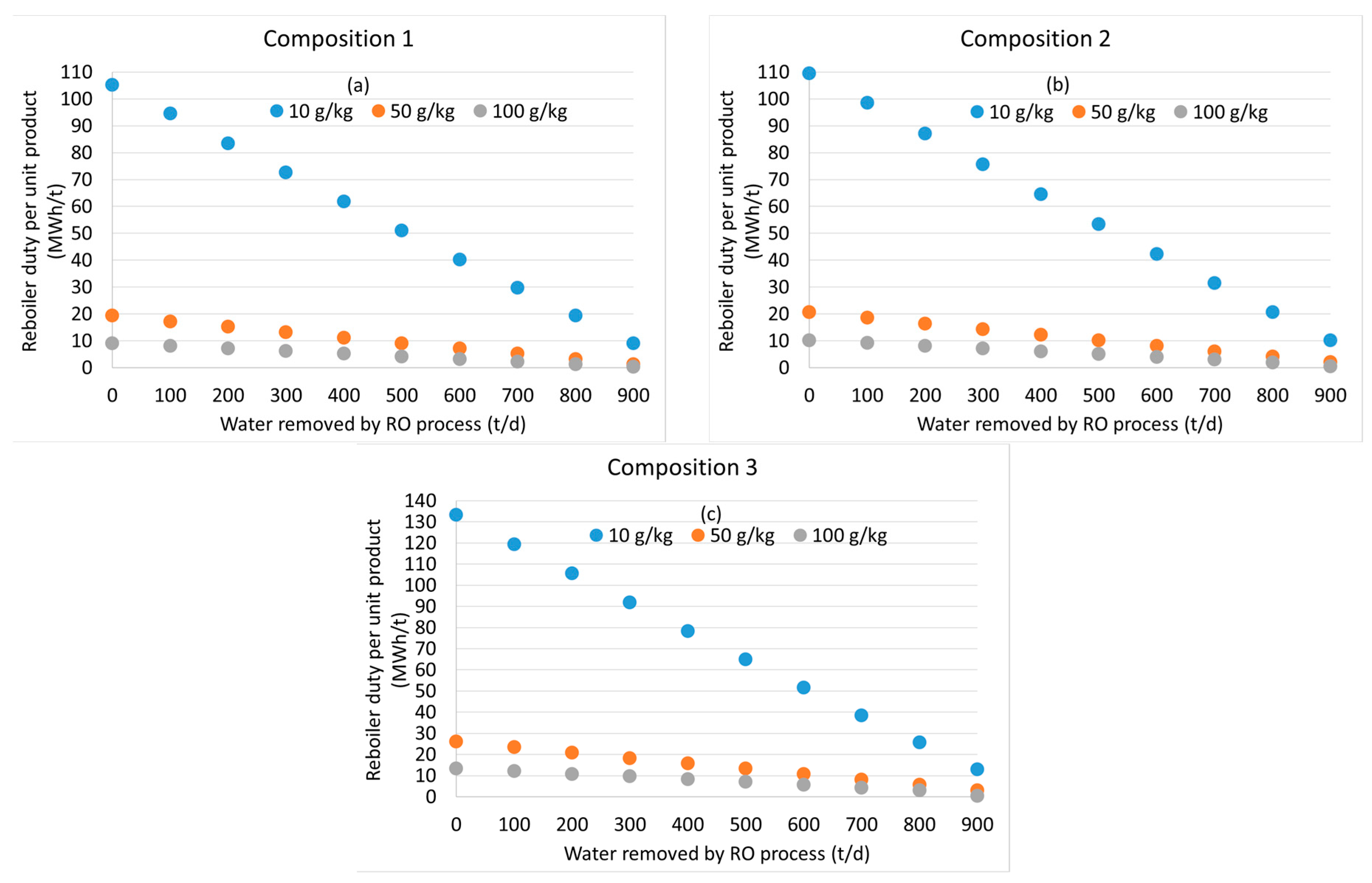
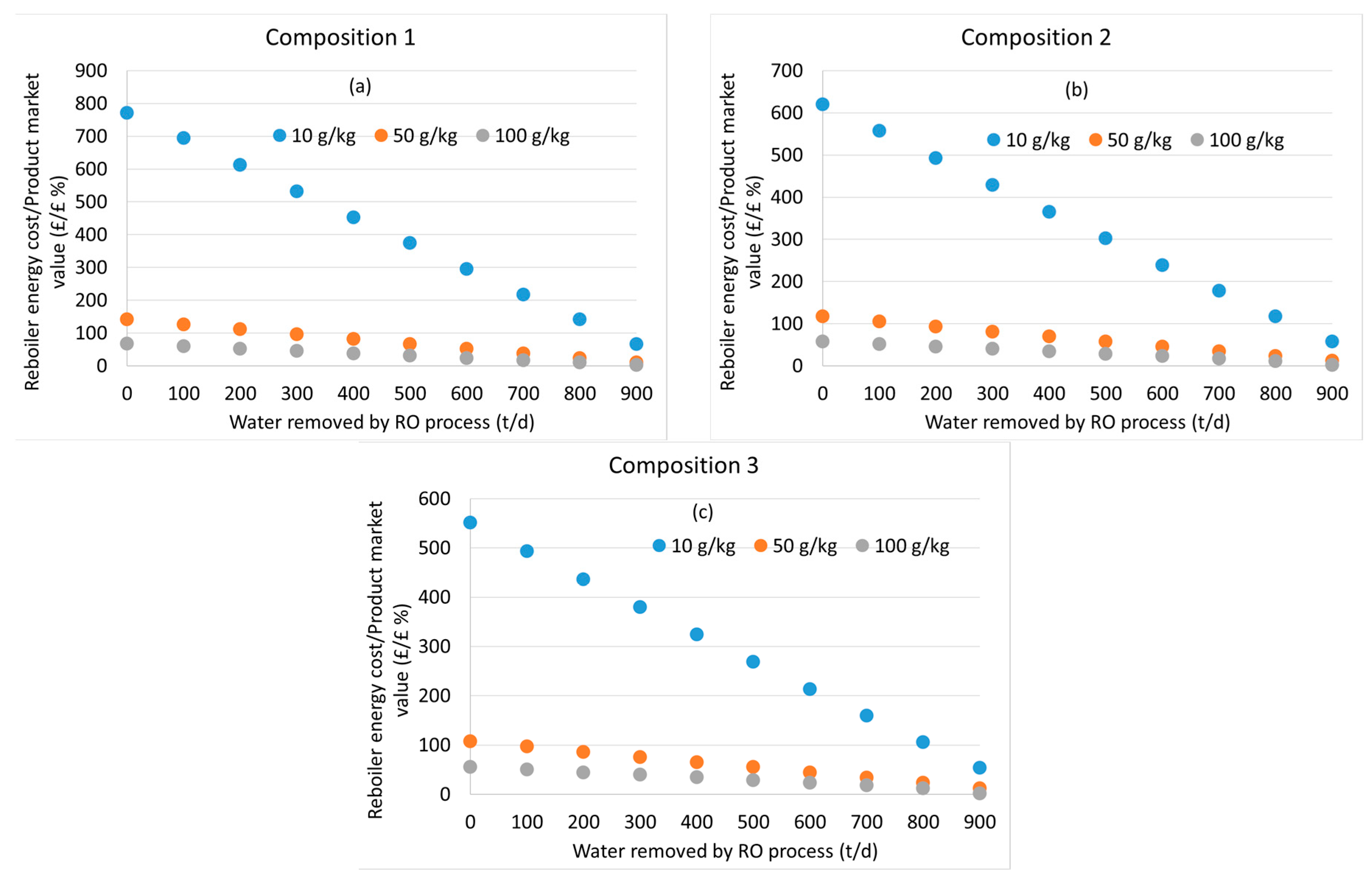
3.2. Distillation after Water Removal with Membranes
3.3. General Discussion and Model Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleerebezem, R.; Joosse, B.; Rozendal, R.; Van Loosdrecht, M.C. Anaerobic digestion without biogas? Rev. Environ. Sci. Biotechnol. 2015, 14, 787–801. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ormeci, B.; Radadiya, P.; Dhar, B.R.; Sangal, A.; Hussain, A. Leach bed reactors for production of short-chain fatty acids: A review of critical operating parameters, current limitations and challenges, and prospects. Chem. Eng. J. 2023, 456, 141044. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.A.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial propionic acid production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Pal, P.; Nayak, J. Acetic Acid Production and Purification: Critical Review Towards Process Intensification. Sep. Purif. Rev. 2017, 46, 44–61. [Google Scholar] [CrossRef]
- Dionisi, D.; Silva, I.M.O. Production of ethanol, organic acids and hydrogen: An opportunity for mixed culture biotechnology? Rev. Environ. Sci. Biotechnol. 2016, 15, 213–242. [Google Scholar] [CrossRef]
- Simonetti, S.; Saptoro, A.; Fernández Martín, C.; Dionisi, D. Product concentration, yield and productivity in anaerobic digestion to produce short chain organic acids: A critical analysis of literature data. Processes 2020, 8, 1538. [Google Scholar] [CrossRef]
- Li, Q.Z.; Jiang, X.L.; Feng, X.J.; Wang, J.M.; Sun, C.; Zhang, H.B.; Xian, M.; Liu, H.Z. Recovery Processes of Organic Acids from Fermentation Broths in the Biomass-Based Industry. J. Microbiol. Biotechnol. 2016, 26, 1–8. [Google Scholar] [CrossRef]
- Murali, N.; Srinivas, K.; Ahring, B.K. Biochemical production and separation of carboxylic acids for biorefinery applications. Fermentation 2017, 3, 22. [Google Scholar] [CrossRef]
- Kiss, A.A.; Lange, J.P.; Schuur, B.; Brilman, D.W.F.; van der Ham, A.G.; Kersten, S.R. Separation technology–Making a difference in biorefineries. Biomass Bioenergy 2016, 95, 296–309. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. Recovery of volatile fatty acids (VFA) from complex waste effluents using membranes. Water Sci. Technol. 2014, 69, 495–503. [Google Scholar] [CrossRef]
- Domingos, J.M.; Martinez, G.A.; Morselli, E.; Bandini, S.; Bertin, L. Reverse osmosis and nanofiltration opportunities to concentrate multicomponent mixtures of volatile fatty acids. Sep. Purif. Technol. 2022, 290, 120840. [Google Scholar] [CrossRef]
- Bóna, Á.; Bakonyi, P.; Galambos, I.; Bélafi-Bakó, K.; Nemestóthy, N. Separation of volatile fatty acids from model anaerobic effluents using various membrane technologies. Membranes 2020, 10, 252. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, C.; Wei, J. Simultaneous acetic acid separation and monosaccharide concentration by reverse osmosis. Biores. Technol. 2013, 131, 349–356. [Google Scholar] [CrossRef]
- Simonetti, S. Anaerobic Fermentation of Food Waste for the Production of Chemicals. Chapter 7. Concentration of Fermentation Products via Reverse Osmosis Membrane Filtration. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2022. [Google Scholar]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, S.; Martin, C.F.; Dionisi, D. Anaerobic fermentation for the production of short chain organic acids: Product concentration, yield and productivity in batch experiments at high feed concentration. J. Environ. Chem. Eng. 2021, 9, 106311. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pilli, S.; Bhunia, P.; Tyagi, R.D.; Surampalli, R.Y.; Zhang, T.C.; Kim, S.-H. Ashok Pandey. Dark fermentation: Production and utilization of volatile fatty acid from different wastes-A review. Chemosphere 2022, 288, 132444. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.T.; Lee, M.J.; Lin, H.M. Multiphase equilibria for mixtures containing acetic acid, water, propylene glycol monomethyl ether, and propylene glycol methyl ether acetate. Ind. Eng. Chem. Res. 2006, 45, 2123–2130. [Google Scholar] [CrossRef]
- Komesu, A.; Oliveira, J.D.; Maciel, M.R.W.; Maciel Filho, R. Simulation of molecular distillation process for lactic acid. J. Chem. Chem. Eng. 2016, 10, 230–234. [Google Scholar]
- Kanchanalai, P.; Lively, R.P.; Realff, M.J.; Kawajiri, Y. Cost and energy savings using an optimal design of reverse osmosis membrane pretreatment for dilute bioethanol purification. Ind. Eng. Chem. Res. 2013, 52, 11132–11141. [Google Scholar] [CrossRef]
- Sinnott, R.; Towler, G. Chemical Engineering Design, 5th ed.; Coulson and Richardson’s Chemical Engineering Series; IChemE: Rugby, UK, 2013. [Google Scholar]
- Available online: www.statista.com (accessed on 8 June 2023).
- Caldera, U.; Breyer, C. Learning curve for seawater reverse osmosis desalination plants: Capital cost trend of the past, present, and future. Water Resour. Res. 2017, 53, 10523–10538. [Google Scholar] [CrossRef]
- UK Government. Statistical Data Set. Gas and Electricity Prices in the Non-Domestic Sector. Available online: https://www.gov.uk/government/statistical-data-sets/gas-and-electricity-prices-in-the-non-domestic-sector (accessed on 8 July 2023).
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Hernandez, S. Analysis of energy-efficient complex distillation options to purify bioethanol. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2008, 31, 597–603. [Google Scholar] [CrossRef]
- Dimian, A.C.; Kiss, A.A. Novel energy efficient process for acetic acid production by methanol carbonylation. Chem. Eng. Res. Des. 2020, 159, 1–12. [Google Scholar] [CrossRef]
- Yoneda, N.; Kusano, S.; Yasui, M.; Pujado, P.; Wilcher, S. Recent advances in processes and catalysts for the production of acetic acid. Appl. Catal. A Gen. 2001, 221, 253–265. [Google Scholar] [CrossRef]
- Komesu, A.; Wolf Maciel, M.R.; Rocha de Oliveira, J.A.; da Silva Martins, L.H.; Maciel Filho, R. Purification of lactic acid produced by fermentation: Focus on non-traditional distillation processes. Sep. Purif. Rev. 2017, 46, 241–254. [Google Scholar] [CrossRef]


| SCOAs | SCOA Composition (% w/w of Total SCOAs) | Mass Flow Rate (kg/d) of Each Component for Total SCOA Concentration of | |||
|---|---|---|---|---|---|
| 10 g/kg | 50 g/kg | 100 g/kg | |||
| Composition 1 | Acetic | 10 | 1.00 × 103 | 5.00 × 103 | 1.00 × 104 |
| Lactic | 80 | 8.00 × 103 | 4.00 × 104 | 8.00 × 104 | |
| Propionic | 10 | 1.00 × 103 | 5.00 × 103 | 1.00 × 104 | |
| Water | 9.90 × 105 | 9.50 × 105 | 9.00 × 105 | ||
| Composition 2 | Acetic | 40 | 4.00 × 103 | 2.00 × 104 | 4.00 × 104 |
| Lactic | 40 | 4.00 × 103 | 2.00 × 104 | 4.00 × 104 | |
| Propionic | 20 | 2.00 × 103 | 1.00 × 104 | 2.00 × 104 | |
| Water | 9.90 × 105 | 9.50 × 105 | 9.00 × 105 | ||
| Composition 3 | Acetic | 60 | 6.00 × 103 | 3.00 × 104 | 6.00 × 104 |
| Lactic | 0 | 0 | 0 | 0 | |
| Propionic | 40 | 4.00 × 103 | 2.00 × 104 | 4.00 × 104 | |
| Water | 9.90 × 105 | 9.50 × 105 | 9.00 × 105 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonetti, S.; Dionisi, D. Modelling the Effect of Water Removal by Reverse Osmosis on the Distillation of Mixtures of Short-Chain Organic Acids from Anaerobic Fermentation. Processes 2023, 11, 2362. https://doi.org/10.3390/pr11082362
Simonetti S, Dionisi D. Modelling the Effect of Water Removal by Reverse Osmosis on the Distillation of Mixtures of Short-Chain Organic Acids from Anaerobic Fermentation. Processes. 2023; 11(8):2362. https://doi.org/10.3390/pr11082362
Chicago/Turabian StyleSimonetti, Serena, and Davide Dionisi. 2023. "Modelling the Effect of Water Removal by Reverse Osmosis on the Distillation of Mixtures of Short-Chain Organic Acids from Anaerobic Fermentation" Processes 11, no. 8: 2362. https://doi.org/10.3390/pr11082362
APA StyleSimonetti, S., & Dionisi, D. (2023). Modelling the Effect of Water Removal by Reverse Osmosis on the Distillation of Mixtures of Short-Chain Organic Acids from Anaerobic Fermentation. Processes, 11(8), 2362. https://doi.org/10.3390/pr11082362







