Mixing Characteristics and Parameter Effects on the Mixing Efficiency of High-Viscosity Solid–Liquid Mixtures under High-Intensity Acoustic Vibration
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Numerical Simulation
2.3. Evaluation of Mixing Uniformity
3. Results and Discussion
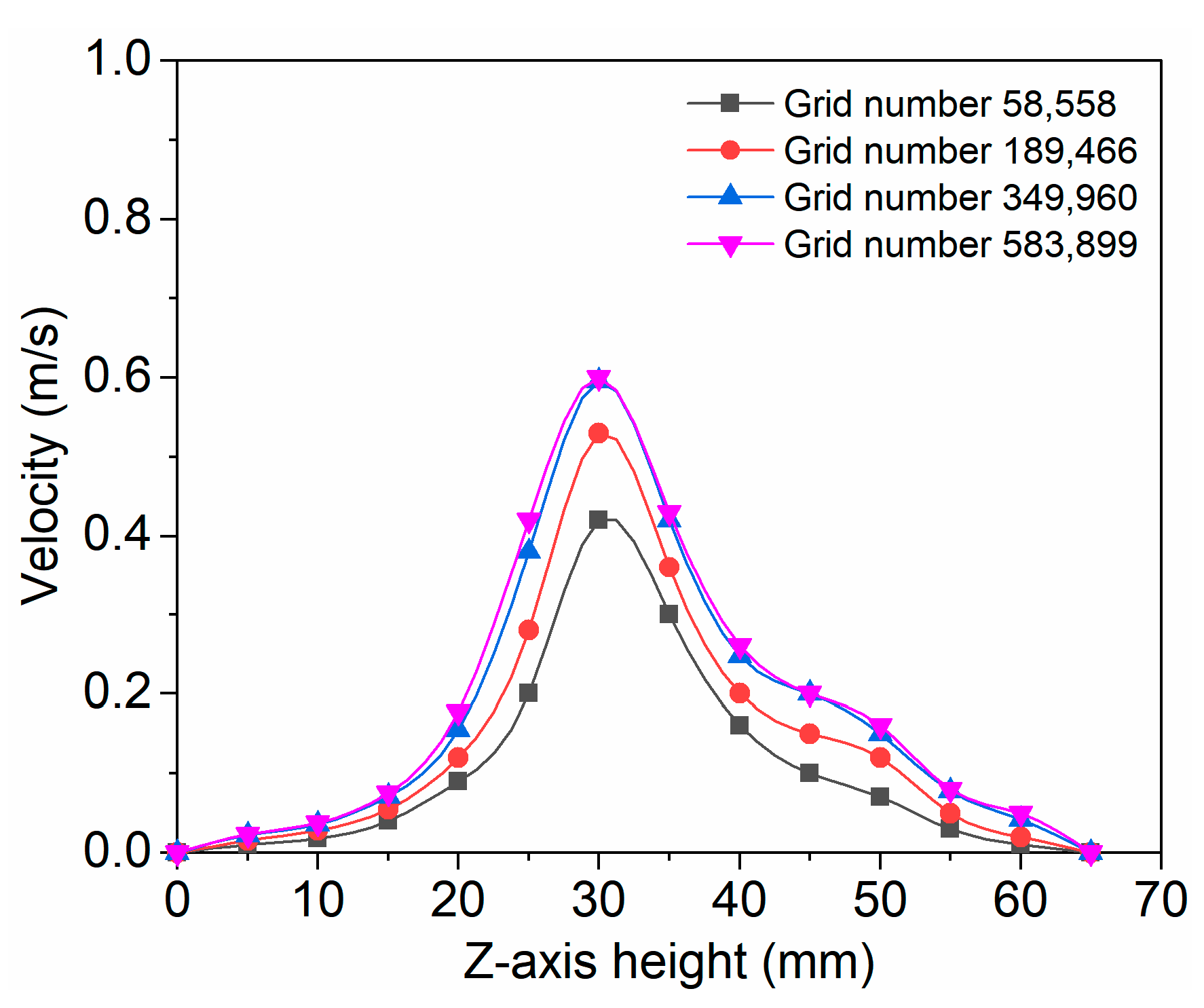
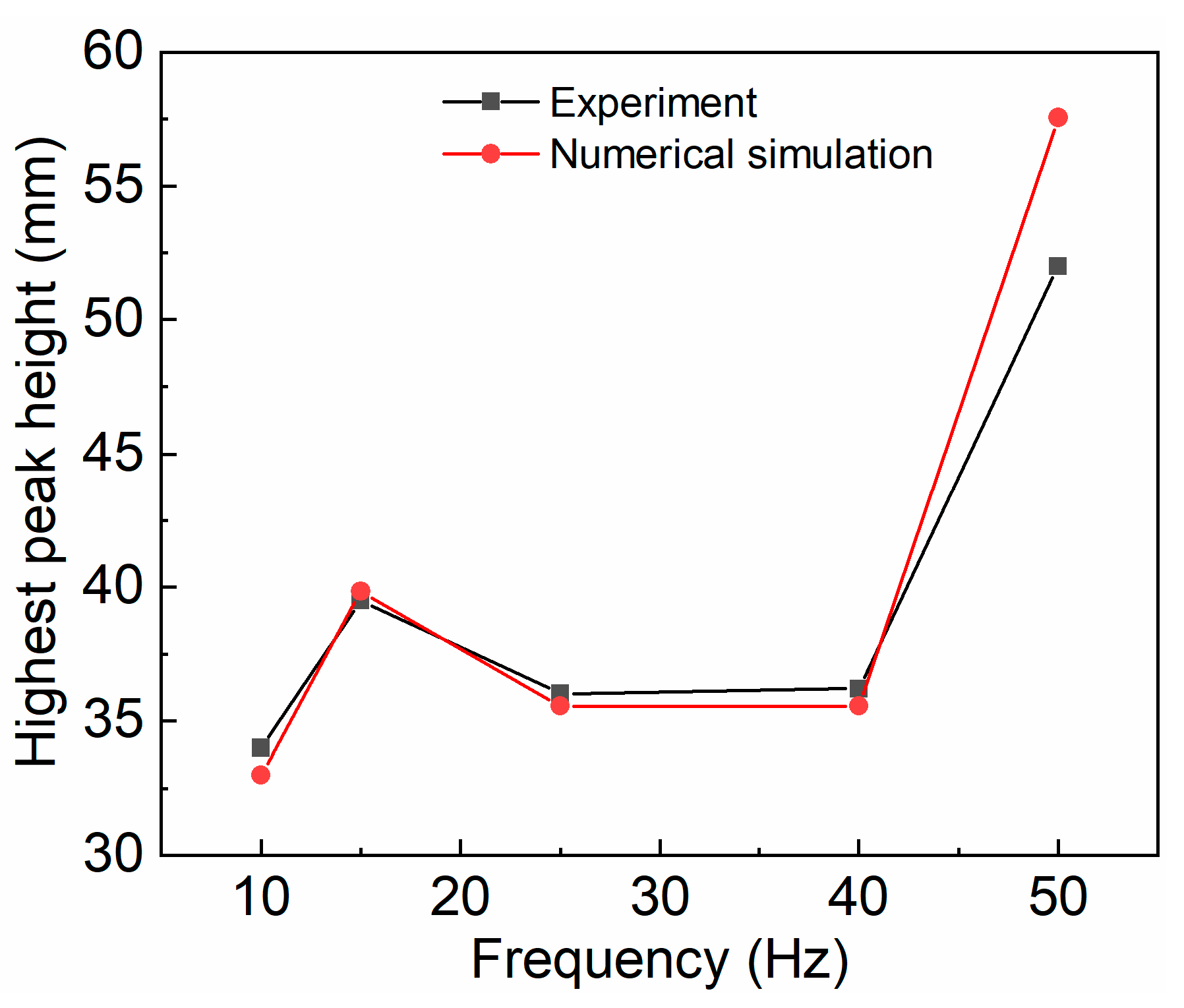
3.1. Model Validation
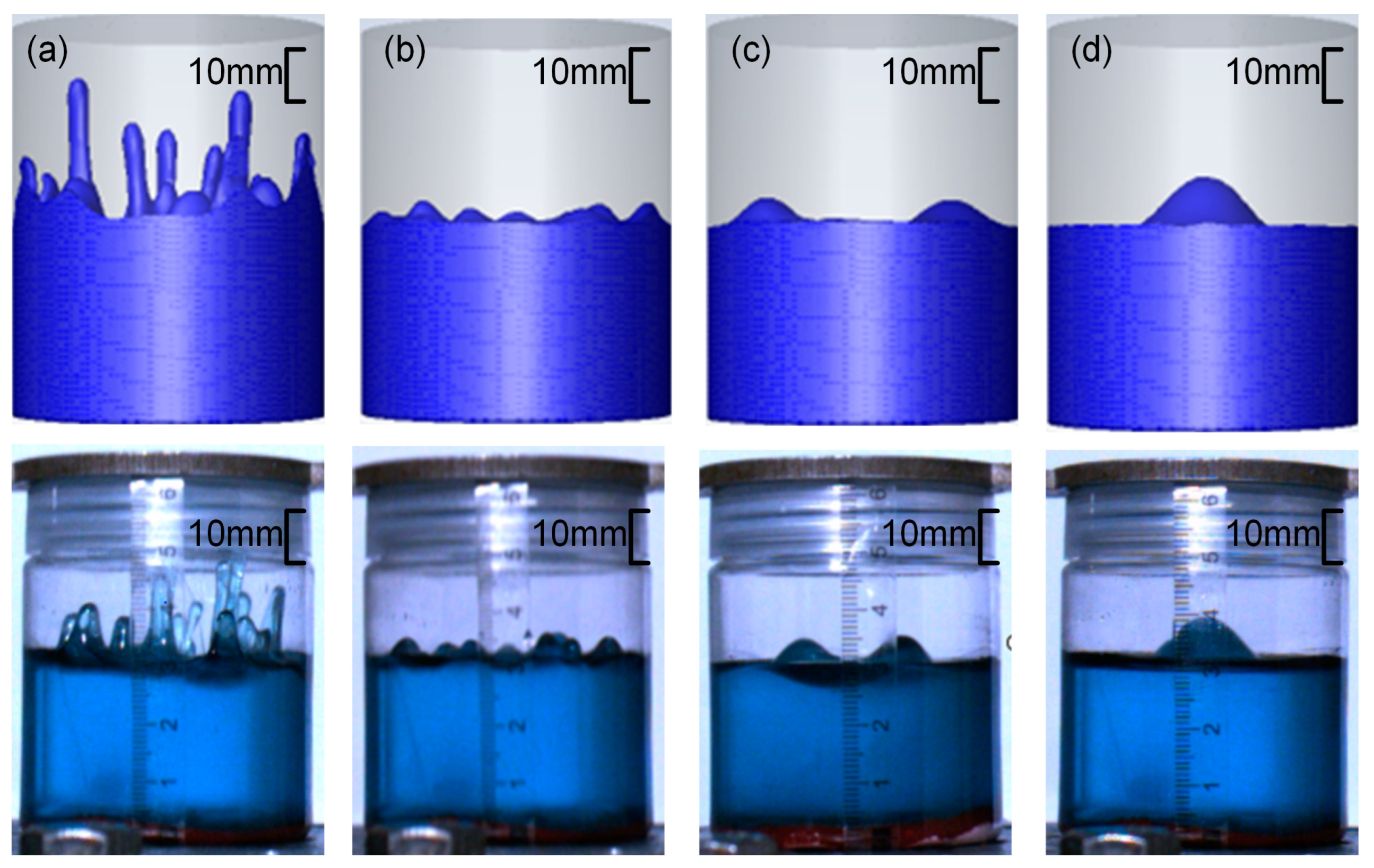
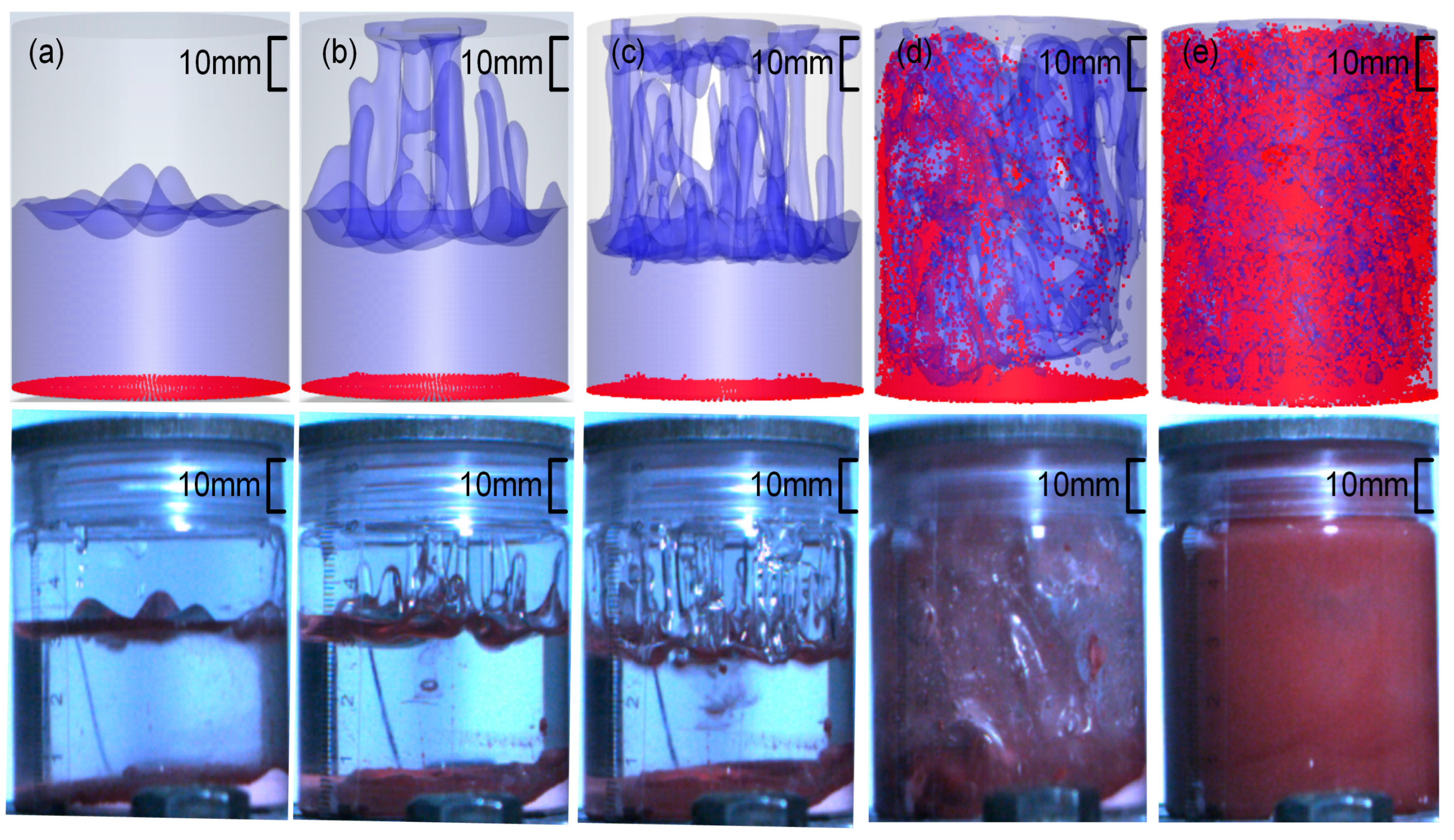
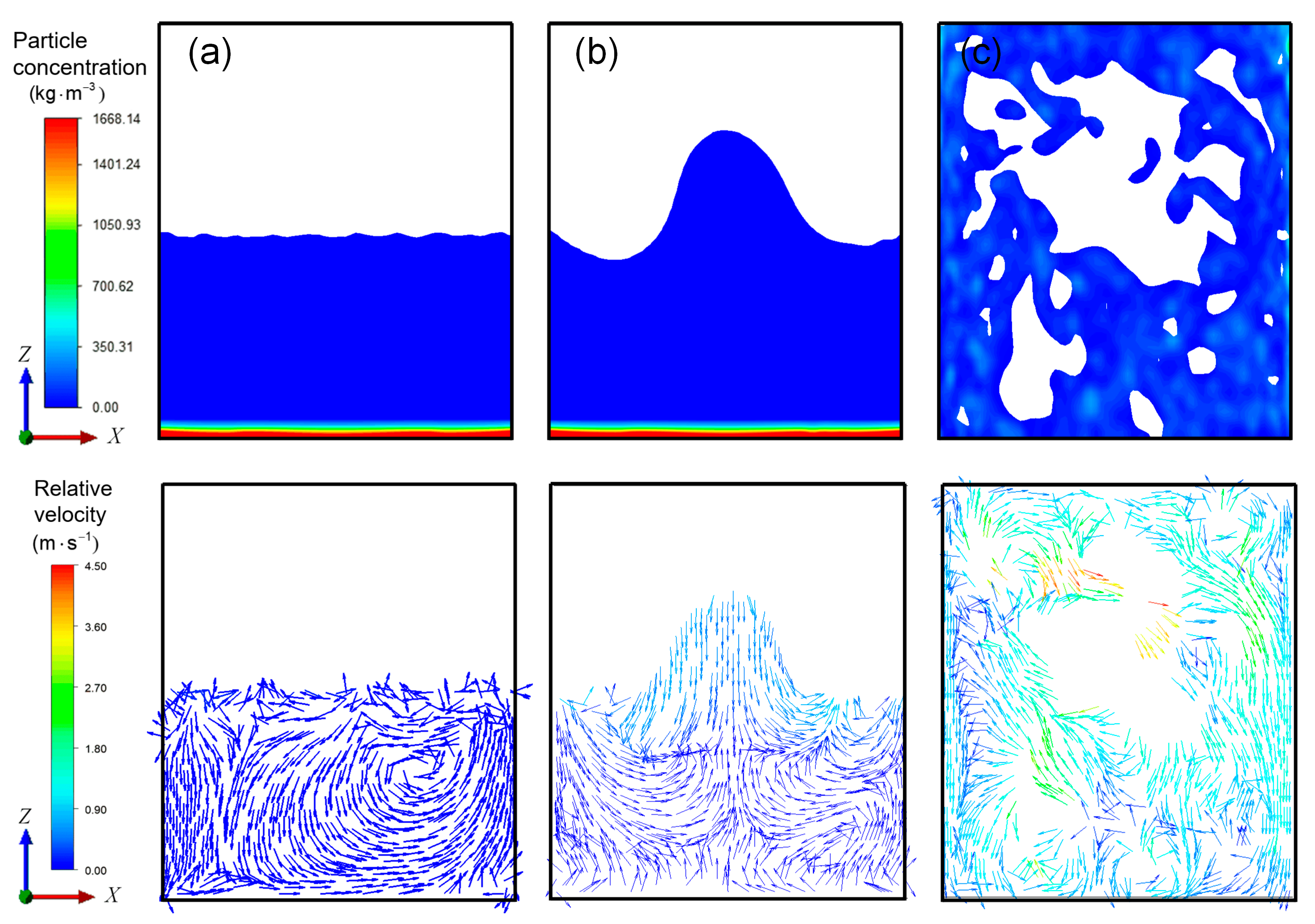
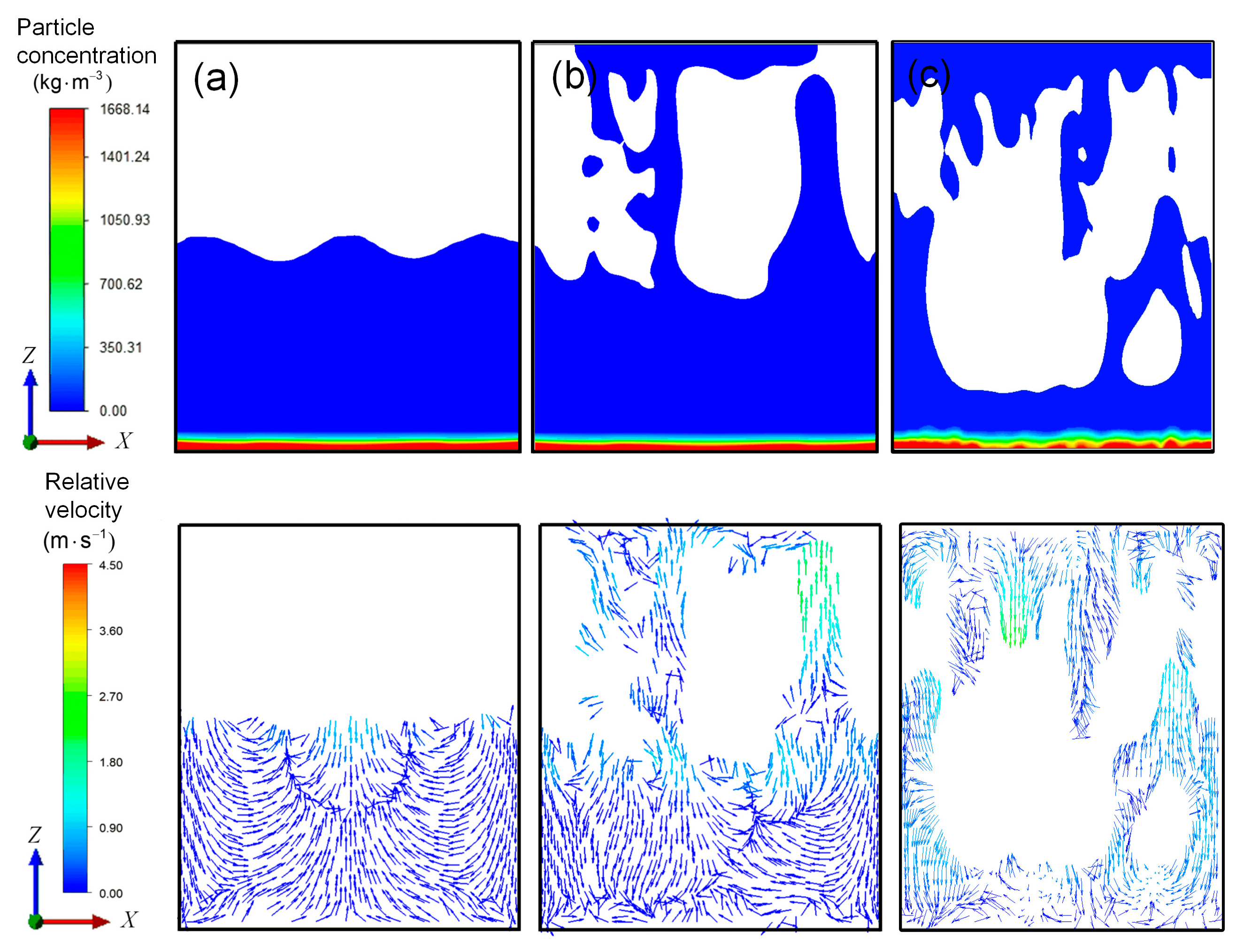
3.2. Flow Field Characteristics of High-Viscosity Fluid Excited by Acoustic Vibration
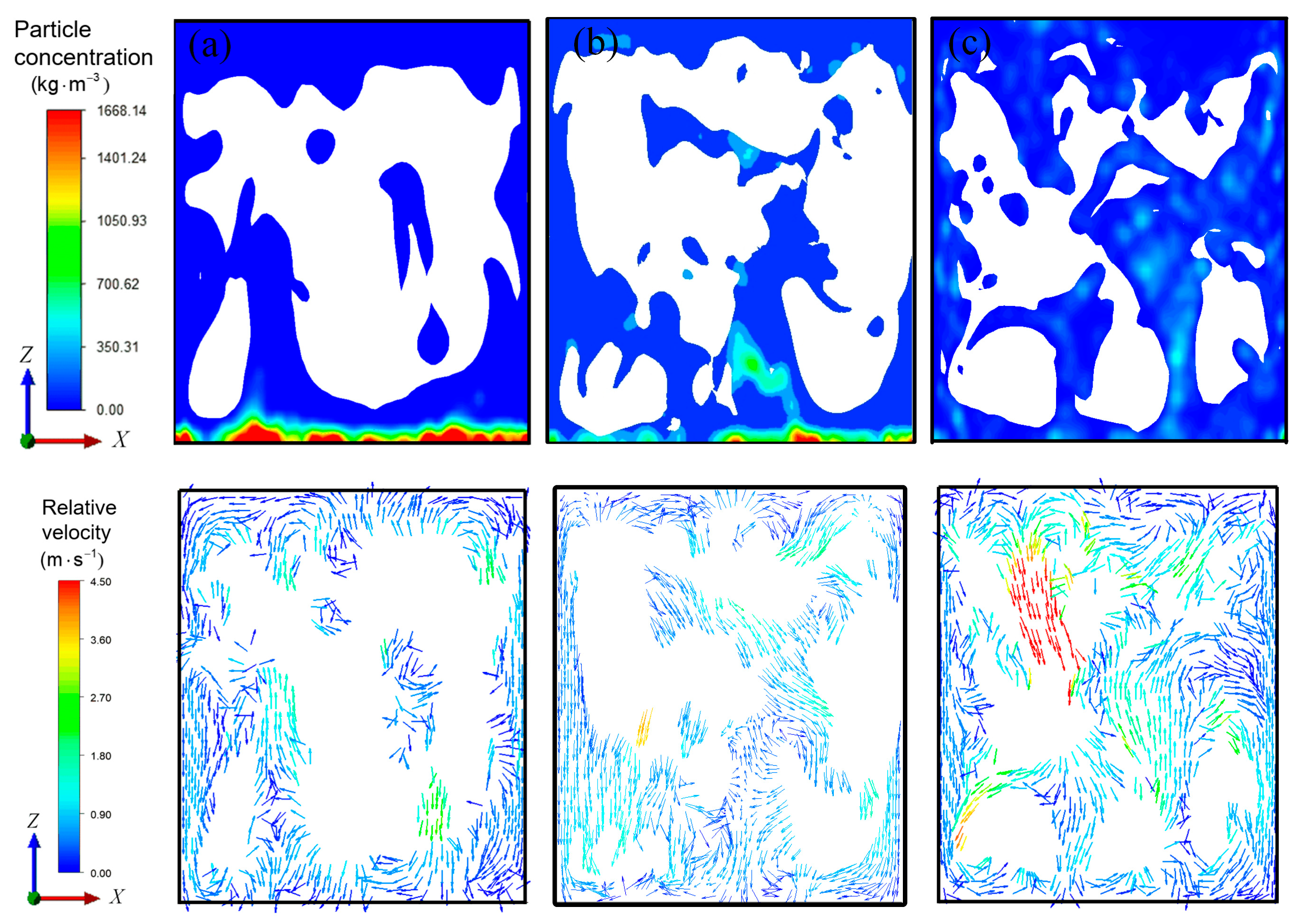
3.3. Characteristic Analysis of the Full-Field Mixing
3.4. Influence of Vibration Parameters on Mixing Efficiency
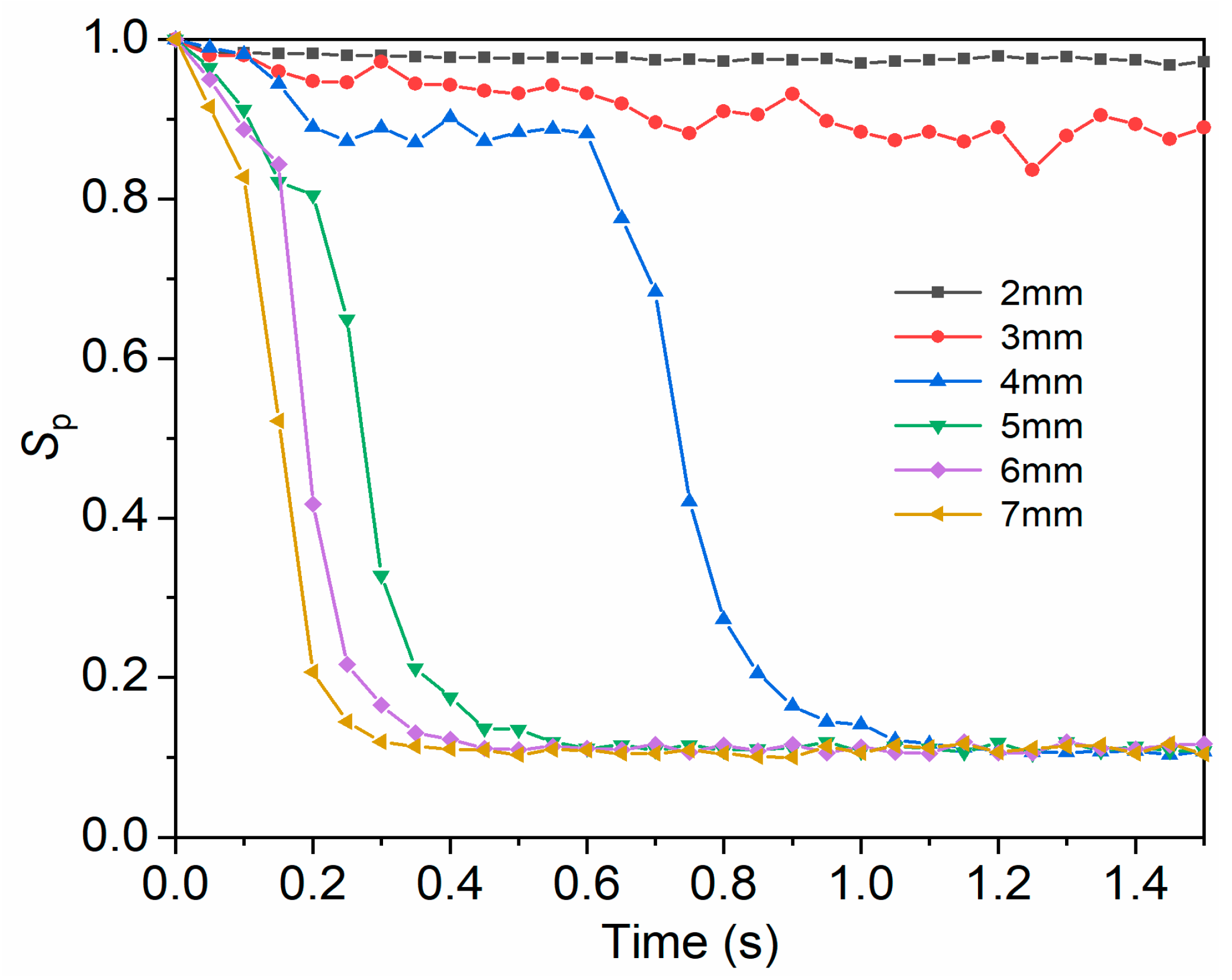
3.4.1. Influence of Amplitude on Mixing Uniformity
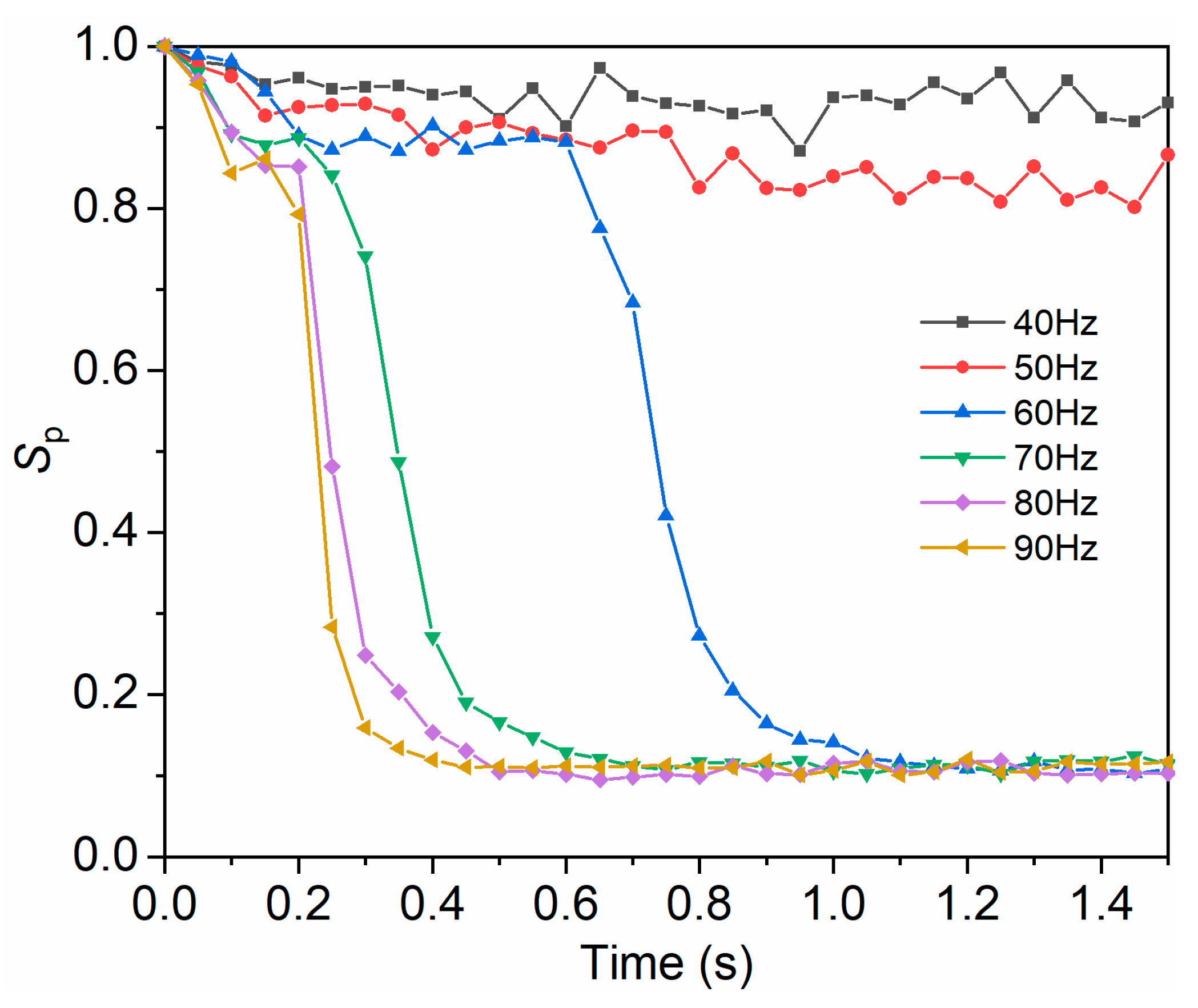
3.4.2. Influence of Frequency on Mixing Uniformity
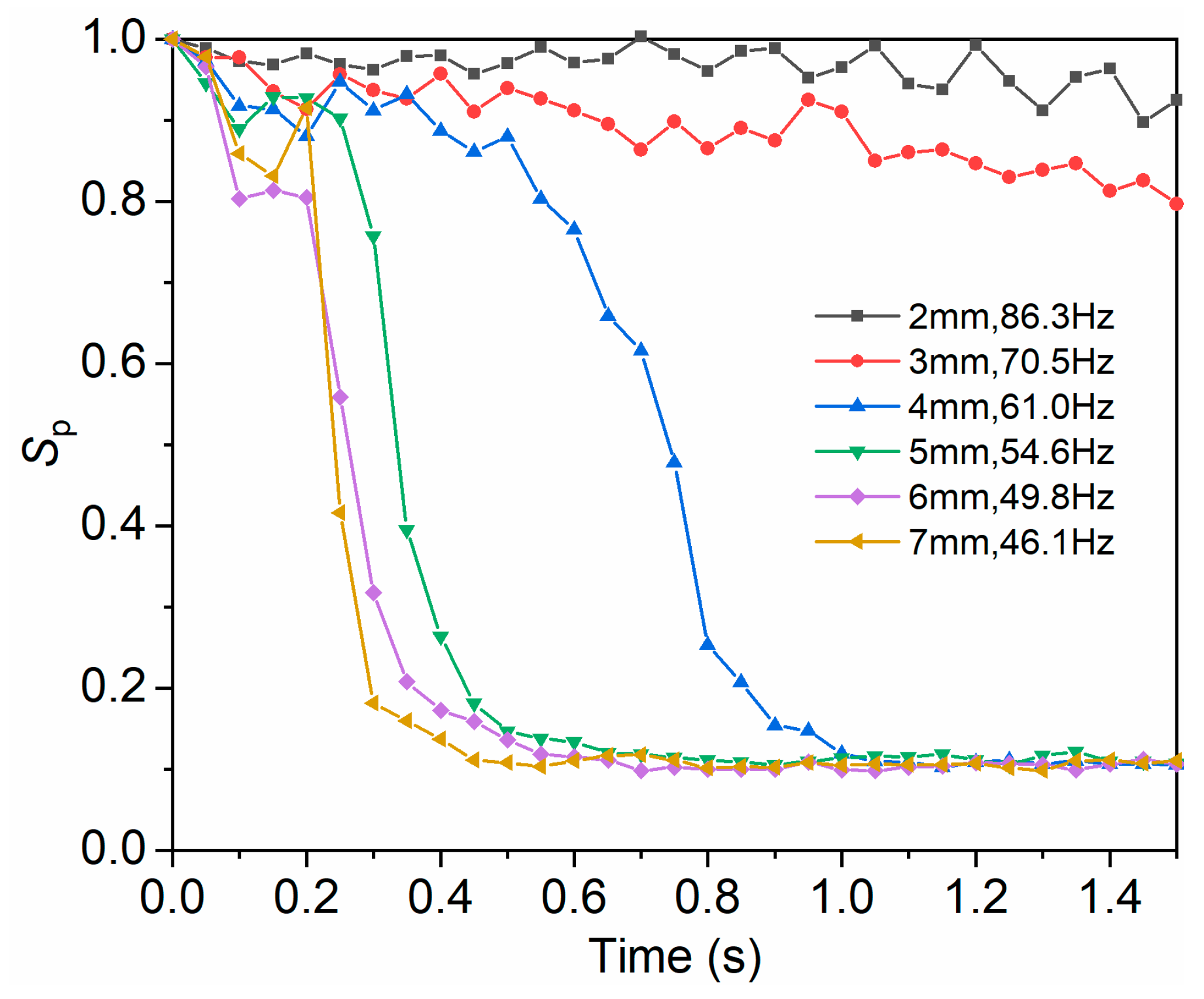
3.4.3. Influence of Amplitude and Frequency on Mixing Uniformity under Constant Acceleration
3.5. Threshold of Vibration Parameters for the Full-Field Mixing
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lysien, K.; Lysien, K.; Stolarczyk, A.; Stolarczyk, A.; Jarosz, T.; Jarosz, T. Solid Propellant Formulations: A Review of Recent Progress and Utilized Components. Materials 2021, 14, 6657. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.P.; Roland, C.M. Solid Propellants. Rubber Chem. Technol. 2019, 92, 1–24. [Google Scholar] [CrossRef]
- Wei, Y.J.; Xu, G.G.; Huang, Q.A.; Liu, X.M. Optimization of HTPB-Bonding System Used for High-Solid-Containing PBX Based on Properties of Materials. Adv. Mater. Res. 2014, 848, 141–145. [Google Scholar] [CrossRef]
- Korotkikh, A.G.; Glotov, O.G.; Arkhipov, V.A.; Zarko, V.E.; Kiskin, A.B. Effect of iron and boron ultrafine powders on combustion of aluminized solid propellants. Combust. Flame 2017, 178, 195–204. [Google Scholar] [CrossRef]
- Arkhipov, V.A.; Gorbenko, M.V.; Gorbenko, T.I.; Savel Eva, L.A. Effect of ultrafine aluminum on the combustion of composite solid propellants at subatmospheric pressures. Combust. Explos. Shock Waves. 2009, 45, 40–47. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Solid propellants: AP/HTPB composite propellants. Arab. J. Chem. 2019, 12, 2061–2068. [Google Scholar] [CrossRef]
- Mahjub, A.; Mazlan, N.M.; Abdullah, M.Z.; Azam, Q. Design Optimization of Solid Rocket Propulsion: A Survey of Recent Advancements. J. Spacecr. Rocket. 2019, 57, 3–11. [Google Scholar] [CrossRef]
- Trache, D.; Klapötke, T.M.; Maiz, L.; Abd-Elghany, M.; DeLuca, L.T. Recent advances in new oxidizers for solid rocket propulsion. Green Chem. 2017, 19, 4711–4736. [Google Scholar] [CrossRef]
- Lin, D.; Wei, H.; Song, E.; Ng, B.; He, T.; Dang, L. Experimental and numerical investigation of the scale-up criterion of solid-viscous fluid mixing in a stirred tank. Can. J. Chem. Eng. 2021, 99, S259–S274. [Google Scholar] [CrossRef]
- Oberti, S.; Neild, A.; Wah Ng, T. Microfluidic mixing under low frequency vibration. Lab Chip 2009, 9, 1435–1438. [Google Scholar] [CrossRef]
- Carlsson, F.; Sen, M.; Löfdahl, L. Fluid mixing induced by vibrating walls. Eur. J. Mech. B. Fluids 2005, 24, 366–378. [Google Scholar] [CrossRef]
- Osorio, J.G.; Muzzio, F.J. Evaluation of resonant acoustic mixing performance. Powder Technol. 2015, 278, 46–56. [Google Scholar] [CrossRef]
- Zoueshtiagh, F.; Narayanan, R.; Amiroudine, S. Mixing generated by Faraday instability between miscible liquids. Phys. Rev. E 2012, 85, 016326. [Google Scholar] [CrossRef]
- Andrews, M.R.; Collet, C.; Wolff, A.; Hollands, C. Resonant Acoustic® Mixing: Processing and Safety. Propellants Explos. Pyrotech. 2019, 45, 77–86. [Google Scholar] [CrossRef]
- Vandenberg, A.; Wille, K. Evaluation of resonance acoustic mixing technology using ultra high performance concrete. Constr. Build. Mater. 2018, 164, 716–730. [Google Scholar] [CrossRef]
- Davey, R.; Wilgeroth, J.; Burn, A. Processing Studies of Energetic Materials using Resonant Acoustic Mixing Technology. Propellants Explos. Pyrotech. 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Osorio, J.G.; Sowrirajan, K.; Muzzio, F.J. Effect of resonant acoustic mixing on pharmaceutical powder blends and tablets. Adv. Powder Technol. 2016, 27, 1141–1148. [Google Scholar] [CrossRef]
- Claydon, A.J.; Patil, A.N.; Gaulter, S.; Kister, G.; Gill, P.P. Determination and optimisation of Resonant Acoustic Mixing (RAM) efficiency in Polymer Bonded eXplosive (PBX) processing. Chem. Eng. Process. 2022, 173, 108806. [Google Scholar] [CrossRef]
- Bale, S.; Clavin, K.; Sathe, M.; Berrouk, A.S.; Knopf, F.C.; Nandakumar, K. Mixing in oscillating columns: Experimental and numerical studies. Chem. Eng. Sci. 2017, 168, 78–89. [Google Scholar] [CrossRef]
- Zhan, X.; Zhibin, S.; Yu, H.; Shen, B.; Shi, T.; Li, X. Characterization of Fluid Mixing in a Closed Container under Horizontal Vibrations. Can. J. Chem. Eng. 2018, 97, 1931–1938. [Google Scholar] [CrossRef]
- Khan, I.U.; Guo, R.; Farooq, U.; Adhikari, S.; Zhou, H. Parametric Effects on the Mixing Efficiency of Resonant Acoustic Mixing Technology for High-Viscosity Mixture: A Numerical Study. Processes 2023, 11, 266. [Google Scholar] [CrossRef]
- Li, L.; Gu, Z.; Xu, W.; Tan, Y.; Fan, X.; Tan, D. Mixing mass transfer mechanism and dynamic control of gas-liquid-solid multiphase flow based on VOF-DEM coupling. Energy 2023, 272, 127015. [Google Scholar] [CrossRef]
- Nemati, M.; Shateri Najaf Abady, A.R.; Toghraie, D.; Karimipour, A. Numerical investigation of the pseudopotential lattice Boltzmann modeling of liquid–vapor for multi-phase flows. Physica A 2018, 489, 65–77. [Google Scholar] [CrossRef]
- Pukkella, A.K.; Vysyaraju, R.; Tammishetti, V.; Rai, B.; Subramanian, S. Improved mixing of solid suspensions in stirred tanks with interface baffles: CFD simulation and experimental validation. Chem. Eng. J. 2019, 358, 621–633. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Yin, G.; Ong, M.C.; Li, G.; Han, F. Experimental Investigation and Numerical Modeling of Two-Phase Flow Development and Flow-Induced Vibration of a Multi-Plane Subsea Jumper. J. Mar. Sci. Eng. 2022, 10, 1334. [Google Scholar] [CrossRef]
- Garoosi, F.; Hooman, K. Numerical simulation of multiphase flows using an enhanced Volume-of-Fluid (VOF) method. Int. J. Mech. Sci. 2022, 215, 106956. [Google Scholar] [CrossRef]
- Gu, D.; Cheng, C.; Liu, Z.; Wang, Y. Numerical simulation of solid-liquid mixing characteristics in a stirred tank with fractal impellers. Adv. Powder Technol. 2019, 30, 2126–2138. [Google Scholar] [CrossRef]
- Leporini, M.; Marchetti, B.; Corvaro, F.; di Giovine, G.; Polonara, F.; Terenzi, A. Sand transport in multiphase flow mixtures in a horizontal pipeline: An experimental investigation. Petroleum 2019, 5, 161–170. [Google Scholar] [CrossRef]
- Toghraie, D.; Mashayekhi, R.; Arasteh, H.; Sheykhi, S.; Niknejadi, M.; Chamkha, A.J. Two-phase investigation of water-Al2O3 nanofluid in a micro concentric annulus under non-uniform heat flux boundary conditions. Int. J. Numer. Methods Heat Fluid Flow 2019, 30, 1795–1814. [Google Scholar] [CrossRef]
- Sengia, J.; James, A.; Singh, R.; Bale, S. Size effect of oscillating columns on mixing: A CFD study. Eur. J. Mech. B. Fluids 2019, 77, 230–238. [Google Scholar] [CrossRef]
- Valha, J.; Lewis, J.; Kubie, J. A numerical study of the behaviour of a gas–liquid interface subjected to periodic vertical motion. Int. J. Numer. Methods Fluids 2002, 40, 697–721. [Google Scholar] [CrossRef]
- Wang, C.; Ji, C.; Zou, J. Simulation and experiment on transitional behaviours of multiphase flow in a hydrocyclone. Can. J. Chem. Eng. 2015, 93, 1802–1811. [Google Scholar] [CrossRef]
- Wang, R.; Jia, S.; He, Z. Numerical Investigation on the Effects of Impeller Structures in Hot Metal Desulfurization Processes by Mechanical Stirring. Metals 2022, 12, 229. [Google Scholar] [CrossRef]
- Cong, D.; Jian-Zhong, L.; Zhen-Yu, H.; Jun-Hu, Z.; Ke-Fa, C. VOF Method for Impinging-Jet Atomizers. Energy Fuels 2010, 24, 451–455. [Google Scholar] [CrossRef]
- Hirt, C.W.; Nichols, B.D. Volume of fluid (VOF) method for the dynamics of free boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Brackbill, J.U.; Kothe, D.B.; Zemach, C. A continuum method for modeling surface tension. J. Comput. Phys. 1992, 100, 335–354. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, F.; Wang, Z.; Zhang, W. Numerical Modeling of Capillary Flows in a Non-Uniform Cross-Sectional Cavity Basedon VOF and CSF. Semicond. Technol. 2011, 36, 169–172. [Google Scholar] [CrossRef]
- Zahari, N.M.; Zawawi, M.H.; Sidek, L.M.; Mohamad, D.; Itam, Z.; Ramli, M.Z.; Syamsir, A.; Abas, A.; Rashid, M. Introduction of discrete phase model (DPM) in fluid flow: A review. AIP Conf. Proc. 2018, 2030, 020234. [Google Scholar] [CrossRef]
- Kharoua, N.; AlShehhi, M.; Khezzar, L. Prediction of Black Powder distribution in junctions using the Discrete Phase Model. Powder Technol. 2015, 286, 202–211. [Google Scholar] [CrossRef]
- Morel, C. Interfacial Forces and Momentum Exchange Closure. In Mathematical Modeling of Disperse Two-Phase Flows; Fluid Mechanics and Its Applications; Springer: Cham, Switzerland, 2015; Volume 114, pp. 159–191. [Google Scholar] [CrossRef]
- Zhu, S.; Xiaopeng, W.; Chen, S.; Xu, X. Simulation of dispersion characteristics of resonant acoustic mixing with low solid content of powder. Chem. Ind. Eng. Prog. 2019, 38, 4414–4422. [Google Scholar] [CrossRef]
- Tian, S.; Barigou, M. An improved vibration technique for enhancing temperature uniformity and heat transfer in viscous fluid flow. Chem. Eng. Sci. 2015, 123, 609–619. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, P.; Wang, F.; Zhou, S. Experimental Study on Mixing Uniformity of Injection On-Line Mixer of Crop Protection Equipment. Int. J. Heat Technol. 2021, 39, 1143–1152. [Google Scholar] [CrossRef]




| Authors | Research Theme | Key Finding |
|---|---|---|
| Osorio et al. [17] | Mixing characteristics of powder by RAM experiment | Powder and tablet properties were significantly affected by acceleration, mixing time and total energy input. |
| Claydon et al. [18] | The effects of process parameters on RAM efficiency | Partial vacuum application without degassing is beneficial for mixing. |
| Bale et al. [19] | Free surface stability when mixing in an oscillating column | The mixing time is highly nonlinear with respect to the vibration frequency. |
| Zhan et al. [20] | Two miscible high-viscosity liquids under vertical vibration | Fluid injection can promote the mixing of the two liquids. |
| Khan et al. [21] | Parametric effects on the mixing efficiency of RAM technology | Amplitude and frequency both have significant influences on the mixing efficiency of the RAM process. |
| Li et al. [22] | Mixing mass transfer mechanism of gas–liquid–solid multiphase flow | The appropriate inflation rate can improve particle suspension effects and promote interphase mixing mass transfer. |
| Nemati et al. [23] | Modeling of liquid–vapor for multi-phase flows | The scheme for the interparticle interaction force term, as well as the force term incorporation method, matters to achieve more accurate and stable results. |
| Pukkella et al. [24] | Mixing of solid suspensions in stirred tanks | The effect of the novel interface baffle design on the mixing uniformity in solid–liquid suspensions is explored. |
| Li et al. [25] | Two-phase flow development and flow-induced vibration | Vibration amplitudes are highly related to the gas content rate and mixing velocity. |
| Garoosi and Hooman [26] | Numerical simulation of multiphase flows using an enhanced VOF method | The proposed model has robust convergence behavior in strongly coupled multiphysics problems. |
| Gu et al. [27] | CFD simulation of solid–liquid mixing characteristics in a stirred tank | Larger particle diameter and higher initial solid particle loading resulted in less homogenous distribution of solid particles. |
| Leporini et al. [28] | Sand transport in multiphase flow mixtures | Variation of the sand concentration and particle size in air–water–sand flow causes a change in sand deposition characteristics. |
| Toghraie et al. [29] | Numerical study of convective heat transfer using two-phase mixture model | The nanofluid in a micro concentric annulus and its hydro-thermal effects are simulated. |
| Parameters | Values |
|---|---|
| Density of glycerin (kg·m−3) | 1260 |
| Viscosity of glycerin (kg·m−1·s−1) | 0.8 |
| Density of air (kg·m−3) | 1.225 |
| Viscosity of air (kg·m−1·s−1) | 1.79 × 10−5 |
| Surface tension between air and glycerin (N·m−1) | 0.063 |
| Density of sand (kg·m−3) | 2650 |
| Particle size of sand (m) | 6.3 × 10−5 |
| Condition | Acceleration (m/s2) | Amplitude (mm) | Frequency (Hz) |
|---|---|---|---|
| a | 588.6 | 2 | 86.3 |
| b | 588.6 | 3 | 70.5 |
| c | 588.6 | 4 | 61.0 |
| d | 588.6 | 5 | 54.6. |
| e | 588.6 | 6 | 49.8 |
| f | 588.6 | 7 | 46.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, X.; Yu, L.; Jiang, Y.; Jiang, Q.; Shi, T. Mixing Characteristics and Parameter Effects on the Mixing Efficiency of High-Viscosity Solid–Liquid Mixtures under High-Intensity Acoustic Vibration. Processes 2023, 11, 2367. https://doi.org/10.3390/pr11082367
Zhan X, Yu L, Jiang Y, Jiang Q, Shi T. Mixing Characteristics and Parameter Effects on the Mixing Efficiency of High-Viscosity Solid–Liquid Mixtures under High-Intensity Acoustic Vibration. Processes. 2023; 11(8):2367. https://doi.org/10.3390/pr11082367
Chicago/Turabian StyleZhan, Xiaobin, Lei Yu, Yalong Jiang, Qiankun Jiang, and Tielin Shi. 2023. "Mixing Characteristics and Parameter Effects on the Mixing Efficiency of High-Viscosity Solid–Liquid Mixtures under High-Intensity Acoustic Vibration" Processes 11, no. 8: 2367. https://doi.org/10.3390/pr11082367
APA StyleZhan, X., Yu, L., Jiang, Y., Jiang, Q., & Shi, T. (2023). Mixing Characteristics and Parameter Effects on the Mixing Efficiency of High-Viscosity Solid–Liquid Mixtures under High-Intensity Acoustic Vibration. Processes, 11(8), 2367. https://doi.org/10.3390/pr11082367







