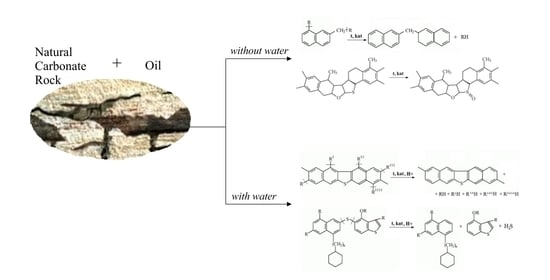
Catalytic Conversion of Oil in Model and Natural Reservoir Rocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Analysis
2.3. SARA Analysis
2.4. CHNS Analysis
2.5. IR Spectroscopy
2.6. EPR Spectroscopy
2.7. Gas–Liquid Chromatography
3. Results
3.1. Evaluation of the Catalytic Activity of Metal Oxides
3.2. The Change in SARA Composition of Oil in Natural Rock at Low Temperature
3.3. Changes in the Composition of High-Molecular Oil Components




4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polishchuk, Y.M.; Yashchenko, I.G. High-viscosity oils: Analytical review of regularities of spatial and temporal changes of their properties. Pet. Eng. 2006, 4, 27–34. [Google Scholar]
- Armas, J.D.; Ortega, L.C.; Scott, C.E.; Maini, B.; Pereira-Almao, P. In-Situ upgrading technology: Nanocatalyst concentration levels effects and hydrocarbons paths in the porous medium. J. Pet. Sci. Eng. 2022, 208 Part D, 109594. [Google Scholar] [CrossRef]
- Muslimov, R.K.; Romanov, G.V.; Kayukova, G.P. Comprehensive Development of Heavy Oils and Natural Bitumens of the Permian System of the Republic of Tatarstan; FEN: Kazan, Russia, 2012; 396p. [Google Scholar]
- Xu, H.; Pu, C.; Wu, F. Low frequency vibration assisted catalytic aquathermolysis of heavy crude oil. Energy Fuels 2012, 26, 5655–5662. [Google Scholar] [CrossRef]
- Kholmurodov, T.; Aliev, F.A.; Mirzaev, O.; Dengaev, A.V.; Tajik Arash Vakhin, A.V. Hydrothermal in-reservoir upgrading of heavy oil in the presence of non-ionic surfactants. Processes 2022, 10, 2176. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-Situ heavy oil aquathermolysis in the presence of nanodispersed catalysts based on transition metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Hart, A.; Adam, M.; Robinson, J.P.; Rigby, S.P.; Wood, J. Tetralin and Decalin H-Donor Effect on Catalytic Upgrading of Heavy Oil Inductively Heated with Steel Balls. Catalysts 2020, 10, 393. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.; Shah, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of the CAPRI process for heavy oil upgrading: Effect of hydrogen and guard bed. Ind. Eng. Chem. Res. 2013, 52, 15394–15406. [Google Scholar] [CrossRef]
- Method of Recoverable Oil Increasing (MROI) Due to Activation of Natural Nanocatalysts in Oil-Field Waters Using a Flow Chemical Reactor (FCR) on the Bottom-Well. Available online: https://www.eemkzn.ru/uploads/2c4ad7aef27b920cd9b5e2c5e37a139b.pdf (accessed on 26 May 2023).
- Qi, Y.; Cai, C.; Sun, P.; Wang, D.; Zhu, H. Crude oil cracking in deep reservoirs: A review of the controlling factors and estimation methods. Pet. Sci. 2023, in press. [CrossRef]
- Okhotnikova, E.S.; Ganeeva, Y.M.; Barskaya, E.E.; Yusupova, T.N. The influence of carbonate rock on the processes of oil transformation. Pet. Sci. Technol. 2018, 36, 1080–1084. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Lu, N.; Xu, T.; Zhu, G.; Wang, K. A review on upgrading and viscosity reduction of heavy oil and bitumen by underground catalytic cracking. Energy Rep. 2021, 7, 4249–4272. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Wang, Z.Y.; Zhang, S.C.; Wang, H.J. Cracking condition of crude oil under different geological environment. Sci. China. Ser. D Earth Sci. 2008, 51, 77–83. [Google Scholar] [CrossRef]
- Petrov, S.M.; Kayukova, G.P.; Vakhin, A.V.; Petrova, A.N.; Ibrahim, A.Y.I.; Onishchenko, Y.V.; Nurgaliyev, D.K. Catalytic effects research of carbonaceous rock under conditions of in-situ oxidation of super-sticky naphtha. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1624–1629. [Google Scholar]
- Shabalin, K.V.; Foss, L.E.; Borisova, Y.Y.; Borisov, D.N.; Yakubova, S.G.; Yakubov, M.R. Study of the heavy oil asphaltenes oxidation products composition using EPR and IR spectroscopy. Pet. Sci. Technol. 2020, 38, 992–997. [Google Scholar] [CrossRef]
- Junaid, A.S.M.; Street, C.; Wang, W.; Rahman, M.M.; An, W.; McCaffrey, W.C.; Kuznicki, S.M. Integrated extraction and low severity upgrading of oilsands bitumen by activated natural zeolite catalysts. Fuel 2012, 94, 457–464. [Google Scholar] [CrossRef]
- ASTM D6560-17; Standard Test Method for Determination of Asphaltenes (Heptane Insolubles) in Crude Petroleum and Petroleum Products. ASTM: West Conshohocken, PA, USA, 2022.
- GOST11244-2018; Petroleum. Method for Determination of Potential Content of Distillation and Residue Oils·GOST 11244-76: Petroleum. State Committee of the Russian Federation for Standardization: Moscow, Russia, 2018.
- Okhotnikova, E.S.; Ganeeva, Y.M.; Barskaya, E.E.; Yusupova, T.N.; Ismagilova, Z.R.; Mukhametzyanova, A.A. Influence of carbonate rock on the processes of transformation of oil components. Bull. Technol. Univ. 2017, 20, 61–64. [Google Scholar]
- Galanov, S.I.; Sidorova, O.I.; Litvak, E.A.; Kosyreva, K.A. Manganese-containing catalysts for the processing of associated petroleum gases into olefins. Bull. Tomsk Polytech. Univ. 2012, 320, 124–128. [Google Scholar]
- Ganeeva, Y.M.; Yusupova, T.N.; Okhotnikova, E.S. Thermal analysis methods to study the reservoir bitumens. J. Therm. Anal. 2020, 139, 273–278. [Google Scholar] [CrossRef]
- Abdelsalam, Y.I.I.; Aliev, F.A.; Khamidullin, R.F.; Dengaev, A.V.; Katnov, V.E.; Vakhin, A.V. Catalytic Low-Temperature Thermolysis of Heavy Oil in the Presence of Fullerene C60 Nanoparticles in Aquatic and N2 Medium. Catalysts 2023, 13, 347. [Google Scholar] [CrossRef]
- Vakhin, A.V. Rock Mineral Components Effect on Heavy and Shale Oil Transformation During Aquathermolysis. Energies 2022, 15, 6047. [Google Scholar] [CrossRef]
- Tarasevich, B.N. IR Spectra of the Main Classes of Organic Compounds. Reference materials; Moscow State University: Moscow, Russia, 2012; 54p. [Google Scholar]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Vakhin, A.V.; Khelkhal, M.A.; Maksimov, A.L. Special Issue “Heavy Oil In Situ Upgrading and Catalysis”. Catalysts 2023, 13, 99. [Google Scholar] [CrossRef]
- Gazizullin, I.F.; Simonov, Y.I.; Skvortsova, Z.N.; Traskin, V.Y. Calcite Deformation in the Presence of Water. Colloid J. 2015, 77, 577–581. [Google Scholar] [CrossRef]
- Nasyrova, Z.R.; Kayukova, G.P.; Shunina, E.N.; Islamova, G.G.; Batalin, G.A.; Morozova, E.V.; Vakhin, A.V.; Nurgaliev, D.K. Thermal Decomposition of Kerogen in High-Carbon Domanic Rock of the Romashkino Oilfield in Sub- and Supercritical Water. Energy Fuels 2022, 36, 3549–3562. [Google Scholar] [CrossRef]
- Zhang, Y.; Schuler, B.; Liu, F.; Ruiz-Morales, Y.; Gross, L.; Pomerantz, A.; Pauchard, V.; Banejee, S.; Mullins, O. 5 Asphaltenes: Structures and applications. In The Chemistry of Oil and Petroleum Products; Fingas, M., Ed.; De Gruyter: Berlin, Germany, 2022; pp. 203–306. [Google Scholar] [CrossRef]
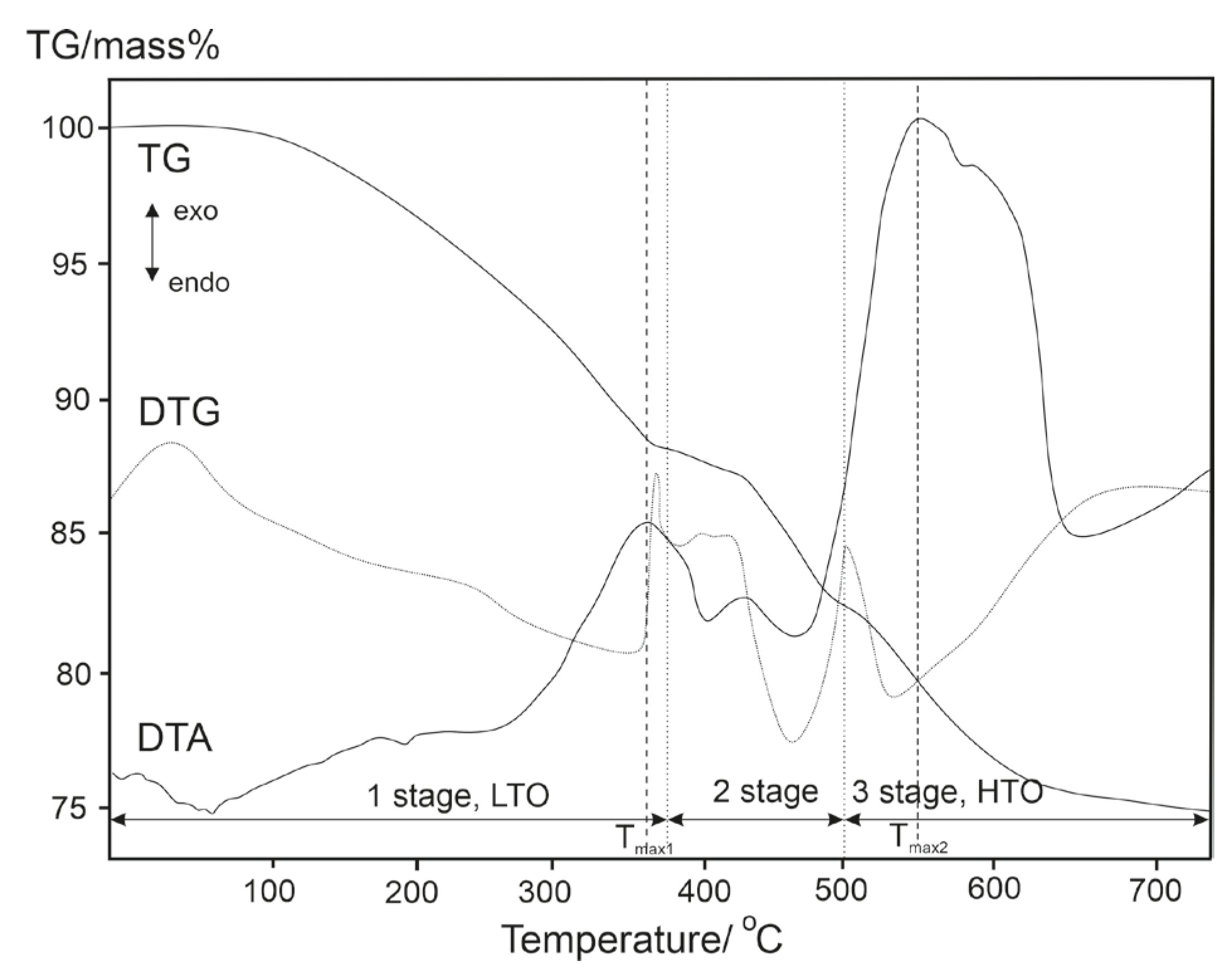

| Sample | Temperature, °C | Tmax1, °C | Tmax2, °C | ||
|---|---|---|---|---|---|
| Stage 1, LTO | Stage 2 | Stage 3, HTO | |||
| 268–387 | 387–501 | 501–636 | 354 | 549 |
| 250–373 | 373–491 | 491–595 | 341 | 539 |
| 255–376 | 376–493 | 493–607 | 356 | 543 |
| 263–380 | 380–497 | 497–625 | 348 | 544 |
| 256–378 | 378–497 | 497–609 | 348 | 537 |
| Mass losses, % wt. | |||||
| 12 | 6 | 7 | ||
| 13 | 7 | 6 | ||
| 12 | 6 | 7 | ||
| 13 | 6 | 7 | ||
| 13 | 6 | 6 | ||
| Sample | Content, % wt. | ||||
|---|---|---|---|---|---|
| Saturates | Aromatics | Nonpolar Resin | Polar Resin | Asphaltene | |
| Initial oil | 24.5 | 46.4 | 13.0 | 9.3 | 6.8 |
| Oil ex dry | 24.6 | 36.9 | 21.8 | 9.5 | 7.2 |
| Oil ex wet | 27.3 | 47.2 | 16.2 | 4.6 | 4.7 |
| Parameter | Sample | Content, % wt. | ||
|---|---|---|---|---|
| Nonpolar Resin | Polar Resin | Asphaltene | ||
| C | Initial oil | 79.2 | 77.0 | 78.9 |
| Oil ex dry | 81.1 | 78.7 | 76.0 | |
| Oil ex wet | 81.4 | 81.1 | 81.4 | |
| H | Initial oil | 5.7 | 5.1 | 5.9 |
| Oil ex dry | 5.7 | 5.3 | 5.7 | |
| Oil ex wet | 9.3 | 9.2 | 8.0 | |
| N | Initial oil | 0.4 | 0.8 | 1.2 |
| Oil ex dry | 0.3 | 0.8 | 0.7 | |
| Oil ex wet | 0.8 | 0.8 | 1.3 | |
| S | Initial oil | 10.6 | 10.9 | 9.5 |
| Oil ex dry | 11.2 | 11.4 | 11.8 | |
| Oil ex wet | 7.9 | 8.8 | 9.2 | |
| O, Me | Initial oil | 4.1 | 6.2 | 4.5 |
| Oil ex dry | 1.7 | 3.8 | 5.8 | |
| Oil ex wet | 0.1 | 0.1 | 0.2 | |
| C/H | Initial oil | 13.9 | 15.1 | 13.4 |
| Oil ex dry | 14.2 | 14.9 | 13.4 | |
| Oil ex wet | 8.8 | 8.8 | 10.2 | |
| Spectral Coefficient | Sample | Values of Spectral Coefficients | ||
|---|---|---|---|---|
| Nonpolar Resin | Polar Resin | Asphaltene | ||
| A | Initial oil | 0.7 | 0.5 | 0.4 |
| Oil ex dry | 0.4 | 0.4 | 0.2 | |
| Oil ex wet | 0.4 | 0.4 | 0.2 | |
| R | Initial oil | 2.7 | 2.0 | 1.6 |
| Oil ex dry | 2.3 | 2.1 | 1.6 | |
| Oil ex wet | 1.9 | 2.3 | 1.7 | |
| O1 | Initial oil | 0.6 | 0.1 | 0.3 |
| Oil ex dry | 0.5 | 1.0 | 0.4 | |
| Oil ex wet | 0.5 | 1.3 | 0.2 | |
| O2 | Initial oil | 1.0 | 1.4 | 0.7 |
| Oil ex dry | 1.1 | 1.6 | 1.3 | |
| Oil ex wet | 0.9 | 2.2 | 1.3 | |
| Sample | Content, c.u. | SFR/VP | |
|---|---|---|---|
| SFR | VP | ||
| Initial oil | 1.88 | 1.26 | 1.5 |
| Oil ex dry | 1.60 | 0.97 | 1.6 |
| Oil ex wet | 1.89 | 1.24 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okhotnikova, E.S.; Barskaya, E.E.; Ganeeva, Y.M.; Yusupova, T.N.; Dengaev, A.V.; Vakhin, A.V. Catalytic Conversion of Oil in Model and Natural Reservoir Rocks. Processes 2023, 11, 2380. https://doi.org/10.3390/pr11082380
Okhotnikova ES, Barskaya EE, Ganeeva YM, Yusupova TN, Dengaev AV, Vakhin AV. Catalytic Conversion of Oil in Model and Natural Reservoir Rocks. Processes. 2023; 11(8):2380. https://doi.org/10.3390/pr11082380
Chicago/Turabian StyleOkhotnikova, Ekaterina S., Ekaterina E. Barskaya, Yulia M. Ganeeva, Tatyana N. Yusupova, Aleksey V. Dengaev, and Alexey V. Vakhin. 2023. "Catalytic Conversion of Oil in Model and Natural Reservoir Rocks" Processes 11, no. 8: 2380. https://doi.org/10.3390/pr11082380